Abstract
Background
Atypical cerebrospinal fluid (CSF) patterns, involving an increase in the concentration of phosphorylated-tau (P-tau) proteins but normal amyloid-β concentration, are not uncommon in patients with mild neurocognitive disorders and suspected Alzheimer’s disease (AD). In these conditions, however, AD diagnosis may be ruled out in the absence of any amyloid deposition at positron-emission tomography (PET). This pilot cross-sectional study was aimed to determine whether this negativity of amyloid PET can be predicted by CSF profiles in such patients.
Methods
Twenty-five patients (73 [68–80] years, 10 women) with mild neurocognitive disorders, suspected AD and an increase in the CSF concentration of P-tau proteins but normal Aβ42 concentration and Aβ42/Aβ40 ratio were prospectively included and referred to a 18F-florbetaben PET. The latter was considered as definitively negative with the conjunction of both visual (brain amyloid plaque load score) and quantified (standard uptake value ratios) criteria. Predictors of a negative PET were searched among current CSF biomarkers (Aß42, Aß40, T-tau, P-tau, Aß42/Aß40, Aß42/p-tau).
Results
Amyloid PET was negative in 15 patients (60%) with a CSF Aß42 concentration being the sole independent predictor of this negativity. The criterion of an Aß42 concentration in the very high range (> 843 pg/mL), observed in 60% (15/25) of the study patients, was associated with a negative amyloid PET in 93% (14/15) of cases.
Conclusions
In mild neurocognitive disorders patients with suspected AD and showing an increase in CSF P-tau protein level, amyloid PETs are commonly negative, when Aß42 concentration is in the very high range. In such case, AD diagnosis based on biomarkers can be ruled out with reasonable certainty, without the need for additional CSF second-line assays or results from amyloid PET.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Background
The dual criterion of a brain increase in phosphorylated-tau (P-tau) proteins, combined with the presence of an amyloid deposition in the brain, was recently proposed by the National Institute on Aging and Alzheimer’s Association (NIA-AA) for the definition of Alzheimer’s disease (AD) [1]. The combination of such increase in P-tau proteins in cerebrospinal fluid (CSF), which are considered as relatively specific biomarkers of AD [2], with normal amyloid-β concentration correspond to an atypical CSF pattern. These CSF profiles are far from being uncommon in patients suspected of AD [3], even when taking into account the Aβ42/Aβ40 ratio to improve the accuracy of the CSF analysis [3, 4]. However, according to the NIA-AA criteria, AD diagnosis can be ruled out by a definite assessment of an absence of any amyloid deposition in the brain in these patients [1].
Interestingly, this assessment may now be provided by amyloid positron-emission tomography (PET) brain imaging. 18F-Florbetaben is one of the PET tracers of amyloid plaques which may be used in clinical routine [5] following its approval by the United States Food and Drug Administration and of the European Medicines Agency for this purpose [6]. In particular, 18F-florbetaben PET is associated with a very high negative predictive value for AD diagnosis (96%), especially when using not only visual but also quantitative analyses [5]. Such additional quantitative assessments have clearly been shown to improve the interpretation of 18F-florbetaben PET in this setting [7,8,9,10,11]. Nevertheless, a negative 18F-florbetaben PET is now established as a reliable indicator of the absence of sufficient plaque pathology in the brain to support a diagnosis of AD [5].
In light with the above, this pilot cross-sectional study was aimed to determine whether CSF profiles could predict the negativity of a 18F-florbetaben PET and thus, help to rule out AD diagnosis in mild neurocognitive disorders patients suspected of AD with abnormal CSF levels of P-tau-proteins but normal CSF amyloid-β levels.
Methods
Population
Among the 800 CSF analyses performed between December 2015 and September 2017 in the Department of Biochemistry, Molecular Biology and Nutrition of the University Hospital of Nancy in patients with mild neurocognitive disorders [12] and suspected AD, according to the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association (NINCDS ADRDA) criteria [13, 14], 25 exhibited an increase in CSF P-tau concentrations (> 60 pg/mL) along with normal Aß42 concentration (> 600 pg/mL) and Aß42/Aß40 ratio (> 0.07), according to routine cut-off values [3, 15]. The patients presenting this CSF biomarker profile were included in this ancillary analysis of the MAF (Maladie d’Alzheimer Florbetaben) study (NCT02556502) [16] with the following main additional inclusion criteria: age ≥ 18 years old, absence of formal or relative contraindication to 18F-florbetaben PET, affiliation to a health care system, no guardianship or curatorship, and absence of pregnancy. In addition, patients unable to undergo 18F-florbetaben PET due to agitation or confusion, as well as those unable to sign the informed consent form, were not included. Eligible patients were referred to 18F-florbetaben PET scanning less than 1 year after the CSF analysis.
CSF biomarkers
CSF was collected in the clinical setting by lumbar puncture of the L3–L4 or L4–L5 intervertebral space after local anesthesia in non-fasting patients, as part of the investigation of the patient’s cognitive dysfunction. Two levels of supplier-provided internal quality controls were analyzed in each series, with the laboratory, furthermore, participating in the Alzheimer’s Association Quality Control Program by analyzing, four times yearly, two levels of external quality controls and one longitudinal control. Since 2009, the laboratory results have systematically remained within the ± 2 standard deviations interval for each level of control. According to standard procedure, CSF was collected in a 5-mL Gosselin polypropylene tube. All samples were transported within 4 h at 4 °C to the Biochemistry Laboratory of the University Hospital of Nancy (Central Laboratory) and were immediately centrifuged and stored at − 80 °C until assayed.
Aβ42, Aβ40, T-tau and P-tau concentrations were determined using commercially available sandwich ELISA procedures (Innotest®, Fujirebio, Ghent, Belgium) according to the manufacturer’s instructions. Biomarker concentrations were proportional to optical density at 450 nm. A CSF pool sampled in the same polypropylene tubes and stored at − 80 °C was used as internal quality control in each experiment. Standards, samples and controls were run in duplicates. Samples were re-assayed if the variation coefficient between both values was > 10%.
Aβ42 peptide, Tau and P-Tau protein values of 600, 350 and 60 pg/mL, respectively, have been proposed as pathological thresholds while an Aβ42/Aβ40 ratio above 0.07 is considered pathological [3, 4, 15, 17, 18].
Amyloid PET recording
All 18F-florbetaben PET images were recorded on the same Biograph™ six hybrid PET/computed tomography (CT) system (Siemens Medical Solutions, Erlangen, Germany) after the intravenous injection of a bolus of 300 MBq (± 10%) of 18F-florbetaben. Ninety minutes later, the imaging protocol was initiated by a low-dose brain CT scan for attenuation correction (110 keV, 40 mAs, matrix size 512 × 512, 3 mm slice thickness and a pitch of 1), immediately followed by a 20-min brain PET recording. PET images were reconstructed with an iterative three-dimensional ordered subset expected minimization (OSEM) method, corrected for attenuation and diffusion and displayed through 2.7-mm isotropic voxels [19].
Amyloid PET analysis
Only amyloid PETs classified as negative upon both visual and quantitative analyses were considered as definitely negative (the characterization of the patients with a definite negative amyloid PET was the primary objective of the present study). Accordingly, amyloid PETs were considered positive in the instances of positive visual and/or quantitative analyses.
The visual analysis was obtained from two experienced nuclear physicians (CM, AV) who were blinded to all other patient data. Amyloid PET results were interpreted in a consensual manner as either “positive” or “negative” by conventional means [6], according to the amount of 18F-florbetaben uptake observed on the lateral temporal cortex, frontal cortex, posterior cingulate cortex/precuneus and parietal cortex. Only brain amyloid plaque load (BAPL) scores higher than 1 were considered as reflecting positivity, as currently recommended [6].
The quantitative analysis was obtained with the use of the low-dose CT images for spatial normalization and subsequent PET quantification [20]. More precisely, each set of CT images was spatially normalized into the standard Montreal National Institute (MNI) space through the segmentation–normalization algorithm from the SPM12 software (Wellcome Trust Centre for Neuroimaging, London) [20]. Thereafter, each set of PET images was registered into the MNI-152 space, using the deformation matrix obtained for the spatial normalization of the CT scan, and was segmented for the gray-matter volume, using the segmentation of the CT scan.
Standard uptake values ratios (SUVr) were obtained for each patient in a conventional way, with the averaged activity from six ROIs being divided by the mean activity from a cerebellar reference region [21]. These six ROIs were located on the dorsolateral and medial frontal cortex, the cingulum, the precuneus, the inferior and superior parietal lobules, the lateral occipital cortex and the lateral temporal cortex, and were automatically defined through the Automated Anatomical Labeling atlas [5, 22]. The criterion of a SUVr < 1.478 was used to identify the negative 18F-florbetaben PET, according to that previously recommended [5].
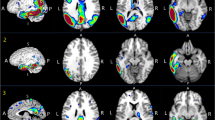
Typical axial slices of positive and negative amyloid PET are displayed in Fig. 1.
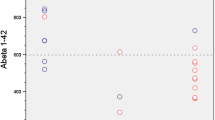
Individual patient values of brain amyloid plaque load (BAPL) scores (upper panel), standardized uptake value ratios (SUVr) (medium panel) and Aβ42 cerebrospinal fluid concentrations (lower panel) for the 25 study patients classified according to increasing values of first BALP score and second SUVr values. The blue columns indicate normal values for BALP score, SUVr and Aβ42 concentration > 843 pg/mL, whereas the red columns indicate abnormal values for BALP score, SUVr and Aβ42 concentration ≤ 843 pg/mL. Axial slices of negative (patient #1) and positive (patient #25) amyloid PET are additionally displayed in the upper portion of this figure
Statistical analysis
Continuous variables are expressed as median and interquartile range [IQR] with two-group comparisons performed with Mann–Whitney tests. Categorical variables are expressed as percentages and were compared using chi-square tests. The concordance between the results of visual and quantitative PET analyses was additionally assessed with a kappa coefficient.
A multivariate analysis was performed using ascending logistic regression models with the negative/positive status of amyloid PET as the outcome variable and the CSF and clinical parameters listed in Table 1 and showing a p value < 0.10 at univariate analysis as explanatory variables. The criteria of p values ≤ 0.10 and p > 0.10 were used to, respectively, enter and remove the variables from the model. The validity of assumptions of the models was verified, with the variation inflation factor and conditional index being used for multicollinearity diagnosis.
The predictive value of the CSF concentration biomarkers was additionally assessed by receiver operating characteristic (ROC) curves. The Aß42 cut-off value was considered as optimal for identifying patients with negative PET when the product of paired values for sensitivity and specificity reached its maximum. For all univariate tests, a p value < 0.05 was considered as significant. Statistical analyses were performed with SAS version 9.4 (SAS Institute Inc., Cary, N.C., USA) and SPSS 20 (SPSS, Chicago, IL, USA).
Results
Patient characteristics are detailed in Table 1. Of the 25 study patients, 10 (40%) were women, the median age was 73 years and the median of the Mini-Mental State Examination (MMSE) score was 23. According to the Clinical Dementia Rating (CDR) [23], none of the patients were classified with a score of 0 (no dementia) or 3 (severe dementia).
Identification of definitely negative amyloid PET
Fifteen among the 25 amyloid PET scans (60%) were considered to be definitively negative and thus without any abnormal amyloid deposition according to both visual and quantitative criteria. The concordance rate between visual and quantitative identifications of negative amyloid PET was 88% (22/25) with a corresponding kappa coefficient of 0.73 (p < 0.001). As detailed in Fig. 1, amyloid PET was negative quantitatively but positive visually in a single patient for whom the visual BAPL score was of only 2, just above the normal limit, whereas all other positive PETs were associated with BALP scores of 3. Amyloid PET was negative visually but positive quantitatively in two patients for whom the SUVr were also just above the normal limit of 1.478—i.e. 1.523 and 1.629 (see Fig. 1).
Prediction of a definitely negative PET
As detailed in Table 1, there were non-significant trends toward higher Mini-Mental State Examination (p = 0.09) and educational level (p = 0.07) in patients with definitely negative PET. Moreover, the CSF concentrations of Aß40 and Aß42, as well as the Aß42/Aß40 and Aß42/p-tau concentration ratios, were significant predictors of a negative amyloid PET on univariate analysis (respective areas under the curve of 0.85, 0.95, 0.79 and 0.90). On multivariate analysis, however, Aß42 concentration was the sole independent predictor of a definitively negative PET. No other clinical or CSF variable was able to provide significant additional predictive information.
The ROC curve of the identification of definitely negative PET according to the level of CSF Aß42 concentration is displayed in Fig. 2. The threshold of 843 pg/mL provided the best sensitivity specificity product and is highlighted on this curve.
As shown in Fig. 1, only one patient with an Aß42 concentration > 843 pg/mL had a positive amyloid PET although this positivity was based on a SUVr value of 1.523, just above the normal limit of 1.478, whereas the visual analysis was negative (BALP score of 1). This figure also shows that an Aß42 concentration ≤ 843 pg/mL was associated with a negative PET in a single patient. As a result, for the identification of a definitely negative PET, the criterion of an Aß42 concentration > 843 pg/mL was associated with a global accuracy of 92% (23/25).
Discussion
In the particular population of mild neurocognitive disorders patients with suspected AD and an abnormal CSF P-tau-protein level but normal CSF amyloid-β, our study shows that amyloid PET is commonly negative if the Aß42 concentration is in the very high range. More precisely, in patients with Aß42 concentration higher than 843 pg/mL and thus far above the threshold limit of normal (600 pg/mL), 93% (14/15) had a definitely negative amyloid PET, a strong criterion for ruling out the diagnosis of AD [5]. This percentage is in agreement with the high negative predictive value for AD diagnosis (96%) currently documented with 18F-florbetaben PET in patients with suspected AD [5]. In the present study, a positive 18F-florbetaben PET was associated with an Aß42 concentration higher than 843 pg/mL in only one patient for whom this PET positivity was furthermore at the borderline of significance with a slightly abnormal quantitative analysis and a negative visual analysis (Fig. 1).
This relationship between Aß42 concentration and amyloid PET status is in accordance with the previous observations of an inverse relationship between the amount of abnormal amyloid PET uptake and CSF Aß42 levels [24,25,26,27,28,29]. Aß42 has also been previously shown to be the best CSF biomarker of total brain amyloid load at autopsy, presumably since Aß42 is a major component of brain amyloid plaques in vivo [30].
On univariate analysis, however, the CSF concentration of Aß40 as well as the Aß42/Aß40 and Aß42/P-tau ratios were additional predictors of a negative amyloid PET (Table 1), although this is likely the result of all these biomarkers being linked to the process of amyloid plaque load. By contrast, the CSF concentrations of T-tau and P-tau proteins, which are linked to the neurodegenerative and not to the amyloid components of the AD process, were not univariate predictors of a negative amyloid PET (Table 1). On multivariate analysis, however, Aß42 concentration was the sole independent predictor of the amyloid PET status, with no other clinical or CSF variable able to provide any significant predictive value in addition to that provided by CSF Aß42 concentration.
Although this remains to be confirmed on a larger scale, our results suggest that in patients with an Aß42 concentration far above the threshold limit of normal (> 843 pg/mL in the present study), AD diagnosis based on biomarkers [1] can be ruled out with reasonable certainty, even in the presence of an abnormal CSF concentration of P-tau protein. Indeed, in this particular instance, no other additional investigation such as second-line assays of CSF Aß42/Aß40 or Aß42/p-tau ratios, or amyloid PET would be required to determine the risk of AD. Both diagnostic and therapeutic strategies could thus be reoriented to other mild neurocognitive disorders not due to AD (primary age-related tauopathy, argyrophilic grain disease, Pick’ disease, corticobasal degeneration or progressive supranuclear palsy, etc.) [31].
By contrast, the risk of AD would remain significant in patients in whom Aß42 concentrations are normal but close to the abnormal range (from 600 to 843 pg/mL in the present study), especially when associated with mild neurocognitive disorders and an increase in CSF P-tau proteins [16]. This was indeed the case of ten patients herein among whom one had a definitely negative amyloid PET although nine had not (Fig. 1).
To rule out the diagnosis of AD with a high degree of certainty, amyloid PET was considered as definitively negative in the present study only if both visual and quantitative criteria were negative, which represented a non-negligible proportion of our specific population (n = 15 patients, 60%). Such visual [6] and quantitative [7,8,9,10,11] analyses have already been shown to provide excellent diagnostic performances for AD diagnosis, with the visual BAPL score commonly used in this setting [6], and with the SUV ratios for which the abnormal range was recently defined according to histopathological criteria of AD [5]. These visual and quantitative assessments showed an excellent concordance herein, with a kappa coefficient of 0.73, in accordance with previously published data [9, 11], although three discordance cases were nonetheless documented. These three cases hovered at the borderline of the visual and quantitative thresholds defining a negative 18F-florbetaben PET (see Fig. 1), although the presence of these discordances strengthens our choice of considering both visual and quantitative criteria for the definite diagnosis of a negative amyloid PET.
The main limitations of the present study are its single-center and cross-sectional nature and the limited number of study patients, thus restricting the generalization of our results. In addition, further longitudinal studies would be useful to assess the time evolution of Aß42 concentrations, amyloid PET status and, ultimately, the rate of AD conversion, in those patients with mild neurocognitive disorders and atypical CSF biomarker profiles. The interest of patient monitoring through serial amyloid PET has, moreover, already been suggested in such atypical cases [32].
Conclusions
Altogether, this pilot study shows that in mild neurocognitive disorders patients with suspected AD and with abnormal CSF P-tau-protein levels but with normal CSF amyloid-β levels, amyloid PET is commonly negative when the CSF concentration of Aß42 is additionally in the very high range. In such case, AD diagnosis based on biomarkers can be ruled out with reasonable certainty, without the need for additional CSF second-line assays or results from amyloid PET.
References
Jack CR, Bennett DA, Blennow K et al (2018) NIA-AA research framework: toward a biological definition of Alzheimer’s disease. Alzheimers Dement J Alzheimers Assoc 14:535–562. https://doi.org/10.1016/j.jalz.2018.02.018
Koopman K, Le Bastard N, Martin J-J et al (2009) Improved discrimination of autopsy-confirmed Alzheimer’s disease (AD) from non-AD dementias using CSF P-tau (181P). Neurochem Int 55:214–218. https://doi.org/10.1016/j.neuint.2009.02.017
Sauvée M, DidierLaurent G, Latarche C et al (2014) Additional use of Aβ42/Aβ40 ratio with cerebrospinal fluid biomarkers P-tau and Aβ42 increases the level of evidence of Alzheimer’s disease pathophysiological process in routine practice. J Alzheimers Dis JAD 41:377–386. https://doi.org/10.3233/JAD-131838
Gabelle A, Dumurgier J, Vercruysse O et al (2013) Impact of the 2008–2012 French Alzheimer plan on the use of cerebrospinal fluid biomarkers in research memory center: the PLM Study. J Alzheimers Dis JAD 34:297–305. https://doi.org/10.3233/JAD-121549
Sabri O, Sabbagh MN, Seibyl J et al (2015) Florbetaben PET imaging to detect amyloid beta plaques in Alzheimer’s disease: phase 3 study. Alzheimers Dement J Alzheimers Assoc 11:964–974. https://doi.org/10.1016/j.jalz.2015.02.004
Sabri O, Seibyl J, Rowe C, Barthel H (2015) Beta-amyloid imaging with florbetaben. Clin Transl Imaging 3:13–26. https://doi.org/10.1007/s40336-015-0102-6
Pontecorvo MJ, Arora AK, Devine M et al (2017) Quantitation of PET signal as an adjunct to visual interpretation of florbetapir imaging. Eur J Nucl Med Mol Imaging 44:825–837. https://doi.org/10.1007/s00259-016-3601-4
Harn NR, Hunt SL, Hill J et al (2017) Augmenting amyloid PET interpretations with quantitative information improves consistency of early amyloid detection. Clin Nucl Med 42:577–581. https://doi.org/10.1097/RLU.0000000000001693
Thurfjell L, Lilja J, Lundqvist R et al (2014) Automated quantification of 18F-flutemetamol PET activity for categorizing scans as negative or positive for brain amyloid: concordance with visual image reads. J Nucl Med 55:1623–1628. https://doi.org/10.2967/jnumed.114.142109
Nayate AP, Dubroff JG, Schmitt JE et al (2015) Use of standardized uptake value ratios decreases interreader variability of [18F] florbetapir PET brain scan interpretation. AJNR Am J Neuroradiol 36:1237–1244. https://doi.org/10.3174/ajnr.A4281
Bullich S, Seibyl J, Catafau AM et al (2017) Optimized classification of18F-Florbetaben PET scans as positive and negative using an SUVR quantitative approach and comparison to visual assessment. NeuroImage Clin 15:325–332. https://doi.org/10.1016/j.nicl.2017.04.025
American Psychiatric Association (2013) Diagnostic and statistical manual of mental disorders (DSM-5®). American Psychiatric Pub, Washington DC
McKhann GM, Knopman DS, Chertkow H et al (2011) The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement J Alzheimers Assoc 7:263–269. https://doi.org/10.1016/j.jalz.2011.03.005
McKhann G, Drachman D, Folstein M et al (1984) Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s disease. Neurology 34:939–944
Gervaise-Henry C, Watfa G, Albuisson E et al (2017) Cerebrospinal fluid Aβ42/Aβ40 as a means to limiting tube- and storage-dependent pre-analytical variability in clinical setting. J Alzheimers Dis JAD 57:437–445. https://doi.org/10.3233/JAD-160865
Manca C, Hopes L, Kearney-Schwartz A et al (2019) Assessment of 18F-florbetaben amyloid PET imaging in patients with suspected Alzheimer’s disease and isolated increase in cerebrospinal fluid tau proteins. J Alzheimers Dis. https://doi.org/10.3233/JAD-181146
Dumurgier J, Vercruysse O, Paquet C et al (2013) Intersite variability of CSF Alzheimer’s disease biomarkers in clinical setting. Alzheimers Dement 9:406–413. https://doi.org/10.1016/j.jalz.2012.06.006
Lehmann S, Schraen S, Quadrio I et al (2014) Impact of harmonization of collection tubes on Alzheimer’s disease diagnosis. Alzheimers Dement J Alzheimers Assoc 10:S390–S394. https://doi.org/10.1016/j.jalz.2013.06.008
Chetouani A, Chawki MB, Hossu G et al (2018) Cross-sectional variations of white and grey matter in older hypertensive patients with subjective memory complaints. NeuroImage Clin 17:804–810. https://doi.org/10.1016/j.nicl.2017.12.024
Presotto L, Iaccarino L, Sala A et al (2018) Low-dose CT for the spatial normalization of PET images: a validation procedure for amyloid-PET semi-quantification. NeuroImage Clin 20:153–160. https://doi.org/10.1016/j.nicl.2018.07.013
Catafau AM, Bullich S, Seibyl JP et al (2016) Cerebellar amyloid-β plaques: how frequent are they, and do they influence 18F-florbetaben SUV ratios? J Nucl Med 57:1740–1745. https://doi.org/10.2967/jnumed.115.171652
Barthel H, Gertz H-J, Dresel S et al (2011) Cerebral amyloid-β PET with florbetaben (18F) in patients with Alzheimer’s disease and healthy controls: a multicentre phase 2 diagnostic study. Lancet Neurol 10:424–435. https://doi.org/10.1016/S1474-4422(11)70077-1
Morris JC (1993) The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology 43:2412–2414
Fagan AM, Mintun MA, Mach RH et al (2006) Inverse relation between in vivo amyloid imaging load and cerebrospinal fluid Aβ42 in humans. Ann Neurol 59:512–519. https://doi.org/10.1002/ana.20730
Forsberg A, Engler H, Almkvist O et al (2008) PET imaging of amyloid deposition in patients with mild cognitive impairment. Neurobiol Aging 29:1456–1465. https://doi.org/10.1016/j.neurobiolaging.2007.03.029
Grimmer T, Riemenschneider M, Förstl H et al (2009) Beta amyloid in Alzheimer’s disease: increased deposition in brain is reflected in reduced concentration in cerebrospinal fluid. Biol Psychiatry 65:927–934. https://doi.org/10.1016/j.biopsych.2009.01.027
Tolboom N, van der Flier WM, Yaqub M et al (2009) Relationship of cerebrospinal fluid markers to 11C-PiB and 18F-FDDNP binding. J Nucl Med 50:1464–1470. https://doi.org/10.2967/jnumed.109.064360
Jagust WJ, Landau SM, Shaw LM et al (2009) Relationships between biomarkers in aging and dementia. Neurology 73:1193–1199. https://doi.org/10.1212/WNL.0b013e3181bc010c
Landau SM, Lu M, Joshi AD et al (2013) Comparing positron emission tomography imaging and cerebrospinal fluid measurements of β-amyloid. Ann Neurol 74:826–836. https://doi.org/10.1002/ana.23908
Tapiola T, Alafuzoff I, Herukka S-K et al (2009) Cerebrospinal fluid β-amyloid 42 and tau proteins as biomarkers of Alzheimer-type pathologic changes in the brain. Arch Neurol 66:382–389. https://doi.org/10.1001/archneurol.2008.596
Lebouvier T, Pasquier F, Buée L (2017) Update on tauopathies. Curr Opin Neurol 30:589–598. https://doi.org/10.1097/WCO.0000000000000502
Vlassenko AG, McCue L, Jasielec MS et al (2016) Imaging and cerebrospinal fluid biomarkers in early preclinical alzheimer disease. Ann Neurol 80:379–387. https://doi.org/10.1002/ana.24719
Acknowledgements
The authors thank Pierre Pothier for critical review of the manuscript.
Funding
This work was carried out thanks to the support of the Nancyclotep Imaging Platform and Nancy University Hospital.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Ethical standards
The study was approved by the French ethics committee CPP Est III on September 15th, 2015, as well as received the authorization from the national competent authority (ANSM) on September 18th 2015, and adhered to the Declaration of Helsinki.
Informed consent
All patients provided written informed consent for participation in the study. All patients provided written informed consent for publication.
Availability of data and materials
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Manca, C., Rivasseau Jonveaux, T., Roch, V. et al. Amyloid PETs are commonly negative in suspected Alzheimer’s disease with an increase in CSF phosphorylated-tau protein concentration but an Aβ42 concentration in the very high range: a prospective study. J Neurol 266, 1685–1692 (2019). https://doi.org/10.1007/s00415-019-09315-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-019-09315-y