Abstract
Objectives
To investigate a diversity of stroke-like episodes (SLEs) in mitochondrial encephalomyopathy, lactic acidosis, and stroke-like episodes (MELAS), and report a disseminated form of SLEs (D-SLEs) attributed to a cluster of disseminated small cortical lesions.
Methods
We retrospectively reviewed the clinical information of 27 MELAS patients seen at Kitasato University Hospital between January 1990 and April 2018. Among those, we selected 13 patients with m.3243A>G mutation [median age at onset, 35 years (11–68 years), two pediatric onset < 17 years] who had at least one SLE. SLEs were classified into classic or non-classic based on characteristic features of stroke-like lesions.
Results
44 SLEs were identified during a median observational period of 119 months (3–240 months). Among those, 29 (65.9%) were classic SLEs (C-SLEs) mainly attributed to a single continuous lobular lesion incongruent to vascular territory and occasionally accompanied by a gradual spread associated with hyperperfusion and persistent seizure activity. The remaining 15 were non-classic attributed to sparsely distributed (n = 10), disseminated (n = 4) or cerebellar lesions (n = 1). C-SLEs developed in all patients but non-classic SLEs in 5; D-SLEs developed in 4 patients accounting for 4 of 44 SLEs (9.1%). Non-classic SLEs developed more frequently in pediatric-onset than in adult-onset patients (12/15 vs. 3/29, p < 0.0001). SLEs began with acute onset of symptoms in 42 SLEs (95.5%), but D-SLEs of 2 adult-onset patients began with ill-defined subacute-onset fluctuating encephalopathy.
Conclusions
This study showed a diversity of SLEs in patients with m.3243A>G mutation. Further studies are required to elucidate the pathophysiological mechanisms of non-classic SLEs including D-SLEs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mitochondrial encephalomyopathy, lactic acidosis, and stroke-like episodes (MELAS) is a mitochondrial disorder characterized by migraine-like headache, seizure, encephalopathy, and stroke-like episodes (SLEs) [1]. More than 40 pathogenic mitochondrial DNA (mtDNA) or nuclear DNA mutations have been identified associated with MELAS or MELAS/other phenotype overlap syndromes [2], among those, the m.3243A>G mutation in the MT-LT1 gene is the most common pathogenic mutation [3].
The term “SLEs” has been used to denote episodic events mimicking ischemic stroke, but it has not been clearly defined yet [2]. The molecular mechanism of MELAS mutations has been elucidated [4, 5]; however, the pathogenesis of SLEs remains unknown. Ischemic [6], metabolic [7], or non-ischemic neurovascular cellular hypotheses [2, 8, 9] have been proposed. SLEs often begin with an acute onset of headache, vomiting, visual symptoms, seizures, or other symptoms [1, 2, 8, 9]. Stroke-like lesions (SLLs) in MELAS are usually a single, moderate to large-sized lesion incongruent to vascular territory [2, 8, 9], often gradually spread to adjacent cortex associated with persistent seizure activities [10], and are accompanied by vasogenic edema [11] presumably attributed to endothelial dysfunction of the blood–brain barrier (BBB); however, a phenotypic diversity of SLEs has not been fully investigated.
Here, we report a diversity of SLEs in patients with m.3243A>G mutation and a disseminated form of SLEs (D-SLEs) attributed to a cluster of disseminated small cortical lesions, which have not been described yet.
Methods
Identification of patients and clinical information
We retrospectively reviewed the clinical information of 27 patients with MELAS [with mutation of m.3243A>G (n = 24), m.13513G>A (n = 1), m.617G>A (n = 1), or m.5541C > T (n = 1)] who were seen at Kitasato University Hospital between January 1, 1990 and April 1, 2018. The diagnosis of MELAS was made based on the clinical symptoms, neurological examination, serum and cerebrospinal fluid (CSF) examination, imaging studies, and genomic mutation analysis [8]. Among those, we selected 13 patients with m.3243A>G mutation [median age at onset of 1st SLE, 35 years (range 11–68 years)] who developed at least one SLE to evaluate SLEs associated with m.3243A>G mutation. Thus, the remaining 14 patients (11 with m.3243A>G mutation who did not develop SLEs and the other 3 with other mtDNA mutations) were not included. Some of the clinical features of patients 1–7 were previously reported [2, 8,9,10]. All SLEs identified during observational period from the onset of 1st SLE to the last follow-up were included. A detailed clinical information of SLEs including neurological manifestations, brain magnetic resonance imaging (MRI), electroencephalogram (EEG), cerebral blood flow (CBF) studies, and treatment was obtained from the referring physicians and authors.
Definition of SLEs and SLLs
SLEs were defined as clinical episodes with acute or subacute onset of neurological manifestations, of which clinically relevant brain lesions were confirmed by either brain MRI, CT, or autopsy. Symptoms alone without relevant acute brain lesion were not regarded as SLEs. SLLs were defined as acute ischemic-like lesions corresponding to new onset of neurological manifestations, but apparent ischemic lesions due to lacunar stroke, artery-to-artery or cardiogenic embolism, or arterial dissection were not regarded as SLLs.
A phenotype of each SLE was classified into classic or non-classic based on characteristic features of SLLs mainly contributing to the neurological manifestations. Classic SLEs (C-SLEs) were defined as those attributed to classic SLLs characterized by continuous lobular edematous lesions extending along the cerebral cortex and incongruent to vascular territory [2, 8,9,10]. Non-classic SLEs were defined as those attributed to SLLs other than classic SLLs. Subtentorial lesion (such as cerebellar lesion) was regarded as non-classic SLL.
Evaluation of SLEs and SLLs
Brain MRIs, three-dimensional time-of-flight MR angiography (3D-TOF MRA), arterial spin labelling (ASL)-MRI, single-photon-emission CT (SPECT), and EEG obtained within 30 days of the onset of symptoms of each SLE were reviewed.
SLLs were assessed using diffusion-weighted images (DWI), T1-weighted, T2-weighted, fluid-attenuated inversion recovery (FLAIR) images, MRA, and CBF studies. Brain MRI was obtained with a 1.5 T (or 3.0 T). CBF was evaluated with ASL-MRI and/or CBF-SPECT. ASL-MRI was performed using pseudo-continuous ASL (pCASL) with postlabelling delay of 1525 ms. In CBF-SPECT, 99mTc-d,l-hexamethyl-propyleneamine oxime (HMPAO), 99mTc-ethyl cysteinate dimer (ECD), or N-isoprpyl-p-123I iodoamphetamine (IMP) was used as a flow tracer.
The association between neurological manifestations, SLLs, vasodilatation, hyperperfusion, and paroxysmal discharges were assessed. The clinical neuroimaging features of SLEs were compared between pediatric-onset (age at onset of 1st SLE, < 17 years) and adult-onset patients (≥ 17 years), and between C-SLEs and non-classic SLEs.
Treatments, and short-term and long-term outcomes
Treatment was determined by the physicians and different depending on the time of presentation. Treatments were classified as (1) symptomatic (e.g. anti-epileptic drugs [AEDs]), (2) intravenous edaravone (a free radical scavenger; 30 mg twice daily for adult and 0.5 mg/kg, twice daily for pediatric patients), (3) intravenous l-arginine (20 g/day for adult, and 10 g/day for pediatric patients), and oral l-arginine (0.2–0.5 g/kg/day), and (4) intravenous corticosteroids (betamethasone 4–12 mg/day, short tapering off).
Short-term outcome of each SLE was evaluated based on the clinical improvement of neurological manifestations attributed to newly appearing SLLs. If the symptoms substantially improved and the patient’s disability returned to the baseline level before the symptom onset, the short-term outcome was considered to be “good” but otherwise “poor”. Long-term outcome of neurological disability at the last follow-up was evaluated by the modified Rankin scale (mRS). Good outcome was defined as a mRS score of 0–2, and poor outcome was defined as a mRS score of 3 or higher.
Standard protocol approvals and patient consents
This retrospective observational study was approved by the Institutional Review Board of Kitasato University (B17-351). A written consent was obtained from all patients for genetic study, and we notified information to the subjects and made public, with respect to implementing the research using their existing information and the opportunities to refuse utilization of their information.
Statistical analysis
The Fisher exact test was performed for comparison of categorical variables, and the Mann–Whitney test was used for continuous variables. The statistical significance was set at p < 0.05. We used JMP, version 11.2.0 (SAS Institute Inc.) for statistical analyses.
Results
Clinical features and neuroimaging patterns of SLEs
A total of 44 SLEs were identified from 13 patients with m.3243A>G mutation during a median observational period of 119 months (range 3–240 months). The clinical features of each SLE are summarized in Table 1. Patient 1 is a mother of patient 2, and patient 8 is an elder sister of patient 9. Both patients 8 and 9 were pediatric onset. Individual patients developed median SLEs of 2 (range 1–8) during their observational period.
The common neurological manifestations of SLEs included headache (29/44, 65.9%), followed by seizure (25/44, 56.8%), visual symptoms (23/44, 52.3%), decreased level of consciousness (16/44), nausea/vomiting (16/44), psychiatric symptoms (10/44), muscle weakness (9/44) or speech dysfunction (9/44). Epileptic seizure associated with acute SLLs developed not only at the symptom onset but also 5 days or later (range day 5–30); such delayed-onset seizure developed in 12 SLEs (27.3%). Compared to SLEs of adult-onset patients, pediatric-onset patients’ SLEs had more frequently nausea/vomiting (10/15 vs. 6/29, p = 0.0068), but less frequently seizures (5/15 vs. 20/29, p = 0.0303), delayed-onset seizure (1/15 vs. 11/29, p = 0.0352), and speech dysfunction (0/15 vs. 9/29, p = 0.0181), but there was no difference in frequency of headache, visual symptoms, decreased level of consciousness, psychosis, or motor symptoms.
Of 44 SLEs, 29 (65.9%) were C-SLEs while the remaining 15 were non-classic SLEs due to sparsely distributed (n = 10), extensively disseminated (n = 4) or cerebellar lesions (n = 1). Neurological manifestations were attributed to single SLL in 25 of 44 SLEs (56.8%) but multiple SLLs in the remaining 19 SLEs. C-SLEs were more frequently attributed to single SLL than non-classic SLEs (24/29 vs. 1/15, p < 0.0001). C-SLEs developed in all patients but non-classic SLEs in 5; D-SLEs developed in 4 of 13 patients (30.8%) accounting for 4 of 44 SLEs (9.1%) (Figs. 1, 2, 3, 4, Online Resource 1–4). Two patients required an implantable cardiac pacemaker during their follow-up period, but no patient with multiple SLLs had evidence of cardiogenic embolism. Compared to SLEs of adult-onset patients, pediatric-onset patients’ SLEs were more frequently attributed to non-classic SLLs (12/15 vs. 3/29, p < 0.0001) and multiple SLLs (14/15 vs. 5/29, p < 0.0001). Regarding the symptoms, nausea/vomiting developed more frequently (9/15 vs. 7/29, p = 0.0255), and delayed-onset seizure less frequently (1/15 vs. 11/29, p = 0.0352) in non-classic SLEs than in C-SLEs, but there was no difference in other symptoms (such as headache or seizure) between them.
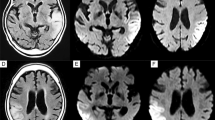
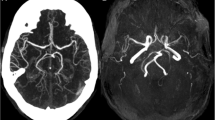
Neuroimaging of patient 2 (adult-onset MELAS). Brain MRIs (a, b) and 123I-IMP SPECT (c) obtained on day 2 of the 2nd SLE show slightly increased DWI/FLAIR signals in the left occipito-temporal cortex and marked hyperperfusion. The MRI/MRA (d–f) obtained on day 6 show increased DWI/FLAIR signals along the cerebral cortex with associated edema and vasodilatation of the left posterior cerebral artery branches. Brain MRIs (g–l) obtained during the active stage of the 3rd SLE show disseminated increased DWI/FLAIR signals confined to the cerebral cortexes. a, d, g–i DWIs, b, e, j–l FLAIR images, c123I-IMP-SPECT, and f MRA
Neuroimaging of patient 11 (adult-onset MELAS). Brain MRIs (a–f) obtained during the 1st SLE show disseminated small increased DWI/FLAIR signals confined to the cerebral cortex with subcortical edema. Note abnormal linear enhancement along the gyri of the affected cortexes (g–i), suggesting the same subacute stage of all disseminated lesions with blood–brain barrier breakdown. Brain MRIs (j–l) obtained at the 2nd SLE show two separate classic SLLs in the right medial frontal lobe (open arrows) and the right occipital lobe (closed arrows), both of which are accompanied by focal hyperperfusion (l). a–c, j DWIs, d–f, k FLAIR images, g–i post-enhanced T1-weighted images, l ASL-MRI
Neuroimaging of patient 8 (pediatric-onset MELAS). Brain MRIs (a–c) obtained on day 3 of the 3rd SLE show multifocal asymmetric increased FLAIR signals disseminated to both cerebral hemispheres, some of which in the right occipital lobe join with edema resulting in continuous lesions resembling classic SLL on FLAIR images. Brain MRIs (d–f) obtained on day 6 of the 4th SLE show a single classic stroke-like lesion in the left occipito-temporo-parietal lobe. Brain MRIs (g–i) obtained on day 3 of the 7th SLE show small increased DWI/FLAIR signals sparsely distributed to the right parietal cortex. Although a cluster of adjoining lesions may resemble classic SLLs on FLAIR image, the imaging pattern is different between classic SLL and a cluster of adjoining lesions. a–f, j–l FLAIR images, and g–i DWIs
Neuroimaging of patient 9 (pediatric-onset MELAS). Brain MRIs (a–f) obtained on day 1 of the 1st SLE show punctate or lineal increased DWI/FLAIR signals along the cerebral cortex of bilateral medial occipital cortexes. Brain MRIs (g–l) obtained on day 2 of the 2nd SLE show numerous punctate lesions on DWI (g–i). Note that a cluster of disseminated lesions on DWI is shown as continuous lesions resembling classic SLLs on FLAIR images (j–l); however, the imaging pattern is different from classic lesion (see Figs. 1d, e, 3d–f). a–c, g–i DWIs and d–f, j–l FLAIR images
Paroxysmal discharges and focal hyperperfusion were seen in 20 of 24 SLEs (83.3%), and 25 of 26 SLEs (96.2%), respectively. Initial brain lesion spread to adjacent cortex over a few weeks in at least 14 of 44 SLEs (31.8%). Such a progressive spread was more frequently seen in SLEs with delayed-onset seizure than in those without (8/12 vs. 6/32, p = 0.0043) and exclusively in C-SLEs but not in non-classic SLEs (14/29 vs. 0/15, p = 0.0013). Progressive spread was often accompanied by focal hyperperfusion (14/14, 100%) and seizure activity (10/13, 76.9%). A MRA did not show vasospasm but rather vasodilatation of the branches of middle cerebral artery or posterior cerebral artery ipsilateral to acute SLLs in nine SLEs (Fig. 1f). Accordingly, C-SLEs were mainly attributed to single continuous lobular lesions incongruent to vascular territory, extending along the cerebral cortex, and occasionally accompanied by a gradual spread of SLL associated with hyperperfusion and persistent seizure activity, while non-classic SLEs were mainly attributed to multiple sparsely distributed or disseminated small cortical lesions (Figs. 1, 2, 3, 4).
SLEs began with acute onset of symptoms in 42 of 44 SLEs (95.5%), but D-SLEs of 2 adult-onset patients began with ill-defined subacute-onset fluctuating encephalopathy. D-SLEs developed in two adult-onset and two pediatric-onset patients. D-SLEs of the adult-onset patients were characterized by non-specific psychosomatic symptoms such as fatigue, dizziness, headache, decreased concentration, speech alteration, tremor, and disinhibited behaviours. These symptoms were initially thought to be psychogenic or residual non-specific symptoms, resulting in a marked delay of the initiation of therapy; no treatment was initiated until a hospitalization 5 months after the symptoms onset in patient 2. In patient 11, it took 3 months to be recognized as a recurrence of SLEs (Online Resource 1). In patient 11, brain MRIs obtained 16 months after the symptoms onset showed extensive gadolinium enhancement along the gyri of the affected cortexes, indicating not only focal disruption of the BBB, but also same subacute stage of all disseminated lesions (Fig. 2g–i). An EEG also showed periodic synchronous discharges (PSDs) when the patient was being confused (Online Resource 4). While D-SLEs in two pediatric patients began with acute onset of headache and vomiting, both patients were immediately transferred to a hospital and treated with intravenous l-arginine and edaravone on the day of the symptom onset. The clinical pictures of D-SLEs were different between adult-onset and pediatric-onset patients. On brain MRIs, disseminated adjacent small lesions joined with each other causing FLAIR hyperintensity along the cerebral cortex similar to classic SLLs (Fig. 3a–c). A cluster of disseminated spotty DWI hyperintensities was also shown as continuous FLAIR hyperintensities resembling classic SLL (Fig. 4g–l). However, imaging pattern was different between classic SLL and a cluster of adjoining lesions.
Treatment, and short-term and long-term outcomes
During the acute stage, each SLE was treated with AEDs (41/44, 93.2%), intravenous edaravone (23/44, 52.3%), intravenous l-arginine (21/44, 47.7%), or combination of these drugs (Online Resource 5). After recovery of symptoms, 12 patients were treated with oral AEDs, and 5 with oral l-arginine. Neurological symptoms improved in 42 of 44 SLEs (95.5%) but patients 1 and 5 died during acute stage of the SLEs (Table 1). Other three patients died independently of SLEs. The median mRS at the last follow-up was 5 (range 2–6).
Discussion
This study showed the following findings: (1) SLEs were attributed to diverse SLLs ranging from a single classic lesion to disseminated small cortical lesions, (2) non-classic SLEs may be more frequent in pediatric-onset than in adult-onset patients, (3) slowly progressive spread was associated with delayed-onset seizure but exclusively seen in C-SLEs, and (4) ill-defined but subacute onset of fluctuating encephalopathy in adult-onset MELAS could be due to a cluster of disseminated small cortical lesions.
It remains controversial whether SLEs are caused by ischemia or not, but endothelial dysfunction is currently considered to play an important role [2, 11,12,13,14,15]. Persistent seizure activity is also implicated in the pathophysiology of slowly progressive spread, by increasing an energy demand under a genetically determined oxidative phosphorylation defect, which causes an imbalance between energy demand and supply, resulting in cortical laminar necrosis in the most vulnerable layer of the cortex [2, 8,9,10]. Extensive oxygen extraction fraction (OEF) reduction in SLLs, as well as in the normal-appearing brain regions have been demonstrated using MR OEF imaging, and more severe dysfunction of the mitochondria is implied at the onset of SLEs [16]. The association between slowly progressive spread and delayed-onset seizure supports hyperexcitability hypothesis. Migraine-like headache at onset could be explained by activation of the trigeminovascular system [17]. Elevated CSF levels of calcitonin gene-related peptide (CGRP), which is a potent vasodilator released from activated perivascular trigeminal nerve endings and implicated in migraine pathophysiology [18], are demonstrated at the early stage of SLEs with prominent vasodilatation of the ipsilateral intracranial arteries (at the 1st SLE of patient 7) [2]. Thus, vasodilatation of intracranial arteries associated with acute SLLs may be explained in part by activation of the trigeminovascular system.
This study clearly demonstrated a diversity of SLEs in patients with m.3243A>G mutation. We divided SLEs into classic and non-classic SLEs. C-SLEs are usually attributed to a single continuous lobular lesion incongruent to vascular territory and occasionally accompanied by a gradual spread of SLL. These C-SLEs are mainly based on SLEs of adult-onset MELAS [2, 8,9,10,11]. In contrast, non-classic SLEs were predominantly seen in SLEs of pediatric-onset patients. Interestingly, these sparsely or disseminated lesions were also confined to the cerebral cortex. Preferential cortical involvement suggests the presence of common pathophysiological mechanism between classic and non-classic lesions. In our series of patients with m.3243A>G mutation, no patient had acute ischemic-like lesion in basal ganglia or white matter, but we previously reported a patient with m.13513G>A mutation whose MRI showed symmetric basal ganglia lesions [9]. We also reported a patient with m.617G>A who developed recurrent embolic strokes associated with carotid artery stenosis as a phenotype of microangiopathy [19]. Thus, a phenotype of acute brain lesions may be differently associated with mtDNA mutation.
In one patient, acute cerebellar lesion developed. Symptomatic cerebellar SLLs are not common in MELAS, thus we excluded such subtentorial lesion from classic SLLs; however, multiple SLLs in the cerebellum have been reported in patients with m.3243 A>G mutation [20], in which plasma protein extravasation is demonstrated as evidence of BBB breakdown in those who had vascular cytochrome c oxidase-deficiency and thinning of the endothelial cell layer. Therefore, cerebellar SLLs are presumed to be attributed to endothelial dysfunction of microangiopathy.
Although D-SLEs may be a constellation of clinically under-recognized recurrent multiple SLEs and some of classic SLLs may consist of a cluster of multiple adjacent lesions along the cerebral cortex, it is important to note that D-SLEs could occur in MELAS patients. We noticed that multiple SLLs developed more frequently in SLEs of pediatric-onset patients than in those of adult-onset patients, suggesting a potential onset age-related diversity of SLEs. Although SLLs usually accumulate following recurrent SLEs, D-SLEs have not been reported yet. The frequency of D-SLEs is unclear, but we identified D-SLEs in four patients (30.8%) with m.3243A>G mutation, accounting for 9.1% of our series of all identified SLEs. Therefore, D-SLEs may not be rare. In our adult-onset patients, D-SLEs began with subacute-onset fluctuating mental status changes accompanied by psychosomatic symptoms; in both D-SLEs the symptoms were not initially recognized as a relapse of SLEs, accordingly initiation of therapy was markedly delayed because of the lack of knowledge on D-SLEs. While in both pediatric-onset patients, D-SLEs began with acute onset of headache with vomiting like other C-SLEs; therefore, the attacks were easily recognized by their parents and both patients received acute therapy on the day of symptom onset.
The pathophysiology underlying D-SLEs is unknown. Disseminated cortical lesions might develop simultaneously as a cluster of ill-defined SLEs, but cardiogenic embolisms were unlikely because of no evidence of coagulopathy, arrhythmia, or cardiomyopathy in our patients with non-classic SLLs. Diffuse cerebral vasospasm was also unlikely because no such vasospasm was demonstrated in any patient, but vasodilatation with hyperperfusion was seen in one adult-onset patient during the active stage of D-SLE. Preferential cortical involvement in D-SLEs implies that the pathophysiological mechanisms may be similar to those of cortical laminar necrosis attributed to incomplete global ischemia, anoxia, hypoglycaemia, immunosuppressive treatment [21] or prolonged status epilepticus [22]. Simultaneous development of extensive cortical lesions suggests the presence of critical diffuse metabolic derangement in the affected cerebral cortex, which is the most vulnerable to increased energy demand. In one adult-onset patient, PSDs were seen during protracted course of the D-SLEs. Thus, increase in energy demand may play an important role in D-SLEs as well as in C-SLEs in MELAS patients with pre-existing extensive OEF reduction even in normal-appearing cerebral cortex [16]. Suppression of increase in energy demand and prevention of seizure are both important during acute stage of SLEs; however, potential toxic effects of AEDs on mitochondria should be taken into account in MELAS patients because mitochondrial-toxic AEDs may trigger or worsen mitochondrial disorders. Hence, AEDs that interfere with mitochondrial function should be used carefully or avoided [23].
There are limitations; this is a retrospective study based on a small number of patients with m.3243A>G mutation. Patients with other MELAS mutations were not included. The subjects included only two pediatric-onset patients. A mutation load of biopsied muscle specimen was not determined. Only a few CBF or EEG data are available in non-classic SLE. The outcome was not evaluated based on a standard clinical rating scale because of retrospective long-term observational neuroimaging studies. Other issues include necessary upgrades of the different imaging modalities with long duration of follow-up, including some biases in data collection. The classification of SLEs (divided into either classic or non-classic including D-SLEs) is based on clinical long-term observation focussing on the phenotypes of SLEs, which had been carefully observed over 28 years at this hospital by the authors.
Despite these limitations, this study clearly showed a phenotypic diversity of SLEs in patients with m.3243G>A mutation, and that D-SLEs develop in any patient independent of age of onset. Early recognition of D-SLEs is crucial for prompt initiation of treatment to correct ongoing diffuse metabolic derangement of the brain. Although the pathophysiological mechanism of SLEs remains unclear, acute treatment strategy should focus on underlying mechanisms, which may be different between C-SLEs and non-classic ones. These observations deserve further study to confirm a potentially onset age-related diversity of SLEs and D-SLEs as a distinctive phenotype of SLEs in MELAS.
Change history
09 April 2019
The author would like to correct the errors in the publication of the original article. The corrected details are given below for your reading.
References
Pavlakis SG, Phillips PC, DiMauro S, De Vivo DC, Rowland LP (1984) Mitochondrial myopathy, encephalopathy, lactic acidosis, and strokelike episodes: a distinctive clinical syndrome. Ann Neurol 16:481–488. https://doi.org/10.1002/ana.410160409
Iizuka T, Sakai F (2010) Pathophysiology of stroke-like episodes in MELAS: neuron-astrocyte uncoupling in neuronal hyperexcitability. Future Neurol 5:61–83. https://doi.org/10.2217/fnl.09.71
Goto Y, Nonaka I, Horai S (1990) A mutation in the tRNA(Leu)(UUR) gene associated with the MELAS subgroup of mitochondrial encephalomyopathies. Nature 348:651–653
Kirino Y, Suzuki T (2005) Human mitochondrial diseases associated with tRNA wobble modification deficiency. RNA Biol 2:41–44
Suzuki T, Nagao A, Suzuki T (2011) Human mitochondrial diseases caused by lack of taurine modification in mitochondrial tRNAs. Wiley Interdiscip Rev RNA 2:376–386. https://doi.org/10.1002/wrna.65
Ohama E, Ohara S, Ikuta F, Tanaka K, Nishizawa M, Miyatake T (1987) Mitochondrial angiopathy in cerebral blood vessels of mitochondrial encephalomyopathy. Acta Neuropathol 74:226–233. https://doi.org/10.1007/BF00688185
Sparaco M, Bonilla E, Dimauro S, Powers JM (1993) Neuropathology of mitochondrial encephalopathies due to mitochondrial DNA defects. J Neuropathol Exp Neurol 52:1–10
Iizuka T, Sakai F, Suzuki N, Hata T, Tsukahara S, Fukuda M, Takiyama Y (2002) Neuronal hyperexcitability in stroke-like episodes of MELAS syndrome. Neurology 59:816–824. https://doi.org/10.1212/WNL.59.6.816
Iizuka T, Sakai F (2005) Pathogenesis of stroke-like episodes in MELAS: analysis of neurovascular cellular mechanisms. Curr Neurovasc Res 2:29–45
Iizuka T, Sakai F, Kan S, Suzuki N (2003) Slowly progressive spread of the stroke-like lesions in MELAS. Neurology 61:1238–1244. https://doi.org/10.1212/01.WNL.0000091888.26232.FE
Yoneda M, Maeda M, Kimura H, Fujii A, Katayama K, Kuriyama M (1999) Vasogenic edema on MELAS: a serial study with diffusion-weighted MR imaging. Neurology 53:2182–2184. https://doi.org/10.1212/WNL.53.9.2182
Koga Y, Povalko N, Nishioka J, Katayama K, Kakimoto N, Matsuishi T (2010) MELAS and l-arginine therapy: pathophysiology of stroke-like episodes. Ann N Y Acad Sci 1201:104–110. https://doi.org/10.1111/j.1749-6632.2010.05624.x
Davidson MM, Walker WF, Hernandez-Rosa E (2009) The m.3243A>G mtDNA mutation is pathogenic in an in vitro model of the human blood brain barrier. Mitochondrion 9:463–470. https://doi.org/10.1016/j.mito.2009.08.006
Matsuzaki M, Takahashi R, Nakayama T, Shishikura K, Suzuki H, Hirayama Y, Osawa M, Oda H (2010) Disruption of endothelial tight junctions in a patient with mitochondrial encephalomyopathy, lactic acidosis and stroke-like episodes (MELAS). Neuropediatrics 41:72–74. https://doi.org/10.1055/s-0030-1261886
Koga Y, Povalko N, Inoue E, Nakamura H, Ishii A, Suzuki Y, Yoneda M, Kanda F, Kubota M, Okada H, Fujii K (2018) Therapeutic regimen of l-arginine for MELAS: 9-year, prospective, multicenter, clinical research. Neurol J 265:2861–2874. https://doi.org/10.1007/s00415-018-9057-7
Xie S (2014) MR OEF imaging in MELAS. Methods Enzymol 547:433–444. https://doi.org/10.1016/B978-0-12-801415-8.00021-7
Iizuka T, Sakai F, Endo M, Suzuki N (2003) Response to sumatriptan in headache of MELAS syndrome. Neurology 61:577–578. https://doi.org/10.1212/01.WNL.0000078931.87815.B3
Edvinsson L (2017) The trigeminovascular pathway: role of CGRP and CGRP receptors in migraine. Headache 57(Suppl 2):47–55. https://doi.org/10.1111/head.13081
Iizuka T, Goto Y, Miyakawa S, Sato M, Wang Z, Suzuki K, Hamada J, Kurata A, Sakai F (2009) Progressive carotid artery stenosis with a novel tRNA phenylalanine mitochondrial DNA mutation. J Neurol Sci 278:35–40. https://doi.org/10.1016/j.jns.2008.11.016
Lax NZ, Pienaar IS, Reeve AK, Hepplewhite PD, Jaros E, Taylor RW, Kalaria RN, Turnbull DM (2012) Microangiopathy in the cerebellum of patients with mitochondrial DNA disease. Brain 135:1736–1750. https://doi.org/10.1093/brain/aws110
Bargalló N, Burrel M, Berenguer J, Cofan F, Buñesch L, Mercader JM (2000) Cortical laminar necrosis caused by immunosuppressive therapy and chemotherapy. AJNR Am J Neuroradiol 21:479–484
Donaire A, Carreno M, Gómez B, Fossas P, Bargalló N, Agudo R, Falip M, Setoaín X, Boget T, Raspall T, Obach V, Rumiá J (2006) Cortical laminar necrosis related to prolonged focal status epilepticus. J Neurol Neurosurg Psychiatry 77:104–106. https://doi.org/10.1136/jnnp.2004.058701
Finsterer J, Zarrouk Mahjoub S (2012) Mitochondrial toxicity of antiepileptic drugs and their tolerability in mitochondrial disorders. Expert Opin Drug Metab Toxicol 8:71–79. https://doi.org/10.1517/17425255.2012.644535
Acknowledgements
The authors thank all of the patients involved in this study and acknowledge the efforts of research staff, who worked on the clinical and neuroimaging data collection.
Funding
None.
Author information
Authors and Affiliations
Contributions
YH, NK, TI: conception of the work, data acquisition, data interpretation, drafting and revising the manuscript for intellectual content. JK, HS, DI, EK, TA, YO, TG, KN: data acquisition, data interpretation, and revising the manuscript for intellectual content.
Corresponding author
Ethics declarations
Conflicts of interest
KN received grants from Daiichi Sankyo Co., Ltd., Otsuka Pharmaceutical Co., Ltd., Dainippon Sumitomo Pharma Co., Ltd., and Eisai Co., Ltd. TI received a grant from Japan Epilepsy Research Foundation. Other authors report no disclosures.
Ethical standards
The study was approved by the Institutional Review Board of Kitasato University (B17-351).
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hongo, Y., Kaneko, J., Suga, H. et al. A cluster of disseminated small cortical lesions in MELAS: its distinctive clinical and neuroimaging features. J Neurol 266, 1459–1472 (2019). https://doi.org/10.1007/s00415-019-09283-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-019-09283-3