Abstract
Recently, there has been a surge in awareness of the gastrointestinal microbiome (GM) and its role in health and disease. Of particular note is an association between the GM and Parkinson’s disease (PD) and the realisation that the GM can act via a complex bidirectional communication between the gut and the brain. Compelling evidence suggests that a shift in GM composition may play an important role in the pathogenesis of PD by facilitating the characteristic ascending neurodegenerative spread of α-synuclein aggregates from the enteric nervous system to the brain. Here, we review evidence linking GM changes with PD, highlighting mechanisms supportive of pathological α-synuclein spread and intestinal inflammation in PD. We summarise existing patterns and correlations seen in clinical studies of the GM in PD, together with the impacts of non-motor symptoms, medications, lifestyle, diet and ageing on the GM. Roles of GM modulating therapies including probiotics and faecal microbiota transplantation are discussed. Encouragingly, alterations in the GM have repeatedly been observed in PD, supporting a biological link and highlighting it as a potential therapeutic target.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Parkinson’s disease (PD) is an incurable and progressive neurodegenerative disorder, affecting 1–2 per 1000 (0.4–2.0% of people above the age of 65) of the population worldwide [1]. PD is a multisystem disorder that contributes to significant morbidity and healthcare burden [2, 3]. It is predominantly associated with the irreversible degeneration of dopaminergic neurons in the substantia nigra and other brain regions [4], often characterised by widespread Lewy body (LB) formation in the central and peripheral nervous systems. Features characteristic of clinical disease include tremor, rigidity, bradykinesia and postural instability [5]. Premotor and non-motor symptoms of PD include constipation, hyposmia, REM-sleep behaviour disorder (RBD), as well as cognitive, neuropsychiatric, autonomic and sensory disturbances [6]. These symptoms can emerge years, or even decades, prior to the manifestation of motor symptoms, but often go unrecognised [7].
Recently, the human gastrointestinal microbiome (GM) has been proposed to be an integral link for the pathogenesis of many neurodegenerative diseases [8]. Evidence exists for a bidirectional interaction between the GM and the central nervous system (CNS), known as the ‘microbiota–gut–brain axis’ (MGBA) [9]. Multiple MGBA pathways exist, including microbially produced molecules with neuroendocrine activity (e.g. serotonin, gamma-aminobutyric acid) [10] and CNS-regulated physiological functions, such as intestinal motility influence on the microbial ecosystem [11]. These connections create a feedback loop between human physiological and microbial community states, forming the basis for neurodegenerative diseases of dysbiosis. Consequently, dysbiosis of the GM portrays an interesting lead to explore the pathogenesis of PD [12, 13] and presents as a novel diagnostic and therapeutic target [14].
The human gastrointestinal microbiome
The human GM contains in the order of 1 × 1014 bacteria [15], with a biomass comparable to the human brain at 1–2 kg [16]. The GM predominantly comprises bacteria, but also archaea, fungi, viruses and other simple eukaryotic organisms [8]. However, the metabolic activity of the GM is overwhelmingly dominated by bacteria. Their primary role is to anaerobically breakdown carbohydrates that are incompatible with human digestive processes. Other cellular microbes contribute other specific metabolic activities (e.g. methanogenesis by archaea) and bacteriophage viruses modulate the genetic background of bacterial growth in the gastrointestinal tract.
Establishment of the GM begins at birth and is vital for postnatal immune and enteric nervous system (ENS) development [17]. The GM also forms an integral defence barrier against potentially hazardous external stimuli and is highly adaptive with regard to changes in diet, lifestyle and the surrounding environment [18]. Diet, exercise, probiotic supplements and changes in hygiene can modify the composition of the GM [19], as can recolonisation following antibiotics and invasive pathogenic colonisation [20]. Despite constant environmental variation in the gut, the GM composition remains relatively stable during adulthood [21]. Although in some instances, dietary changes have been shown to induce variability over 3–4 days [22].
Gut microbiome and the nervous system
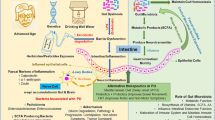
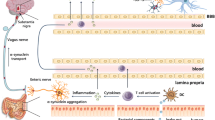
The MGBA connects the gastrointestinal tract to the CNS [23, 24], with neurosynaptic pathways of the vagal nerve, autonomic and enteric fibres, in addition to various neuroendocrine and neuroimmune pathways, mediating its integrity [25]. Under physiological conditions, the MGBA is a major participant in signalling pathways that regulate digestive function and metabolic homeostasis. However, various mechanisms that disrupt these pathways can perturb cognition, behaviour, learning, pain and neuropsychiatric features (including anxiety, depression and even neurodevelopmental disorders, such as autism) [15, 26,27,28]. It has been suggested that dysregulation of the MGBA may directly influence PD pathogenesis, with progressive neurodegeneration from the gut to the brain occurring as a consequence of ascending alpha-synuclein (α-Syn) aggregation and LB formation (Fig. 1) [29].
The microbiota–gut–brian axis. Likely implicated pathways in the pathogenesis of Parkinson’s disease. The microbiota–gut–brain axis provides a bidirectional communication between a dysbiotic gastrointestinal microbial population, characterised by increased inflammophiles and the central nervous system, via projections of the vagal nerve, as well as autonomic and enteric fibres. Abnormal changes in microbial metabolites, namely SCFAs, as well as the activation of innate immunity via pro-inflammatory cytokines propagate local inflammation, oxidative stress and increased mucosal permeability, facilitating leakage of bacterial products into the systemic circulation. These processes lead to abnormal enteric nervous system α-synuclein deposition and subsequent prion-like spread to the central nervous system, supported by ongoing activated microglial activity and systemic immune responses
The GM influences neuronal network health and activity by facilitating the absorption of nutrients, vitamins and medications, as well as modulating the immune system [30] and moderating neurotoxic compounds (e.g. ammonia and d-lactate) [31]. It is also independently capable of neurotransmitter synthesis, including dopamine, noradrenaline, serotonin and the neuromodulators γ-aminobutyric acid and short-chain fatty acids (SCFAs) [10, 32,33,34]. These neurotransmitters act to modulate blood flow, affect gut motility and nutrient absorption, as well as supporting the gastrointestinal innate immune system [35]. As an example, dopamine synthesis in the brain is mediated by catecholamine-producing enzymes that are governed by the GM via the MGBA, producing half of the body’s required dopamine [36]. Accordingly, this intricate network shares a crucial role in maintaining the vital physiological interplay between the gastrointestinal tract and the brain [37].
Braak’s hypothesis for Parkinson’s disease
Common clinical features of gastrointestinal dysfunction in PD include abnormal salivation, dysphagia, nausea, impaired gastric emptying and constipation, and occur in 80% of patients [30, 38]. Several large population studies have shown that constipation can precede motor symptoms of PD by up to 20 years, and increases the risk of developing PD [39, 40]. Consistent with the clinico-epidemiological observations, several neuropathological studies have found early accumulation of LBs in the ENS and dorsal motor nucleus of the vagus, with correlations to motor and gastrointestinal symptom severity [41, 42].
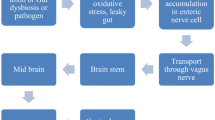
Braak and colleagues proposed a “dual-hit hypothesis”, where α-Syn aggregation commences outside of the brain, within the ENS and olfactory bulb, precipitated by external insults such as toxins and/or microorganisms [43]. They suggested that neuronal α-Syn deposition occurs in a caudorostral gradient, affecting the dorsal motor nucleus of the vagus nerve before progressing to the dopaminergic neurons of the substantia nigra [43]. However, this hypothesis remains controversial, as some studies were unable to replicate the caudorostral progression [44, 45]. Despite this, in support of Braak’s findings, α-Syn has been shown to exhibit prion-like properties, with the ability to misfold, form aggregates and propagate from cell to cell [46, 47]. Further evidence from follow-up cohort studies in Danish [48] and Swedish [49] populations showed that only a full truncal vagotomy could decrease the risk of developing PD compared to a control population. The level of significance was further diminished when the dataset was analysed by an independent research group [45, 50]. In addition, hemivagotomy in a PD mouse model prevented α-Syn accumulation in the ipsilateral dorsal motor nucleus of the vagus [51]. Interestingly, a recent large Swedish database study suggested those with an appendectomy early in life had a 20% reduced risk of developing PD [52]. Despite these findings, phosphorylated α-Syn accumulation in the intestine is not a specific hallmark of PD, having been observed in patients with Lewy body dementia, Alzheimer’s with Lewy bodies and asymptomatic controls [53]. Thus, what constitutes pathological α-Syn species in the gut remains ill defined [54].
Inflammation and gut permeability
A critical aspect of host–microbiome interaction is the barrier functions of the gut epithelium [55]. Disruption of the barrier can trigger positive feedback loops involving intestinal inflammation, reactive oxygen/nitrogen species in the gut lumen and a shift in microbial composition favouring inflammophiles [56, 57]. Key to aberrant GM influence through the MGBA is destabilisation of the protective gastrointestinal barrier, resultant of translocation of bacteria or their products, such as lipopolysaccharides. This leads to oxidative stress and intestinal inflammation, which induces increased mucosal permeability [30, 58], as well as α-Syn aggregation in the ENS [59,60,61].
Intestinal permeability or ‘gut leakiness’ has been shown to be increased in PD patients compared to healthy controls [62,63,64] and mouse models of PD [65], correlating with an increase in enteric α-Syn deposition and tissue oxidative stress [62]. However, findings suggestive of increased intestinal permeability in PD require cautious evaluation. For example, Clairembault et al. [55] showed that the intestinal epithelial barrier was morphologically altered in PD but showed no change in the permeability of the intestinal epithelial barrier. Despite this, other disorders known to have increased intestinal permeability, such as inflammatory bowel disease, have recently been associated with an increased risk of PD. This further supports a role for gastrointestinal inflammation in the development of PD [66,67,68]. Moreover, priming of the gastrointestinal innate immune system by the GM can strengthen the inflammatory response to α-Syn [11].
Microbes vary in their cell structure and propensity to initiate pattern-recognition receptor signalling pathways, leading to inflammation. Evidence suggests that increased levels of Escherichia coli [62] and the proteobacteria Ralstonia [69], as well as lower plasma lipopolysaccharide-binding protein [70], reflect higher endotoxin exposure and drive intestinal inflammation. This inflammation is characterised by increased expression of the pro-inflammatory cytokines TNF-alpha, IFN-gamma, IL-6, and IL-1 beta, as well as an increased activation of enteric glial cells, consistent with colonic biopsies of PD patients [60]. Moreover, Perez-Pardo et al. [71] suggested that toll-like-receptor-4 signalling pathways may be implicated in facilitating intestinal inflammation, disrupting the intestinal barrier and facilitating neuroinflammation, as shown in PD patients and a PD mouse.
Further, SCFAs (acetate, propionate and butyrate) are major metabolic products of the GM, with lower faecal SCFA concentrations reported in PD patients compared to healthy controls [72]. SCFAs have multiple impacts, with nutritional and signalling roles being especially important in humans [73]. In particular, butyrate is a major energy substrate for colonocytes, playing an important role in gut health processes, such as mucin production and tight-junction formation [74]. Beyond their nutritional role, SCFAs are also a primary target for several G protein-coupled receptors that feed into immunometabolic regulatory circuits. The relative concentration and distribution of SCFA types have profound influence over gut function and homeostatic processes.
Several studies have indicated a decreased abundance of Lachnospiraceae in PD patients [69, 75, 76], known for their abundant production of SCFAs. Sampson et al. [77] suggested that SCFAs were a major factor inducing microglial activation and accelerating α-synucleinopathy in mouse models, thus enhancing PD pathophysiology [78]. However, depletion of SCFA-producing organisms in the gut has been observed in a variety of other conditions, suggesting this may be a marker of illness or associated with ageing [72], rather than a specific cause or biomarker of PD [75]. Consistently, controversy remains over the validity of decreased SCFAs leading to a pro-inflammatory gut environment in PD [78].
Gut infections in Parkinson’s disease
The role of Helicobacter pylori in gastritis and dyspepsia is well known. However, in PD, it also appears to be associated with greater severity of motor features [79]. Several studies have suggested that antimicrobial treatment for H. pylori could improve PD motor symptoms and motor response complications, as well as levodopa absorption and bioavailability [80]. However, a Cochrane review concluded that current evidence remained insufficient to recommend H. pylori eradication in PD, due to a lack of informative clinical trials [81]. The prevalence of small intestinal bacterial overgrowth is estimated to range from 25–54% in PD [82, 83] and is associated with worse motor severity [83], longer daily off times and more episodes of delayed-on and dose failures [84]. A small-randomised controlled trial suggested that small intestinal bacterial overgrowth eradication in PD could result in improved motor fluctuations, without affecting the pharmacokinetics of levodopa [84].
Associations between gastrointestinal microbiota community structure and Parkinson’s disease
Over the last 4 years, there have been numerous studies exploring the association between GM and PD, stimulated by greater awareness of gastrointestinal dysfunction in prodromal and early PD, as well as a better understanding of the MGBA [85, 86]. To date, 14 mainly cross-sectional studies from seven countries in the northern hemisphere have reported GM alterations in PD [12, 13, 69, 70, 72, 75, 76, 87,88,89,90,91,92,93]. At the level of taxonomic profiles, no obvious recurrent pattern is seen in these studies. However, emerging trends show correlations between GM alterations in PD and a variety of motor and non-motor symptoms [94]. Considerable variabilities in the patient inclusion/exclusion criteria, molecular, bioinformatic and statistical methodologies between the studies were apparent and are summarised in Table 1. Most studies employed bacterial 16S ribosomal DNA amplicon sequencing of different variable regions to identify bacterial groups/genera/species, with three studies using targeted quantitative PCR to analyse preselected bacterial taxa, and one utilised metagenomic shotgun sequencing. PD patient cohort sizes ranged from 24 to 197 individuals, with all but one study comparing to a healthy control group. Exclusion of participants for use of antibiotics within 1 month of sample collection was generally observed. Only one study reported on longitudinal GM differences with a comparison at 2-year follow-up in 28 patients [13].
Each of the studies found significant differences between PD and healthy control groups, and collectively showed significant differences in the overall faecal GM composition in PD patients when compared to non-PD controls. In spite of this, it is not certain whether these changes are the cause or consequence of the gastrointestinal dysfunction associated with PD. Recent evidence from Heintz-Buschart et al. [88] suggests that GM alterations likely pre-date the motor symptoms of PD. The highest number of microbiota abundance differences between PD and controls was reported in the phylum Firmicutes [95], the largest phylum in the human GM. Specifically, increased Lactobacillaceae (family) and Lactobacillus (genus) and decreased Lachnospiraceae (family), Blautia, Roseburia, Dorea and Faecalibacterium (genus) were observed. The most consistent differences were reported in the phylum Verrucomicrobia, principally increased Verrucomicrobiaceae (family), Akkermansia (genus) and Akkermansia muciniphila (species). Moreover, significant changes in abundance of the phylum Bacteroidetes were also reported, with reductions in Prevotellaceae (family), Prevotella (genus) and Prevotella copri (species) (Table 1). Some studies deduced that putative “pro-inflammatory” bacteria were significantly more abundant, while putative beneficial bacteria were less abundant in PD [69].
Several studies have evaluated predicted functional differences based on faecal GM metagenomic data and suggested alterations of several metabolic pathways in PD, when compared to non-PD controls [69, 75, 91]. Kershavarzian et al. [69] showed that a large number of genes involved in metabolism were significantly lower in the PD faecal microbiome, whereas genes involved in lipopolysaccharide biosynthesis and type III bacterial secretion systems were significantly higher in PD patients. Hill-Burns et al. [75] found alterations in pathways involved in carbohydrate, energy, lipid, cofactor, vitamin and xenobiotic metabolism, as well as xenobiotics. The only study that utilised a very comprehensive metagenomics shotgun analysis found differences in microbiota metabolism in PD involving the β-glucuronate and tryptophan metabolism pathways [91]. Furthermore, metabolite studies reported reduced levels of serum lipopolysaccharide-binding protein [70, 96] and faecal SCFA [72] in PD patients.
Evaluating atypical parkinsonism, Barichella et al. [76] suggested the overall gut microbiota composition in multiple system atrophy (MSA) and progressive supranuclear palsy were not significantly distinct when compared to PD. Moreover, changes in several bacteria taxa when compared to controls were similar to PD [76], whilst drug-naïve PD patients had lower abundances of Lachnospiraceae when compared to controls [76]. In another MSA GM study, higher relative abundances of Gram-negative, putative ‘pro-inflammatory’ bacteria from the phylum Bacteroidetes and Proteobacteria were noted in faecal and mucosal samples, whilst putative ‘anti-inflammatory’ butyrate-producing bacteria were less abundant. Further, the relative abundance of a number of genes involved in metabolism were lower in faecal material from patients with MSA, whilst the relative abundance of genes involved in lipopolysaccharide biosynthesis were higher in MSA patients compared to healthy controls [97]. Several bacteria taxa have been associated with various motor response complications and worse motor severity in PD (Table 2).
Significant differences were also found in the GM composition of sigmoid mucosa in PD when compared to controls, although differences were more pronounced in faecal versus mucosal samples [69]. Comparison of nasal microbiota between PD patients and controls did not yield significantly different results, limiting the utility of a nasal microbiota biomarker in PD [88, 98]. One study on oral microbiota found significant differences in overall composition between PD patients and controls and, interestingly, a higher abundance of Prevotellaceae in PD, contrary to the previous findings in the faecal microbiome [98]. Lastly, increased abundance of Anaerotruncus and reduced relative abundance of Prevotellaceae were shown in patients with idiopathic RBD, suggesting a possible prodromal GM change in PD [88].
Of great interest is the pioneering work by Samson and colleagues [77], who have provided the strongest evidence to date for a causal role of GM alterations in PD. These investigators showed that transplantation of gut microbiota from six PD patients to transgenic mice overexpressing α-Syn resulted in worsening of clinically relevant motor. Additionally, mice raised in a germ-free environment developed little PD-related pathophysiology, including motor dysfunction, neuroinflammation and α-Syn pathology [77]. They hypothesised that the clinical effects of gut microbiota were primarily mediated by SCFAs, enabling the activation of microglia and potentially leading to increased neuroinflammation and subsequent exacerbation of PD. Furthermore, they demonstrated that the oral administration of SCFAs in a germ-free PD mouse model resulted in prominent motor symptoms [77].
Non-motor symptoms and the gastrointestinal microbiota
Non-motor symptoms (NMS) generally pre-date motor features in PD, reflecting degeneration of extra-nigral areas prior to involvement of the substantia nigra. Common NMS include disrupted sleep architecture (particularly with RBD), impaired olfaction, behavioural changes, as well as impaired visuospatial abilities and executive function [99]. There is growing evidence of altered GM composition in various PD-related NMS, in addition to other psychiatric disorders, namely anxiety and depression, which are highly prevalent in PD [100]. Recently, it was identified that changes in Anaerotruncus, Akkermansia and several other unclassified bacteria were significantly related to NMS according to MDS-UPDRS part I [88] (Table 2). For instance, an altered abundance of Anaerotruncus spp. was related to depression in PD [88] and patients with anxiety had a higher abundance of Clostridium clariflavum compared to those without anxiety [101]. Furthermore, those with moderate depression were suggested to have higher relative abundance of Christensenella minuta, Clostridium disporicum and Oscillibacter valericigenes compared to those without depression or with mild depression [101]. In other PD studies, depression was also associated with the genera Anaerotruncus spp. [88]. Studies outside of PD have identified significant changes in the GM composition in those with depression. Depression and PD frequently coincide, with depression often contributing significantly to impairment of health-related quality of life in PD [102]. Jiang et al. [103] showed that those who were acutely depressed had higher levels of Bacteroidetes, Proteobacteria and Actinobacteria, and lower levels of Firmicutes; a negative correlation between Faecalibacterium and the severity of depression was also described.
Gastrointestinal symptoms are one of the most common PD NMS, involving the entirety of the gastrointestinal tract and evident throughout the whole disease course [104]. Patients with PD and irritable bowel syndrome (IBS)-like symptoms have been shown to feature more NMS, as well as a lower faecal abundance of Prevotella bacteria than those without IBS-like symptoms [86]. Constipation, a cardinal NMS of PD, has also been shown to predispose to GM alterations, along with increased mucosal permeability and inflammation [105]. Cognitive changes have been linked to varied GM compositions in PD, with positive associations in the abundance of Butyricicoccus and Clostridium XIVb described [90]. In a 2-year longitudinal study, lower counts of Bifidobacterium and Bacteroides fragilis at baseline were associated with worsening hallucinations and delusions, and worsening motivation and initiative [13], respectively.
Parkinson’s disease medications and the gastrointestinal microbiome
An extensive range of medications have been proposed to influence the composition of the GM, including several PD medications [106]. One study reported that treatment with standard PD treatments, catechol-O-methyltransferase (COMT) inhibitors and anticholinergics, resulted in a difference in the composition of the GM, independent of the PD effect [75]. COMT inhibitor usage was also shown to be significantly associated with increased abundance of Lactobacillaceae and reduced abundance of Clostridiales Incertae Sedis IV [12]. The isolated effects of levodopa/carbidopa were difficult to identify due to ubiquitous usage by PD patients [75]. However, levodopa appeared to be significantly associated with Bacillaceae abundance in one study [88]. Qian et al. [90] suggested that the levodopa equivalent daily dose was negatively associated with the genera Dorea and Phascolarctobacterium. Furthermore, Bedarf et al. [91] commented that the use of monoamine oxidase inhibitors, amantadine or a dopamine agonist had no overall influence on taxa abundance or microbial function. Significantly, reduced abundance of Prevotella, reported by several PD–GM studies, could not be explained by PD medications, and was observed in levodopa naïve early-stage PD patients [91]. Importantly, other significant between-group differences in the GM were observed between drug-naïve and treated PD patients [69]. Interestingly, no studies have specifically evaluated the GM composition in advanced disease patients requiring device-assisted therapies, highlighting a need for more studies in pre-treated PD patients. Hopfner et al. [92] reported that in their cohort of 29 PD patients, six were treated with deep brain stimulation, although no GM subgroup analysis was provided.
Lifestyle influences and other potential confounders of the gastrointestinal microbiota in Parkinson’s disease
There is increasing evidence that certain lifestyle factors contribute to PD pathology. Increased urate levels have been shown to provide a protective effect through antioxidant activity, conferring a 33% risk reduction for PD [107]. Furthermore, although debated, epidemiological studies suggest a smoking history may confer approximately 60% reduced risk of developing PD, whilst coffee consumption was found to reduce the risk by 30% [108, 109]. These neuroprotective effects are likely mediated through nicotinic acetylcholine and adenosine A(2A) receptor-mediated activity [30], or through prevention of α-Syn misfolding and fibril formation [110]. Derkinderen et al. [111] suggested that the beneficial effects of smoking and coffee consumption in PD risk could be mediated through the MGBA, specifically by altering the composition of the GM in ways that mitigate intestinal inflammation, thus reducing α-Syn misfolding and deposition. This hypothesis was supported by a study in humans showing gut dysbiosis after smoking cessation [112], with smokers having a higher abundance of faecal Bacteroides and Prevotella. After smoking cessation, a decreased abundance of Proteobacteria and an increased abundance of Firmicutes and Actinobacteria were observed [112].
Effects of coffee consumption may relate to its composition of alkaloids, phenolic compounds, fibres and minerals [30]. SCFAs that arise from coffee’s dietary fibre metabolism may cause an expansion of Bacteroides and Prevotella bacteria [113]. Coffee consumption can also increase the abundance of anti-inflammatory Bifidobacteria and decrease Clostridium spp. and Escherichia coli [114, 115]. Furthermore, consumption of alcohol-containing products, coffee, tea and sugar-sweetened drinks show correlation with altered microbial compositions. For instance, red wine consumption correlates with an increased abundance of F. prausnitzii, known for its anti-inflammatory functions [116]. Furthermore, coffee, tea and red wine consumption have been associated with increased GM diversity [117], supporting lifestyle modification in the influence of PD predisposition.
Stress has also been suggested to promote gut leakiness and affect the microbiota community abundances in several animal and human studies [118, 119]. A recent study by Dodiya et al. [120] showed that stress exacerbated intestinal inflammation, gut leakiness, endotoxemia, neuroinflammation and dopamine loss, in a PD mouse model.
Macronutrients, particularly carbohydrates, have also been shown to influence the composition and function of the GM [121]. For instance, individuals consuming more plant carbohydrates and fibre had a higher abundance of Prevotella/Paraprevotella, compared to those consuming predominantly animal fat and protein who harboured more of the Bacteroides enterotype [122]. Fibre-rich diets appear to enhance the growth of colonic bacteria producing SCFAs, which in turn confer systemic anti-inflammatory effects [72]. For instance, Prevotella, which has been shown at lower abundance in PD [12], is an important contributor to gut SCFA synthesis, as well as folate and thiamine biosynthesis [123], nutrients known to be deficient in PD [124].
Further, the Western diet is known to be high in refined carbohydrates and saturated fats, which may result in a dysbiotic GM, with lower Bifidobacteria, higher Firmicutes and Proteobacteria [125]. High-fat diets have also been shown to induce intestinal and systemic inflammation in animal models by increasing intestinal permeability and altering the GM [126]. Consumption of dairy products has been suggested to variably affect the risk of PD [109]. Drinking sour milk with a lower fat content has been associated with higher GM diversity of beneficial Leuconostoc mesenteroides and Leuconostoc lactis, whilst drinking whole milk was associated with lower diversity [116]. Furthermore, studies evaluating urate metabolism suggested that Prevotella-dominant enterotypes were associated with higher serum urate levels [12].
The effects of age and exercise have also been described in relation to the GM. Age-related changes in the GM reflect decreased diversity of species [127], with corresponding decreases in microbial functional abundance [116]. Biagi et al. [128] suggested that ageing was associated with increasing abundance of subdominant species, including rearrangement in their co-occurrence networks. A significant reduction in the abundance of Lactobacilli, Bacteroides/Prevotella and Faecalibacterium prausnitzii and an increased abundance of Ruminococcus, Atopobium and Enterobacteriaceae were reported in individuals with high frailty scores [129]. Elderly community-dwelling residents were noted to share a more diverse GM and were healthier compared to those in short or long-term residential care [130], reflected by greater inter-individual GM variation, as well as significant relationships between the GM, diet and differences between community and institutional living [131].
Exercise has also been associated with favourable changes in the GM composition, influencing energy homeostasis and regulation [132]. Physical exercise has been suggested to enhance the number of beneficial bacterial species, enrich microbial diversity and improve the development of commensal bacteria, irrespective of diet [132, 133]. Estaki et al. [133] found that fitter individuals had a GM enriched in butyrate-producing taxa, including Roseburia, Clostridiales, Lachnospiraceae and Erysipelotrichaceae, favouring optimal gut health and improved metabolism.
Antibiotics, not surprisingly, have been shown to induce significant changes in the GM, as well as in dopaminergic signalling [134]. Antibiotic therapy affects the diversity of the GM and is associated with reduced abundance of Bifidobacteria, as well as Bacteroides and Prevotella groups [130]. Minocycline has shown neuroprotective efficacy in PD animal models, by limiting nigrostriatal neurodegeneration, as well as blocking dopamine depletion in the striatum and nucleus accumbens [135]. Other studies have also suggested minocycline-related antioxidant and anti-inflammatory properties confer dopaminergic neuroprotection [136]. Minocycline has been further shown to re-balance GM dysbiosis in a hypertension model, by reducing the Firmicutes:Bacteroidetes ratio [137] although no studies have been conducted to directly evaluate minocycline’s effect on the GM in PD patients [15]. Furthermore, minocycline is implicated in modulation of depression, likely due to anti-inflammatory properties and neuroprotective roles [138]. Conversely, minocycline has been suggested to have deleterious effects in PD animal models [139].
Other antibiotics, including ampicillin, ceftriaxone, neomycin, metronidazole and polymyxin B, have also shown potential neuroprotective mechanisms or result in clinical disease changes via MGBA interactions [15]. Potential mechanisms suggest decreased physical activity, as well as modulation of GM abundances, as potential considerations [140]. Despite these small, mainly animal-based studies describing interesting interactions with antibiotic use and the neurobiology of GM–PD, further human clinical trials are required to investigate potential antibiotic influences on the MGBA.
Potential roles of prebiotics and probiotics in Parkinson’s disease
Emerging interest evaluating prebiotic and probiotic use in PD and other neuropsychiatric disorders is providing valuable insight into potential novel mechanisms aimed at favourably modifying various symptoms. Consumption of fermented milk containing Lactobacillus casei Shirota for 5 weeks was shown to improve stool consistency and reduce bloating and abdominal pain in patients with PD [141]. A more recent randomised controlled trial showed that intake of fermented milk containing probiotic strains and prebiotic fibre significantly increased the frequency of complete bowel movements in PD patients [142]. Probiotic studies have reported beneficial health effects by a variety of mechanisms, including enhancing intestinal epithelial integrity, protecting against barrier disruption, stimulating a healthy mucosal immune system and suppressing pathogenic bacterial growth [143].
Human studies are beginning to show that probiotics may be effective in reducing depression and anxiety-like symptoms [144], with those receiving probiotics having higher numbers of faecal–gut microbial species and lowered levels of Bacteroidaceae compared to healthy controls [145]. Improving gastrointestinal function with probiotic supplementation may also potentially improve levodopa absorption [143], and reduce behavioural and cognitive deficits commonly observed in PD, such as anxiety, depression and memory problems [146]. Prebiotics, which include dietary soluble fibres, such as galactooligosaccharides or fructooligosaccharides, can stimulate the growth of intrinsic commensal microbiota and have also been keenly studied for their effects on mood [147]. Recent prebiotic studies have shown promising anxiety-modifying effects after consumption of certain dietary soluble fibres [148] and fermented foods [149], suggesting anxiolytic efficacy. Therefore, probiotic use and specific dietary modifications may provide useful treatment modalities for patients with PD, aiming to favourably modify the GM composition, by reducing ENS inflammation and α-Syn aggregation, whilst improving gastrointestinal function.
Controversies and future directions
Faecal microbiota transplantation (FMT) was described some 2000 years ago in China, in the treatment of human gastrointestinal disease [150]. FMT has been increasingly used in the treatment of resistant Clostridium difficile infection with excellent efficacy and safety [151, 152] and was more recently trialled in non-PD patients, with marked improvement of constipation [153]. However, little is known regarding its utility in PD, with only anecdotal reports of benefit [154]. Evolving interest exploring FMT in PD suggests a possible slowing of disease progression, coupled with potential change in diet and medication requirement, whilst repopulating the gastrointestinal tract with favourable microbiota [155]. Prospective longitudinal controlled trials are needed to validate the safety and efficacy potential of FMT for PD recipients. It is important to note, however, that neither pre/probiotics nor FMT appear to influence the core PD symptoms but, at best, may alter associated disease features such as anxiety, depression and, of course, constipation.
Further, the scope of gut dysbiosis in PD may be rather more complex and also reflect important changes in the gut virome, particularly inferred by bacteriophage viruses. A recent study by Tetz et al. [156] identified an increased abundance of Lactococcus bacteriophage in PD, impacting abundances of lactic acid bacteria, known to produce dopamine and regulate intestinal permeability. Ultimately, the GM may be utilised as an early and minimally invasive PD biomarker, something urgently needed to provide prodromal or preclinical diagnosis of PD. The most compelling results from across the PD–GM studies identify increased abundances of Lactobacillaceae (family), Lactobacillus (genus), Verrucomicrobiaceae (family), Akkermansia (genus) and reduced Prevotellaceae (family), Prevotella (genus), Lachnospiraceae (family), Blautia, Roseburia, Dorea and Faecalibacterium (genus) in PD patients [12, 13, 69, 70, 72, 75, 76, 87,88,89,90,91,92,93]. These findings suggest significant biological variations that need to be further explored by a meta-analysis evaluating the influence of confounders, such as geography and dietary variations, and whether a distinct and consistent GM pattern exists in PD. Furthermore, lowered Prevotella abundance is not entirely specific to PD, as other conditions including type I diabetes [157], colon cancer [158] and autism [159], also share reduced Prevotella abundances. Perhaps inversely, the elevated faecal abundance of Prevotella seen in several studies could be utilised as a useful biomarker to exclude PD. Interestingly, the canonical signature of GM dysbiosis, reflected by typically increased abundances of Proteobacteria bacteria [160], was not clearly shown across the PD–GM studies. Instead, increased abundances of Akkermansia were noted, requiring further evaluation by meta-analysis.
With expanding interest in the GM and neurodegenerative disorders, next-generation sequencing and high-throughput taxonomic classification will be an important approach to identify patterns of differing GM abundances. If coupled with faecal metabolic profiling, the combination of approaches may better identify useful biomarkers. By identifying disease discriminant biomarkers, faecal microbiota screening in the community may one day be utilised to identify individuals at risk of neurological dysfunction, as well as those with prodromal PD. It would be ideal for these clinical efforts to be complemented by well-designed preclinical models aiming to understand the causal link between the GM and PD. This could lead to the development of novel therapeutic approaches aimed at interfering with α-Syn aggregation and clearance [161,162,163].
Conclusion
Across the most recent GM studies in PD, changes in bacterial taxa have been repeatedly shown in association with disease, endorsing a plausible biological link between the GM and PD [164]. With increasing acceptance, it appears that these GM changes are implicated and contribute to PD pathology, rather than a mere consequence of PD-related gut dysfunction. The MGBA interactions are complex and require ongoing study, although evidence suggests that gut mucosal integrity and permeability, SCFA metabolism, oxidative stress and inflammation are cardinal to the process [85]. Ultimately, these mechanisms support α-Syn aggregation within the ENS and subsequent caudorostral cell-to-cell α-Syn transfer, leading to advancing nigrostriatal dopaminergic network failure, with subsequent overt manifestation of disease. An improved understanding of the specific attributes and characteristics of the GM community structures in PD could lead to more targeted antimicrobial therapies and dietary interventions aimed at modulating the GM. However, the frontier is rapidly shifting and future research needs to challenge these observations by exploring such mechanisms that modulate the GM in PD, as a viable therapeutic strategy to slow or arrest the spread of disease. If such means are found, it could unravel a new wave of therapies that target the disease pathophysiology rather than just addressing symptomatic relief, providing a new direction in PD treatment that is urgently needed.
References
Pringsheim T, Jette N, Frolkis A, Steeves TD (2014) The prevalence of Parkinson’s disease: a systematic review and meta-analysis. Mov Disord 29(13):1583–1590
Lubomski M, Rushworth RL, Tisch S (2015) Hospitalisation and comorbidities in Parkinson’s disease: a large Australian retrospective study. J Neurol Neurosurg Psychiatry 86(3):324–330
Lim SY, Tan AH, Fox SH, Evans AH, Low SC (2017) Integrating patient concerns into Parkinson’s disease management. Curr Neurol Neurosci Rep 17(1):3
Rietdijk CD, Perez-Pardo P, Garssen J, van Wezel RJ, Kraneveld AD (2017) Exploring Braak’s hypothesis of Parkinson’s disease. Front Neurol 8:37
Samii A, Nutt JG, Ransom BR (2004) Parkinson’s disease. Lancet 363(9423):1783–1793
Goldman JG, Postuma R (2014) Premotor and nonmotor features of Parkinson’s disease. Curr Opin Neurol 27(4):434–441
Postuma RB, Aarsland D, Barone P, Burn DJ, Hawkes CH, Oertel W et al (2012) Identifying prodromal Parkinson’s disease: pre-motor disorders in Parkinson’s disease. Mov Disord 27(5):617–626
Tremlett H, Bauer KC, Appel-Cresswell S, Finlay BB, Waubant E (2017) The gut microbiome in human neurological disease: a review. Ann Neurol 81(3):369–382
Nicholson JK, Holmes E, Kinross J, Burcelin R, Gibson G, Jia W et al (2012) Host-gut microbiota metabolic interactions. Science 336(6086):1262–1267
Lyte M (2014) Microbial endocrinology: host-microbiota neuroendocrine interactions influencing brain and behavior. Gut Microbes 5(3):381–389
Mukherjee A, Biswas A, Das SK (2016) Gut dysfunction in Parkinson’s disease. World J Gastroenterol 22(25):5742–5752
Scheperjans F, Aho V, Pereira PA, Koskinen K, Paulin L, Pekkonen E et al (2015) Gut microbiota are related to Parkinson’s disease and clinical phenotype. Mov Disord 30(3):350–358
Minato T, Maeda T, Fujisawa Y, Tsuji H, Nomoto K, Ohno K et al (2017) Progression of Parkinson’s disease is associated with gut dysbiosis: two-year follow-up study. PLoS One 12(11):e0187307
Nair AT, Ramachandran V, Joghee NM, Antony S, Ramalingam G (2018) Gut microbiota dysfunction as reliable non-invasive early diagnostic biomarkers in the pathophysiology of Parkinson’s disease: a critical review. J Neurogastroenterol Motil 24(1):30–42
Parashar A, Udayabanu M (2017) Gut microbiota: implications in Parkinson’s disease. Parkinsonism Relat Disord 38:1–7
Zhu B, Wang X, Li L (2010) Human gut microbiome: the second genome of human body. Protein Cell 1(8):718–725
Gomez de Aguero M, Ganal-Vonarburg SC, Fuhrer T, Rupp S, Uchimura Y, Li H et al (2016) The maternal microbiota drives early postnatal innate immune development. Science 351(6279):1296–1302
Zuker CS (2015) Food for the brain. Cell 161(1):9–11
Sommer F, Backhed F (2013) The gut microbiota-masters of host development and physiology. Nat Rev Microbiol 11(4):227–238
Costello EK, Stagaman K, Dethlefsen L, Bohannan BJ, Relman DA (2012) The application of ecological theory toward an understanding of the human microbiome. Science 336(6086):1255–1262
Lozupone CA, Stombaugh JI, Gordon JI, Jansson JK, Knight R (2012) Diversity, stability and resilience of the human gut microbiota. Nature 489(7415):220–230
David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE et al (2014) Diet rapidly and reproducibly alters the human gut microbiome. Nature 505(7484):559–563
Cryan JF, O’Mahony SM (2011) The microbiome-gut-brain axis: from bowel to behavior. Neurogastroenterol Motil 23(3):187–192
Bercik P (2011) The microbiota-gut-brain axis: learning from intestinal bacteria? Gut 60(3):288–289
Grenham S, Clarke G, Cryan JF, Dinan TG (2011) Brain-gut-microbe communication in health and disease. Front Physiol 2:94
Dinan TG, Cryan JF (2015) The impact of gut microbiota on brain and behaviour: implications for psychiatry. Curr Opin Clin Nutr Metab Care 18(6):552–558
Cryan JF, Dinan TG (2012) Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci 13(10):701–712
Hsiao EY, McBride SW, Hsien S, Sharon G, Hyde ER, McCue T et al (2013) Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell 155(7):1451–1463
Braak H, de Vos RA, Bohl J, Del Tredici K (2006) Gastric alpha-synuclein immunoreactive inclusions in Meissner’s and Auerbach’s plexuses in cases staged for Parkinson’s disease-related brain pathology. Neurosci Lett 396(1):67–72
Scheperjans F, Pekkonen E, Kaakkola S, Auvinen P (2015) Linking smoking, coffee, urate, and Parkinson’s disease—a role for gut microbiota? J Parkinson’s Dis 5(2):255–262
Galland L (2014) The gut microbiome and the brain. J Med Food 17(12):1261–1272
Mulak A, Bonaz B (2015) Brain-gut-microbiota axis in Parkinson’s disease. World J Gastroenterol 21(37):10609–10620
Asano Y, Hiramoto T, Nishino R, Aiba Y, Kimura T, Yoshihara K et al (2012) Critical role of gut microbiota in the production of biologically active, free catecholamines in the gut lumen of mice. Am J Physiol Gastrointest Liver Physiol 303(11):G1288–G1295
Valles-Colomer M, Falony G, Darzi Y, Tigchelaar EF, Wang J, Tito RY et al (2019) The neuroactive potential of the human gut microbiota in quality of life and depression. Nat Microbiol 4:623–632
Mittal R, Debs LH, Patel AP, Nguyen D, Patel K, O’Connor G et al (2017) Neurotransmitters: the critical modulators regulating gut-brain axis. J Cell Physiol 232(9):2359–2372
Eisenhofer G, Aneman A, Friberg P, Hooper D, Fandriks L, Lonroth H et al (1997) Substantial production of dopamine in the human gastrointestinal tract. J Clin Endocrinol Metab 82(11):3864–3871
Rhee SH, Pothoulakis C, Mayer EA (2009) Principles and clinical implications of the brain-gut-enteric microbiota axis. Nat Rev Gastroenterol Hepatol 6(5):306–314
Perez-Pardo P, Hartog M, Garssen J, Kraneveld AD (2017) Microbes tickling your tummy: the importance of the gut-brain axis in Parkinson’s disease. Curr Behav Neurosci Rep 4(4):361–368
Abbott RD, Petrovitch H, White LR, Masaki KH, Tanner CM, Curb JD et al (2001) Frequency of bowel movements and the future risk of Parkinson’s disease. Neurology 57(3):456–462
Savica R, Carlin JM, Grossardt BR, Bower JH, Ahlskog JE, Maraganore DM et al (2009) Medical records documentation of constipation preceding Parkinson disease: a case-control study. Neurology 73(21):1752–1758
Cersosimo MG, Benarroch EE (2012) Pathological correlates of gastrointestinal dysfunction in Parkinson’s disease. Neurobiol Dis 46(3):559–564
Lebouvier T, Neunlist M, des Varannes SB, Coron E, Drouard A, N’Guyen JM et al (2010) Colonic biopsies to assess the neuropathology of Parkinson’s disease and its relationship with symptoms. PloS One 5(9):e12728
Braak H, Rub U, Gai WP, Del Tredici K (2003) Idiopathic Parkinson’s disease: possible routes by which vulnerable neuronal types may be subject to neuroinvasion by an unknown pathogen. J Neural Trans 110(5):517–536
Adler CH, Beach TG (2016) Neuropathological basis of nonmotor manifestations of Parkinson’s disease. Mov Disord 31(8):1114–1119
Lionnet A, Leclair-Visonneau L, Neunlist M, Murayama S, Takao M, Adler CH et al (2018) Does Parkinson’s disease start in the gut? Acta Neuropathol 135(1):1–12
Goedert M, Masuda-Suzukake M, Falcon B (2017) Like prions: the propagation of aggregated tau and alpha-synuclein in neurodegeneration. Brain 140(2):266–278
Recasens A, Ulusoy A, Kahle PJ, Di Monte DA, Dehay B (2018) In vivo models of alpha-synuclein transmission and propagation. Cell Tissue Res 373(1):183–193
Svensson E, Horvath-Puho E, Thomsen RW, Djurhuus JC, Pedersen L, Borghammer P et al (2015) Vagotomy and subsequent risk of Parkinson’s disease. Ann Neurol 78(4):522–529
Liu B, Fang F, Pedersen NL, Tillander A, Ludvigsson JF, Ekbom A et al (2017) Vagotomy and Parkinson disease: a Swedish register-based matched-cohort study. Neurology 88(21):1996–2002
Tysnes OB, Kenborg L, Herlofson K, Steding-Jessen M, Horn A, Olsen JH et al (2015) Does vagotomy reduce the risk of Parkinson’s disease? Ann Neurol 78(6):1011–1012
Pan-Montojo F, Schwarz M, Winkler C, Arnhold M, O’Sullivan GA, Pal A et al (2012) Environmental toxins trigger PD-like progression via increased alpha-synuclein release from enteric neurons in mice. Sci Rep 2:898
Killinger BA, Madaj Z, Sikora JW, Rey N, Haas AJ, Vepa Y et al (2018) The vermiform appendix impacts the risk of developing Parkinson’s disease. Sci Transl Med 10(465):eaar5280
Visanji NP, Marras C, Kern DS, Al Dakheel A, Gao A, Liu LW et al (2015) Colonic mucosal a-synuclein lacks specificity as a biomarker for Parkinson disease. Neurology 84(6):609–616
Ruffmann C, Parkkinen L (2016) Gut feelings about alpha-synuclein in gastrointestinal biopsies: biomarker in the making? Mov Disord 31(2):193–202
Clairembault T, Leclair-Visonneau L, Coron E, Bourreille A, Le Dily S, Vavasseur F et al (2015) Structural alterations of the intestinal epithelial barrier in Parkinson’s disease. Acta Neuropathol Commun 3:12
Ha CW, Lam YY, Holmes AJ (2014) Mechanistic links between gut microbial community dynamics, microbial functions and metabolic health. World J Gastroenterol 20(44):16498–16517
Levy M, Kolodziejczyk AA, Thaiss CA, Elinav E (2017) Dysbiosis and the immune system. Nat Rev Immunol 17(4):219–232
Carabotti M, Scirocco A, Maselli MA, Severi C (2015) The gut-brain axis: interactions between enteric microbiota, central and enteric nervous systems. Ann Gastroenterol 28(2):203–209
Houser MC, Tansey MG (2017) The gut-brain axis: is intestinal inflammation a silent driver of Parkinson’s disease pathogenesis? NPJ Parkinson’s Dis 3:3
Devos D, Lebouvier T, Lardeux B, Biraud M, Rouaud T, Pouclet H et al (2013) Colonic inflammation in Parkinson’s disease. Neurobiol Dis 50:42–48
Houser MC, Chang J, Factor SA, Molho ES, Zabetian CP, Hill-Burns EM et al (2018) Stool immune profiles evince gastrointestinal inflammation in Parkinson’s Dis. Mov Disord 33(5):793–804
Forsyth CB, Shannon KM, Kordower JH, Voigt RM, Shaikh M, Jaglin JA et al (2011) Increased intestinal permeability correlates with sigmoid mucosa alpha-synuclein staining and endotoxin exposure markers in early Parkinson’s disease. PLoS One 6(12):e28032
Salat-Foix D, Tran K, Ranawaya R, Meddings J, Suchowersky O (2012) Increased intestinal permeability and Parkinson disease patients: chicken or egg? Can J Neurol Sci 39(2):185–188
Davies KN, King D, Billington D, Barrett JA (1996) Intestinal permeability and orocaecal transit time in elderly patients with Parkinson’s disease. Postgrad Med J 72(845):164–167
Kelly LP, Carvey PM, Keshavarzian A, Shannon KM, Shaikh M, Bakay RA et al (2014) Progression of intestinal permeability changes and alpha-synuclein expression in a mouse model of Parkinson’s disease. Mov Disord 29(8):999–1009
Villumsen M, Aznar S, Pakkenberg B, Jess T, Brudek T (2019) Inflammatory bowel disease increases the risk of Parkinson’s disease: a Danish nationwide cohort study 1977–2014. Gut 68(1):18–24
Weimers P, Halfvarson J, Sachs MC, Saunders-Pullman R, Ludvigsson JF, Peter I et al (2019) Inflammatory bowel disease and Parkinson’s disease: a nationwide Swedish cohort study. Inflamm Bowel Dis 25(1):111–123
Lin JC, Lin CS, Hsu CW, Lin CL, Kao CH (2016) Association between Parkinson’s disease and inflammatory bowel disease: a nationwide Taiwanese retrospective cohort study. Inflamm Bowel Dis 22(5):1049–1055
Keshavarzian A, Green SJ, Engen PA, Voigt RM, Naqib A, Forsyth CB et al (2015) Colonic bacterial composition in Parkinson’s disease. Mov Disord 30(10):1351–1360
Hasegawa S, Goto S, Tsuji H, Okuno T, Asahara T, Nomoto K et al (2015) Intestinal dysbiosis and lowered serum Lipopolysaccharide-binding protein in Parkinson’s disease. PLoS One 10(11):e0142164
Perez-Pardo P, Dodiya HB, Engen PA, Forsyth CB, Huschens AM, Shaikh M et al (2018) Role of TLR4 in the gut-brain axis in Parkinson’s disease: a translational study from men to mice. Gut 68:829–843
Unger MM, Spiegel J, Dillmann KU, Grundmann D, Philippeit H, Burmann J et al (2016) Short chain fatty acids and gut microbiota differ between patients with Parkinson’s disease and age-matched controls. Parkinsonism Relat Disord 32:66–72
Zoetendal EG, de Vos WM (2014) Effect of diet on the intestinal microbiota and its activity. Curr Opin Gastroenterol 30(2):189–195
Peng L, Li ZR, Green RS, Holzman IR, Lin J (2009) Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in Caco-2 cell monolayers. J Nutr 139(9):1619–1625
Hill-Burns EM, Debelius JW, Morton JT, Wissemann WT, Lewis MR, Wallen ZD et al (2017) Parkinson’s disease and Parkinson’s disease medications have distinct signatures of the gut microbiome. Mov Disord 32(5):739–749
Barichella M, Severgnini M, Cilia R, Cassani E, Bolliri C, Caronni S et al (2018) Unraveling gut microbiota in Parkinson’s disease and atypical parkinsonism. Mov Disord 34:396–405
Sampson TR, Debelius JW, Thron T, Janssen S, Shastri GG, Ilhan ZE et al (2016) Gut microbiota regulate motor deficits and neuroinflammation in a model of Parkinson’s disease. Cell 167(6):1469–1480 e12
Mulak A (2018) A controversy on the role of short-chain fatty acids in the pathogenesis of Parkinson’s disease. Mov Disord 33(3):398–401
Tan AH, Mahadeva S, Marras C, Thalha AM, Kiew CK, Yeat CM et al (2015) Helicobacter pylori infection is associated with worse severity of Parkinson’s disease. Parkinsonism Relat Disord 21(3):221–225
Pierantozzi M, Pietroiusti A, Sancesario G, Lunardi G, Fedele E, Giacomini P et al (2001) Reduced l-dopa absorption and increased clinical fluctuations in Helicobacter pylori-infected Parkinson’s disease patients. Neurol Sci 22(1):89–91
Rees K, Stowe R, Patel S, Ives N, Breen K, Clarke CE et al (2011) Helicobacter pylori eradication for Parkinson’s disease. Cochrane Database Syst Rev 9(11):CD008453
Gabrielli M, Bonazzi P, Scarpellini E, Bendia E, Lauritano EC, Fasano A et al (2011) Prevalence of small intestinal bacterial overgrowth in Parkinson’s disease. Mov Disord 26(5):889–892
Tan AH, Mahadeva S, Thalha AM, Gibson PR, Kiew CK, Yeat CM et al (2014) Small intestinal bacterial overgrowth in Parkinson’s disease. Parkinsonism Relat Disord 20(5):535–540
Fasano A, Bove F, Gabrielli M, Petracca M, Zocco MA, Ragazzoni E et al (2013) The role of small intestinal bacterial overgrowth in Parkinson’s disease. Mov Disord 28(9):1241–1249
Scheperjans F (2018) The prodromal microbiome. Mov Disord 33(1):5–7
Mertsalmi TH, Aho VTE, Pereira PAB, Paulin L, Pekkonen E, Auvinen P et al (2017) More than constipation—bowel symptoms in Parkinson’s disease and their connection to gut microbiota. Eur J Neurol 24(11):1375–1383
Li W, Wu X, Hu X, Wang T, Liang S, Duan Y et al (2017) Structural changes of gut microbiota in Parkinson’s disease and its correlation with clinical features. Sci China Life Sci 60(11):1223–1233
Heintz-Buschart A, Pandey U, Wicke T, Sixel-Doring F, Janzen A, Sittig-Wiegand E et al (2018) The nasal and gut microbiome in Parkinson’s disease and idiopathic rapid eye movement sleep behavior disorder. Mov Disord 33(1):88–98
Petrov VA, Saltykova IV, Zhukova IA, Alifirova VM, Zhukova NG, Dorofeeva YB et al (2017) Analysis of gut microbiota in patients with Parkinson’s disease. Bull Exp Biol Med 162(6):734–737
Qian Y, Yang X, Xu S, Wu C, Song Y, Qin N et al (2018) Alteration of the fecal microbiota in Chinese patients with Parkinson’s disease. Brain Behav Immun 70:194–202
Bedarf JR, Hildebrand F, Coelho LP, Sunagawa S, Bahram M, Goeser F et al (2017) Functional implications of microbial and viral gut metagenome changes in early stage l-DOPA-naive Parkinson’s disease patients. Genome Med 9(1):39
Hopfner F, Kunstner A, Muller SH, Kunzel S, Zeuner KE, Margraf NG et al (2017) Gut microbiota in Parkinson disease in a northern German cohort. Brain Res 1667:41–45
Lin A, Zheng W, He Y, Tang W, Wei X, He R et al (2018) Gut microbiota in patients with Parkinson’s disease in southern China. Parkinsonism Relat Disord 53:82–88
Scheperjans F (2016) Gut microbiota, 1013 new pieces in the Parkinson’s disease puzzle. Curr Opin Neurol 29(6):773–780
Gerhardt S, Mohajeri MH (2018) Changes of colonic bacterial composition in Parkinson’s disease and other neurodegenerative diseases. Nutrients 10(6):E708
Pal GD, Shaikh M, Forsyth CB, Ouyang B, Keshavarzian A, Shannon KM (2015) Abnormal lipopolysaccharide binding protein as marker of gastrointestinal inflammation in Parkinson disease. Front Neurosci 9:306
Engen PA, Dodiya HB, Naqib A, Forsyth CB, Green SJ, Voigt RM et al (2017) The potential role of gut-derived inflammation in multiple system atrophy. J Parkinson’s Dis 7(2):331–346
Pereira PAB, Aho VTE, Paulin L, Pekkonen E, Auvinen P, Scheperjans F (2017) Oral and nasal microbiota in Parkinson’s disease. Parkinsonism Relat Disord 38:61–67
Berg D, Lang AE, Postuma RB, Maetzler W, Deuschl G, Gasser T et al (2013) Changing the research criteria for the diagnosis of Parkinson’s disease: obstacles and opportunities. Lancet Neurol 12(5):514–524
Broen MP, Narayen NE, Kuijf ML, Dissanayaka NN, Leentjens AF (2016) Prevalence of anxiety in Parkinson’s disease: a systematic review and meta-analysis. Mov Disord 31(8):1125–1133
Alifirova VM, Zhukova NG, Zhukova IA, Mironova YS, Petrov VA, Izhboldina OP et al (2016) Correlation between emotional-affective disorders and gut microbiota composition in patients with Parkinson’s disease. Vestn Ross Akad Med Nauk 71(6):427–435
Dinan TG, Cryan JF (2017) Gut feelings on Parkinson’s and depression. Cerebrum 2017:4–17
Jiang H, Ling Z, Zhang Y, Mao H, Ma Z, Yin Y et al (2015) Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav Immun 48:186–194
Klingelhoefer L, Reichmann H (2017) The gut and nonmotor symptoms in Parkinson’s disease. Int Rev Neurobiol 134:787–809
Khalif IL, Quigley EM, Konovitch EA, Maximova ID (2005) Alterations in the colonic flora and intestinal permeability and evidence of immune activation in chronic constipation. Dig Liver Dis 37(11):838–849
Maier L, Pruteanu M, Kuhn M, Zeller G, Telzerow A, Anderson EE et al (2018) Extensive impact of non-antibiotic drugs on human gut bacteria. Nature 555(7698):623–628
Shen C, Guo Y, Luo W, Lin C, Ding M (2013) Serum urate and the risk of Parkinson’s disease: results from a meta-analysis. Can J Neurol Sci 40(1):73–79
Hernan MA, Takkouche B, Caamano-Isorna F, Gestal-Otero JJ (2002) A meta-analysis of coffee drinking, cigarette smoking, and the risk of Parkinson’s disease. Ann Neurol 52(3):276–284
Ascherio A, Schwarzschild MA (2016) The epidemiology of Parkinson’s disease: risk factors and prevention. Lancet Neurol 15(12):1257–1272
Tavassoly O, Kakish J, Nokhrin S, Dmitriev O, Lee JS (2014) The use of nanopore analysis for discovering drugs which bind to alpha-synuclein for treatment of Parkinson’s disease. Eur J Med Chem 88:42–54
Derkinderen P, Shannon KM, Brundin P (2014) Gut feelings about smoking and coffee in Parkinson’s disease. Mov Disord 29(8):976–979
Biedermann L, Brulisauer K, Zeitz J, Frei P, Scharl M, Vavricka SR et al (2014) Smoking cessation alters intestinal microbiota: insights from quantitative investigations on human fecal samples using FISH. Inflamm Bowel Dis 20(9):1496–1501
Gniechwitz D, Reichardt N, Blaut M, Steinhart H, Bunzel M (2007) Dietary fiber from coffee beverage: degradation by human fecal microbiota. J Agric Food Chem 55(17):6989–6996
Jaquet M, Rochat I, Moulin J, Cavin C, Bibiloni R (2009) Impact of coffee consumption on the gut microbiota: a human volunteer study. Int J Food Microbiol 130(2):117–121
Nakayama T, Oishi K (2013) Influence of coffee (Coffea arabica) and galacto-oligosaccharide consumption on intestinal microbiota and the host responses. FEMS Microbiol Lett 343(2):161–168
Zhernakova A, Kurilshikov A, Bonder MJ, Tigchelaar EF, Schirmer M, Vatanen T et al (2016) Population-based metagenomics analysis reveals markers for gut microbiome composition and diversity. Science 352(6285):565–569
Mills CE, Tzounis X, Oruna-Concha MJ, Mottram DS, Gibson GR, Spencer JP (2015) In vitro colonic metabolism of coffee and chlorogenic acid results in selective changes in human faecal microbiota growth. Br J Nutr 113(8):1220–1227
Foster JA, Rinaman L, Cryan JF (2017) Stress and the gut-brain axis: regulation by the microbiome. Neurobiol Stress 7:124–136
Galley JD, Nelson MC, Yu Z, Dowd SE, Walter J, Kumar PS et al (2014) Exposure to a social stressor disrupts the community structure of the colonic mucosa-associated microbiota. BMC Microbiol 14:189
Dodiya HB, Forsyth CB, Voigt RM, Engen PA, Patel J, Shaikh M et al (2018) Chronic stress-induced gut dysfunction exacerbates Parkinson’s disease phenotype and pathology in a rotenone-induced mouse model of Parkinson’s disease. Neurobiol Dis. https://doi.org/10.1016/j.nbd.2018.12.012
Conlon MA, Bird AR (2014) The impact of diet and lifestyle on gut microbiota and human health. Nutrients 7(1):17–44
Wu GD, Chen J, Hoffmann C, Bittinger K, Chen YY, Keilbaugh SA et al (2011) Linking long-term dietary patterns with gut microbial enterotypes. Science 334(6052):105–108
Arumugam M, Raes J, Pelletier E, Le Paslier D, Yamada T, Mende DR et al (2011) Enterotypes of the human gut microbiome. Nature 473(7346):174–180
dos Santos EF, Busanello EN, Miglioranza A, Zanatta A, Barchak AG, Vargas CR et al (2009) Evidence that folic acid deficiency is a major determinant of hyperhomocysteinemia in Parkinson’s disease. Metab Brain Dis 24(2):257–269
Fava F, Gitau R, Griffin BA, Gibson GR, Tuohy KM, Lovegrove JA (2013) The type and quantity of dietary fat and carbohydrate alter faecal microbiome and short-chain fatty acid excretion in a metabolic syndrome ‘at-risk’ population. Int J Obes 37(2):216–223
Kim KA, Gu W, Lee IA, Joh EH, Kim DH (2012) High fat diet-induced gut microbiota exacerbates inflammation and obesity in mice via the TLR4 signaling pathway. PLoS One 7(10):e47713
Friedland RP (2015) Mechanisms of molecular mimicry involving the microbiota in neurodegeneration. J Alzheimer’s Dis 45(2):349–362
Biagi E, Franceschi C, Rampelli S, Severgnini M, Ostan R, Turroni S et al (2016) Gut microbiota and extreme longevity. Curr Biol 26(11):1480–1485
van Tongeren SP, Slaets JP, Harmsen HJ, Welling GW (2005) Fecal microbiota composition and frailty. Appl Environ Microbiol 71(10):6438–6442
Bartosch S, Fite A, Macfarlane GT, McMurdo ME (2004) Characterization of bacterial communities in feces from healthy elderly volunteers and hospitalized elderly patients by using real-time PCR and effects of antibiotic treatment on the fecal microbiota. Appl Environ Microbiol 70(6):3575–3581
Voreades N, Kozil A, Weir TL (2014) Diet and the development of the human intestinal microbiome. Front Microbiol 5:494
Monda V, Villano I, Messina A, Valenzano A, Esposito T, Moscatelli F et al (2017) Exercise modifies the gut microbiota with positive health effects. Oxidative Med Cell longev 2017:3831972
Estaki M, Pither J, Baumeister P, Little JP, Gill SK, Ghosh S et al (2016) Cardiorespiratory fitness as a predictor of intestinal microbial diversity and distinct metagenomic functions. Microbiome 4(1):42
Kiraly DD, Walker DM, Calipari ES, Labonte B, Issler O, Pena CJ et al (2016) Alterations of the host microbiome affect behavioral responses to cocaine. Sci Rep 6:35455
Du Y, Ma Z, Lin S, Dodel RC, Gao F, Bales KR et al (2001) Minocycline prevents nigrostriatal dopaminergic neurodegeneration in the MPTP model of Parkinson’s disease. Proc Natl Acad Sci USA 98(25):14669–14674
Faust K, Gehrke S, Yang Y, Yang L, Beal MF, Lu B (2009) Neuroprotective effects of compounds with antioxidant and anti-inflammatory properties in a Drosophila model of Parkinson’s disease. BMC Neurosci 10:109
Yang T, Santisteban MM, Rodriguez V, Li E, Ahmari N, Carvajal JM et al (2015) Gut dysbiosis is linked to hypertension. Hypertension 65(6):1331–1340
Soczynska JK, Kennedy SH, Alsuwaidan M, Mansur RB, Li M, McAndrews MP et al (2017) A pilot, open-label, 8-week study evaluating the efficacy, safety and tolerability of adjunctive minocycline for the treatment of bipolar I/II depression. Bipolar Disord 19(3):198–213
Diguet E, Fernagut PO, Wei X, Du Y, Rouland R, Gross C et al (2004) Deleterious effects of minocycline in animal models of Parkinson’s disease and Huntington’s disease. Eur J Neurosci 19(12):3266–3276
Davey KJ, Cotter PD, O’Sullivan O, Crispie F, Dinan TG, Cryan JF et al (2013) Antipsychotics and the gut microbiome: olanzapine-induced metabolic dysfunction is attenuated by antibiotic administration in the rat. Transl Psychiatry 3:e309
Cassani E, Privitera G, Pezzoli G, Pusani C, Madio C, Iorio L et al (2011) Use of probiotics for the treatment of constipation in Parkinson’s disease patients. Minerva Gastroenterol Dietol 57(2):117–121
Barichella M, Pacchetti C, Bolliri C, Cassani E, Iorio L, Pusani C et al (2016) Probiotics and prebiotic fiber for constipation associated with Parkinson disease: an RCT. Neurology 87(12):1274–1280
Perez-Pardo P, Kliest T, Dodiya HB, Broersen LM, Garssen J, Keshavarzian A et al (2017) The gut-brain axis in Parkinson’s disease: possibilities for food-based therapies. Eur J Pharmacol 817:86–95
Kim N, Yun M, Oh YJ, Choi HJ (2018) Mind-altering with the gut: modulation of the gut-brain axis with probiotics. J Microbiol 56(3):172–182
Kato-Kataoka A, Nishida K, Takada M, Kawai M, Kikuchi-Hayakawa H, Suda K et al (2016) Fermented milk containing Lactobacillus casei Strain shirota preserves the diversity of the gut microbiota and relieves abdominal dysfunction in healthy medical students exposed to academic stress. Appl Environ Microbiol 82(12):3649–3658
Liang S, Wang T, Hu X, Luo J, Li W, Wu X et al (2015) Administration of Lactobacillus helveticus NS8 improves behavioral, cognitive, and biochemical aberrations caused by chronic restraint stress. Neuroscience 310:561–577
Dinan TG, Cryan JF (2017) The microbiome-gut-brain axis in health and disease. Gastroenterol Clin N Am 46(1):77–89
Schmidt K, Cowen PJ, Harmer CJ, Tzortzis G, Errington S, Burnet PW (2015) Prebiotic intake reduces the waking cortisol response and alters emotional bias in healthy volunteers. Psychopharmacology 232(10):1793–1801
Hilimire MR, DeVylder JE, Forestell CA (2015) Fermented foods, neuroticism, and social anxiety: an interaction model. Psychiatry Res 228(2):203–208
Yang Y (2015) Gut microbiota research: highlights and commentary. Zhonghua nei ke za zhi 54(5):396–398
Bakken JS, Borody T, Brandt LJ, Brill JV, Demarco DC, Franzos MA et al (2011) Treating clostridium difficile infection with fecal microbiota transplantation. Clin Gastroenterol Hepatol 9(12):1044–1049
Leffler DA, Lamont JT (2015) Clostridium difficile infection. N Engl J Med 372(16):1539–1548
Tian H, Ding C, Gong J, Ge X, McFarland LV, Gu L et al (2016) Treatment of slow transit constipation with fecal microbiota transplantation: a pilot study. J Clin Gastroenterol 50(10):865–870
Ananthaswamy A (2011) Faecal transplant eases symptoms of Parkinson’s disease. New Sci 209(2796):8–9
Evrensel A, Ceylan ME (2016) Fecal microbiota transplantation and its usage in neuropsychiatric disorders. Clin Psychopharmacol Neurosci 14(3):231–237
Tetz G, Brown SM, Hao Y, Tetz V (2018) Parkinson’s disease and bacteriophages as its overlooked contributors. Sci Rep 8(1):10812
Brown CT, Davis-Richardson AG, Giongo A, Gano KA, Crabb DB, Mukherjee N et al (2011) Gut microbiome metagenomics analysis suggests a functional model for the development of autoimmunity for type 1 diabetes. PLoS One 6(10):e25792
Weir TL, Manter DK, Sheflin AM, Barnett BA, Heuberger AL, Ryan EP (2013) Stool microbiome and metabolome differences between colorectal cancer patients and healthy adults. PLoS One 8(8):e70803
Kang DW, Park JG, Ilhan ZE, Wallstrom G, Labaer J, Adams JB et al (2013) Reduced incidence of Prevotella and other fermenters in intestinal microflora of autistic children. PLoS One 8(7):e68322
Shin NR, Whon TW, Bae JW (2015) Proteobacteria: microbial signature of dysbiosis in gut microbiota. Trends Biotechnol 33(9):496–503
Perni M, Galvagnion C, Maltsev A, Meisl G, Muller MB, Challa PK et al (2017) A natural product inhibits the initiation of alpha-synuclein aggregation and suppresses its toxicity. Proc Natl Acad Sci USA 114(6):E1009–E1017
Collier TJ, Srivastava KR, Justman C, Grammatopoulous T, Hutter-Paier B, Prokesch M et al (2017) Nortriptyline inhibits aggregation and neurotoxicity of alpha-synuclein by enhancing reconfiguration of the monomeric form. Neurobiol Dis 106:191–204
Chan DKY, Xu YH, Chan LKM, Braidy N, Mellick GD (2017) Mini-review on initiatives to interfere with the propagation and clearance of alpha-synuclein in Parkinson’s disease. Transl Neurodegener 6:33
Principi N, Esposito S (2016) Gut microbiota and central nervous system development. J Infect 73(6):536–546
Author information
Authors and Affiliations
Contributions
ML: performed literature review, drafted and reviewed the manuscript. AHT: drafted and reviewed the manuscript. S-YL: reviewed the manuscript. AJH: reviewed the manuscript. RLD: drafted and reviewed the manuscript. CMS: drafted and reviewed the manuscript
Corresponding author
Ethics declarations
Conflicts of interest
This study was not industry sponsored and had no sources of support. No statistical analysis was performed. All authors report no relevant disclosures.
Rights and permissions
About this article
Cite this article
Lubomski, M., Tan, A.H., Lim, SY. et al. Parkinson’s disease and the gastrointestinal microbiome. J Neurol 267, 2507–2523 (2020). https://doi.org/10.1007/s00415-019-09320-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-019-09320-1