Abstract
Introduction
While subthalamic nucleus deep brain stimulation (STN-DBS) and levodopa improve motor symptoms in Parkinson disease (PD) to a similar magnitude, their combined effect remains unclear. We sought to evaluate whether STN-DBS and levodopa yield differential effects on motor outcomes, dyskinesia, and activities of daily living (ADL) when combined compared to when administered alone.
Methods
We conducted a meta-analysis of all studies reporting motor, dyskinesia, and ADL outcomes after bilateral STN-DBS in PD with presurgical Unified Parkinson’s Disease Rating Scale (UPDRS-III) in Medication-OFF and Medication-ON states and postsurgical assessments in four conditions: Stimulation-ON/Medication-ON, Stimulation-ON/Medication-OFF, Stimulation-OFF/Medication-ON, and Stimulation-OFF/Medication-OFF. Dyskinesia duration (UPDRS item 32) and ADL (UPDRS-II) were compared between high and low postsurgical levodopa equivalent daily dose (LEDD) reduction. Random-effects meta-analyses using generic-inverse variance were conducted. Confidence in outcomes effect sizes was assessed.
Results
Twelve studies were included (n = 401 patients). Stimulation-ON/Medication-ON was associated with an UPDRS-III improvement of − 35.7 points [95% confidence interval, − 40.4, − 31.0] compared with Stimulation-OFF/Medication-OFF, − 11.2 points [− 14.0, − 8.4] compared with Stimulation-OFF/Medication-ON, and − 9.5 points [− 11.0, − 8.0] compared to Stimulation-ON/Medication-OFF within 5 years. The difference was maintained beyond 5 years by − 28.6 [− 32.8, − 24.4], − 8.1 [− 10.2, − 5.9], and − 8.0 [− 10.3, − 5.6], respectively. No difference was observed between Stimulation-ON/Medication-OFF and Stimulation-OFF/Medication-ON within and beyond 5 years. Dyskinesia duration and ADL outcomes were similar in high vs. low postsurgical LEDD reduction.
Conclusion
Subthalamic nucleus deep brain stimulation and levodopa independently lessened motor severity in PD to a similar magnitude, but their combined effect was greater than either treatment alone, suggesting therapeutic synergism.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
With over 140,000 patients treated worldwide, subthalamic nucleus deep brain stimulation (STN-DBS) is an established treatment for motor complications in Parkinson disease (PD) [1]. The postSTN-DBS management, however, poses the challenge of identifying the optimal combination of dopaminergic therapies and stimulation settings.
Reduction in levodopa equivalent daily dose (LEDD) and other dopaminergic medications is widely endorsed after STN-DBS, and has become an “anticipated benefit” of this surgical modality. This paradigm stems from the rationale that STN-DBS might reduce PD cardinal symptoms to a similar extent than levodopa (L-dopa) and that decreasing medications reduces postoperative dyskinesia [1, 2]. On the other hand, medication reduction can elicit other problems, such as depression and apathy [3], which creates uncertainty as to the wisdom of aggressively lowering dopaminergic therapies in patients treated with STN-DBS [4]. While STN-DBS and L-dopa have been recognized as providing similar motor benefits, no systematic assessment of these two treatments combined has been performed in long-term studies [5]. In addition, the difference in motor complications and activities of daily living (ADL) between patients with high vs. low postsurgical LEDD reduction remains to be clarified.
In this meta-analysis, we sought to estimate the magnitude of difference between ON and OFF medication states and ON and OFF stimulation states to determine if STN-DBS and L-dopa may yield differential motor, dyskinesia, and ADL outcomes when combined compared to when stimulation and medication are administered alone.
Materials and methods
This meta-analysis was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) and the Meta-analysis of Observational Studies in Epidemiology (MOOSE) guidelines [6, 7]. Observational studies, randomized clinical trials (RCT), and non-randomized clinical trials (n-RCT) were included if meeting the following criteria: (a) surgical selection for bilateral STN-DBS, as per the Core Assessment Program for Surgical Interventional Therapies in PD (CAPSIT-PD) [8]; (b) presurgical assessment of motor symptoms in Medication-OFF (Med-OFF) and Medication-ON (Med-ON) conditions, as per the motor subscale of the Unified Parkinson’s Disease Rating Scale (UPDRS-III); (c) postsurgical assessment of motor symptoms in the following conditions: Stimulation-ON/Medication-ON (Stim-ON/Med-ON), Stimulation-OFF/Medication-OFF (Stim-OFF/Med-OFF), Stimulation-OFF/Medication-ON (Stim-OFF/Med-ON), and Stimulation-ON/Medication-OFF (Stim-ON/Med-OFF), using a supra-maximal L-dopa challenge dose to assess Medication-ON conditions (Supplementary Table 1), as per the CAPSIT-PD protocol [8].
Exclusion criteria were incomplete data reporting (i.e., lacking one or more of the four postsurgical CAPSIT-PD conditions) or sample sizes fewer than five patients. No restrictions were applied to gender, disease duration, disease severity, or DBS manufacturer.
Search methods
We searched for eligible studies in PubMed, Embase, Cochrane Movement Disorders Group Trials Register, Cochrane Central Register of Controlled Trials (CENTRAL), ClinicalTrials.gov, and the System for Information on Grey Literature in Europe (OpenGrey) up to 31 December 2017 using the following search terms: Parkinson disease, Parkinson, deep brain stimulation, DBS, and follow-up (Supplementary Table 2). No language restrictions were applied.
Meta-analysis design
We divided the analyses into short term (< 5 years) and long term (≥ 5 years) after surgery, comparing the change in UPDRS-III in the four possible Stimulation/Medication conditions. In addition to the motor outcome (UPDRS-III), we examined ADL (UPDRS-II) and the change in the proportion of the waking day spent with dyskinesia (UPDRS item 32).
Selection of studies and data extraction
Abstracts were reviewed for eligibility criteria by three investigators (J.A.V., M.S., and A.M.). Pertinent full-text articles were assessed and variables of interest extracted. Particular attention was paid to studies that shared the same population or published data from the same cohort at different time-points. In this scenario, the longest follow-up within each time interval (< 5 years and ≥ 5 years) was used for the analyses. Disagreements were anticipated to be settled by consensus.
Assessment of risk of bias and heterogeneity
Evidence quality was independently assessed by two investigators (J.A.V. and M.S.). For included studies, we used the Cochrane-validated “Quality Assessment Tool for Before–After Studies with No Control Group” [9]. Visual inspection of funnel plots was conducted to assess for publication bias [10]. Subsequently, the overall confidence in the effect for each outcome of interest was assessed following the Grading of Recommendations Assessment, Development and Evaluation (GRADE) methodology [11]. The degree of heterogeneity was deemed considerable if I2 statistic was ≥ 75% and significance test (p value) below 0.1 [12].
Measures of treatment effect
Random-effects meta-analyses using generic-inverse variance were used to pool the mean differences and standard errors of the following outcomes: (a) motor score; (b) dyskinesia duration; (c) ADL, at the prespecified follow-up intervals, with 95% confidence intervals (C.I.) for these pooled estimates. Subgroup analyses were conducted to compare studies with high (≥ median) vs. low (< median) postsurgical LEDD reduction from baseline, within, and beyond 5 years. All the analyses were performed in Review Manager® (RevMan, version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014).
Results
Out of the 1632 records derived from the initial search strategy (Supplementary Fig. 1), 12 observational and non-randomized studies met full criteria [2, 5, 13,14,15,16,17,18,19,20,21,22], which underwent data extraction (Table 1) and assessment for individual risk of bias (Supplementary Table 3). Pooled studies were assessed to determine the overall quality of evidence (Supplementary Table 4). The agreement was met between evaluators in all cases, and no signs of publication biases were observed in funnel plots (Supplementary Fig. 2).
The total study population consisted of 401 PD patients treated with STN-DBS (n = 366 with < 5 years and n = 196 with ≥ 5 years of follow-up). Six patients had undergone the previous neurosurgical procedures (n = 4 in Ostergaard and Sunde [13]; and n = 2 in Schupbach et al. [21]).
Follow-up < 5 years
The Stim-ON/Med-ON condition reduced (improved) UPDRS-III by − 35.7 points (95% CI − 40.4, − 31.0) compared with Stim-OFF/Med-OFF, but also by − 11.2 [− 14.0, − 8.4] compared with Stim-OFF/Med-ON and − 9.5 [− 11.0, − 8.0] compared with Stim-ON/Med-OFF. No difference was observed between Stim-ON/Med-OFF and Stim-OFF/Med-ON conditions (Fig. 1).
STN-DBS and L-dopa effect on UPDRS-III at < 5 years. Inverse variance method was used to calculate mean differences and data were pooled using a random-effects model. Results are shown as point estimates and 95% confidence interval. STN-DBS + L-dopa Stimulation-ON/Medication-ON, STN-DBS Stimulation-ON/Medication-OFF, L-dopa Stimulation-OFF/Medication-ON, OFF state Stimulation-OFF/Medication-OFF, UPDRS-III Unified Parkinson’s Disease Rating Scale part III, IV inverse variance, CI confidence interval
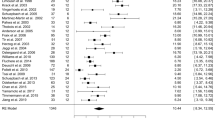
High vs. low postsurgical LEDD reduction (Fig. 2) resulted in a similar improvement in dyskinesia duration [− 1.4 (95% CI − 1.5, − 1.2) vs. − 1.0 (95% C.I. − 1.7, − 0.4); p = 0.33; I2 = 0] and no significant differences in the ADL outcomes [0.6 (95% CI − 0.5, 1.6) vs. − 0.01 (95% CI − 4.1, 4.1); p = 0.79; I2 = 0].
Dyskinesia duration and ADL in higher vs. lower LEDD reduction subgroups. Inverse variance method was used to calculate mean differences and data were pooled using a random-effects model. Results are shown as point estimates and 95% confidence interval. STN-DBS + L-dopa Stimulation-ON/Medication-ON, STN-DBS Stimulation-ON/Medication-OFF, L-dopa Stimulation-OFF/Medication-ON, OFF state Stimulation-OFF/Medication-OFF, UPDRS-II Unified Parkinson’s Disease Rating Scale part II, UPDRS-IV #32 Unified Parkinson’s Disease Rating Scale-Item 32, IV inverse variance, CI confidence interval
Follow-up ≥ 5 years
The Stim-ON/Med-ON condition reduced UPDRS-III by − 28.6 points (95% CI − 32.8, − 24.4) compared to Stim-OFF/Med-OFF, but also by − 8.1 [− 10.2, − 5.9] compared with Stim-OFF/Med-ON and − 8.0 [− 10.3, − 5.6] compared with Stim-ON/Med-OFF. No difference was observed between Stim-ON/Med-OFF and Stim-OFF/Med-ON conditions (Fig. 3).
STN-DBS and L-dopa effect on UPDRS-III at ≥ 5 years. Inverse variance method was used to calculate mean differences and data were pooled using a random-effects model. Results are shown as point estimates and 95% confidence interval. STN-DBS + L-dopa Stimulation-ON/Medication-ON, STN-DBS Stimulation-ON/Medication-OFF, L-dopa Stimulation-OFF/Medication-ON, OFF state Stimulation-OFF/Medication-OFF, UPDRS-III Unified Parkinson’s Disease Rating Scale part III, IV inverse variance, CI confidence interval
High vs. low postsurgical LEDD reduction (Fig. 2) resulted in a similar improvement in dyskinesia duration [− 1.1 (95% CI − 1.3, − 0.9) vs. 1.1 (95% CI − 1.5, − 0.7); p = 0.99; I2 = 0] and no significant differences in the ADL outcomes [5.6 (95% CI 1.0, 10.3) vs. 6.8 (95% CI 3.0, 10.6); p = 0.71; I2 = 0].
Discussion
The results of this meta-analysis demonstrated that while there was similar individual efficacy of STN-DBS and L-dopa, their combined effect on motor severity was additive within and beyond 5 years of follow-up, with a UPDRS-III differential between 9.5 and 11.2 points in the short term and between 8.0 and 8.1 points in the long term. These values are above the 3.25 point threshold considered the minimal clinically important difference (MID) for the UPDRS-III [23]. In addition, no difference was observed in the extent of dyskinesia duration improvement or in the ADL outcome between studies with high vs. low postsurgical LEDD reduction.
Taken together, these data argue against the paradigm of invariably aiming at reducing the dopaminergic tone as part of the postsurgical management of STN-DBS patients. In fact, there is no evidence that greater reduction in dopaminergic therapies might lead to better control of dyskinesia, while harnessing an additive effect between STN-DBS and L-dopa may be particularly relevant at advanced disease stages, in which the main sources of disability are relatively resistant to the conventional medical and surgical therapies alone, such as gait, balance, speech, swallowing, and cognitive impairments [5, 19, 24, 25]. Furthermore, lower reduction in dopaminergic medications, as reported after unilateral STN-DBS [4], might result in lower incidence of apathy and depression [26].
The underlying mechanism behind the additive effect of stimulation and medication might reflect the complementary effects of both intervention, modulating both dopaminergic and non-dopaminergic pathways including, but not limited to, cholinergic and adrenergic circuits [27]. Furthermore, there may be a differential modulation of nigro-striatal dopaminergic pathways between STN-DBS and L-dopa in advanced PD, when L-dopa response may be limited by aberrant synaptic plasticity, reduced density in D3 striatal dopamine receptors [28], and progressive loss of dopaminergic neurons in the caudal putamen [29]. Whether STN-DBS effectiveness might be hampered by advanced degeneration of dopaminergic and non-dopaminergic pathways remains unclear [30].
Some limitations attenuate the strength of our conclusions. First, we included uncontrolled, non-randomized clinical studies of small sample sizes, which lower the confidence in the overall effect. Although heterogeneity was present, a meta-regression was not performed due to the limited number of studies included in the analyses [12]. To minimize these shortcomings, we carefully assessed the individual and overall quality of included studies as per the Cochrane and GRADE handbook recommendations [11]. Second, although standard in the presurgical evaluation of patients, the comparison between Med-OFF and a supra-therapeutic Med-ON condition may not represent an accurate estimate of the patient daily response to L-dopa therapy. Relatedly, not all measurements of UPDRS-III postoperative response to management may have accurately represented ecologically valid settings, such as their functioning at home. In addition, the data on dyskinesia based on UPDRS item 32 lack characterization of semiology, severity, and functional impairment, and may not be sensitive enough to treatment. While the assessment of dyskinesia duration and ADL reflected information gathered from daily-living clinical experience, the possibility exists that subgroup analyses might be underpowered to detect small differences between high and low postsurgical LEDD reduction subgroups. Unfortunately, data from the full UPDRS-IV and from quality of life scales were not consistently available, and would have prevented the construction of a pooled estimate with meta-analysis.
In conclusion, our data confirm the comparable efficacy of STN-DBS and L-dopa, but also suggest an additional benefit to be attained by their combined application, which is greater than each treatment alone. While a postsurgical reduction in dopaminergic therapies may be necessary to ameliorate dopaminergic side effects such as sedation, hallucinations, impulsivity, and orthostatic hypotension, our findings suggest that, for any other reasons, including “simplification” of the daily therapeutic schedule, a significant reduction in dopaminergic tone might preclude the potentially additive effect of STN-DBS and levodopa on PD motor symptoms. Further controlled, prospective studies will be needed to clarify optimal therapeutic strategies, but the available evidence supports the notion that clinicians may be missing an important source of outcome optimization in PD by aggressively reducing medications after bilateral STN-DBS.
Data access and responsibility statement
Drs. Merola and Vizcarra had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
References
Rowland NC, Sammartino F, Lozano AM (2017) Advances in surgery for movement disorders. Mov Disord 32:5–10
Rodriguez-Oroz MC, Obeso JA, Lang AE, Houeto JL, Pollak P, Rehncrona S et al (2005) Bilateral deep brain stimulation in Parkinson’s disease: a multicentre study with 4 years follow-up. Brain 128:2240–2249
Schupbach M, Gargiulo M, Welter ML, Mallet L, Behar C, Houeto JL et al (2006) Neurosurgery in Parkinson disease: a distressed mind in a repaired body? Neurology 66:1811–1816
Okun MS, Wu SS, Fayad S, Ward H, Bowers D, Rosado C et al (2014) Acute and chronic mood and apathy outcomes from a randomized study of unilateral STN and GPi DBS. PLoS One 9:1–16
Zibetti M, Merola A, Rizzi L, Ricchi V, Angrisano S, Azzaro C et al (2011) Beyond nine years of continuous subthalamic nucleus deep brain stimulation in Parkinson’s disease. Mov Disord 26:2327–2334
Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 151:264–269 W64.
Stroup DF (2000) Meta-analysis of observational studies in epidemiology: a proposal for reporting. JAMA 283:2008–2012
Defer G-L, Widner H, Marié R-M, Rémy P, Levivier M (1999) Core assessment program for surgical interventional therapies in Parkinson’s disease (CAPSIT-PD). Mov Disord 14:572–584
NHLBI (2014) Quality assessment tool for before–after (Pre–Post) studies with no control group. https://www.nhlbi.nih.gov/health-pro/guidelines/in-develop/cardiovascular-risk-reduction/tools/before-after. Accessed 1 Jul 2017
Sterne JAC, Sutton AJ, Ioannidis JPA, Terrin N, Jones DR, Lau J et al (2011) Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ 342:d4002
The GRADE Working Group (2013) Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach
Higgins JPT, Green S (eds) (2011) Cochrane handbook for systematic reviews of interventions version 5.1.0 [updated March 2011]. The Cochrane Collaboration. www.handbook.cochrane.org
Østergaard K, Sunde NA (2006) Evolution of Parkinson’s disease during 4 years of bilateral deep brain stimulation of the subthalamic nucleus. Mov Disord 21:624–631
Ząbek M, Sobstyl M, Koziara H, Kądziołka B, Mossakowski Z, Dierzęcki S (2010) Bilateral subthalamic nucleus stimulation in the treatment of advanced Parkinson’s disease. Five years’ personal experience. Neurol Neurochir Pol 44:3–12
Visser-Vandewalle V, Van Der Linden C, Temel Y, Celik H, Ackermans L, Spincemaille G et al (2005) Long-term effects of bilateral subthalamic nucleus stimulation in advanced Parkinson disease: a four year follow-up study. Parkinsonism Relat Disord 11:157–165
Merola A, Romagnolo A, Bernardini A, Rizzi L, Artusi CA, Lanotte M et al (2015) Earlier versus later subthalamic deep brain stimulation in Parkinson’s disease. Parkinsonism Relat Disord 21:972–975
Merola A, Zibetti M, Angrisano S, Rizzi L, Ricchi V, Artusi CA et al (2011) Parkinson’s disease progression at 30 years: a study of subthalamic deep brain-stimulated patients. Brain 134:2074–2084
Moro E, Lozano AM, Pollak P, Agid Y, Rehncrona S, Volkmann J et al (2010) Long-term results of a multicenter study on subthalamic and pallidal stimulation in Parkinson’s disease. Mov Disord 25:578–586
Castrioto A, Lozano A, Poon Y-Y, Lang AE, Fallis M, Moro E (2011) Ten-year outcome of subthalamic stimulation in Parkinson disease. Arch Neurol 68:1550–1556
Piboolnurak P, Lang AE, Lozano AM, Miyasaki JM, Saint-Cyr JA, Poon YYW et al (2007) Levodopa response in long-term bilateral subthalamic stimulation for Parkinson’s disease. Mov Disord 22:990–997
Schupbach WMM, Chastan N, Welter ML, Houeto JL, Mesnage V, Bonnet AM et al (2005) Stimulation of the subthalamic nucleus in Parkinson’s disease: a 5 year follow up. J Neurol Neurosurg Psychiatry 76:1640–1644
Simonin C, Tir M, Devos D, Kreisler A, Dujardin K, Salleron J et al (2009) Reduced levodopa-induced complications after 5 years of subthalamic stimulation in Parkinson’s disease: a second honeymoon. J Neurol 256:1736–1741
Horváth K, Aschermann Z, Ács P, Deli G, Janszky J, Komoly S et al (2015) Minimal clinically important difference on the Motor Examination part of MDS-UPDRS. Parkinsonism Relat Disord 21:1421–1426
Fasano A, Romito LM, Daniele A, Piano C, Zinno M, Bentivoglio AR et al (2010) Motor and cognitive outcome in patients with Parkinson’s disease 8 years after subthalamic implants. Brain 133:2664–2676
Rizzone MG, Fasano A, Daniele A, Zibetti M, Merola A, Rizzi L et al (2014) Long-term outcome of subthalamic nucleus DBS in Parkinson’s disease: from the advanced phase towards the late stage of the disease? Parkinsonism Relat Disord 20:376–381
Thobois S, Lhommée E, Klinger H, Ardouin C, Schmitt E, Bichon A et al (2013) Parkinsonian apathy responds to dopaminergic stimulation of D2/D3 receptors with piribedil. Brain 136:1568–1577
Stemper B, Beric A, Welsch G, Haendl T, Sterio D, Hilz MJ (2006) Deep brain stimulation improves orthostatic regulation of patients with Parkinson disease. Neurology 67:1781–1785
Joyce J, Ryoo H, Beach T, Caviness J, Stacy M, Gurevich E et al (2002) Loss of response to levodopa in Parkinson’s disease and co-occurrence with dementia: role of D3 and not D2 receptors. Brain Res 955:138–152
Porritt MJ, Kingsbury AE, Hughes AJ, Howells DW (2006) Striatal dopaminergic neurons are lost with Parkinson’s disease progression. Mov Disord 21:2208–2211
Fabbri M, Coelho M, Guedes LC, Chendo I, Sousa C, Rosa MM et al (2017) Response of non-motor symptoms to levodopa in late-stage Parkinson’s disease: results of a levodopa challenge test. Parkinsonism Relat Disord 39:37–43
Funding
Nothing to declare.
Author information
Authors and Affiliations
Contributions
Dr. JAV: conception, organization, and execution of research project; design and execution of statistical analysis; writing of the first draft of manuscript. Dr. MS-K: execution of research project; review and critique of the manuscript. Dr. CAA: execution of research project; review and critique of the manuscript. Dr. APD: review and critique of statistical analysis; review and critique of the manuscript. Dr. LL: review and critique of statistical analysis; review and critique of the manuscript. Dr. MSO: review and critique of statistical analysis; review and critique of the manuscript. Dr. AJE: conception of research project; review and critique of statistical analysis; review and critique of the manuscript. Dr. AM: conception and organization of research project; review and critique of statistical analysis; writing of the first draft and review and critique of the manuscript of manuscript.
Corresponding author
Ethics declarations
Ethical standards
The manuscript does not contain clinical studies or patient data.
Conflicts of interest
Dr. Vizcarra reports no conflict of interest. Dr. Situ-Kcomt reports no conflict of interest. Dr. Artusi reports no conflict of interest. Dr. Duker has previously received honoraria but has not received industry support in the last 36 months. Dr. Lopiano has received honoraria for lecturing and travel grants from Medtronic, UCB Pharma, and AbbVie. Dr. Okun serves as a consultant for the National Parkinson Foundation, and has received research grants from NIH, NPF, the Michael J. Fox Foundation, the Parkinson Alliance, Smallwood Foundation, the Bachmann-Strauss Foundation, the Tourette Syndrome Association, and the UF Foundation. His DBS research is supported by: R01 NR014852 and R01NS096008. He has previously received honoraria, but, in the past > 60 months, he has received no support from industry. He has received royalties for publications with Demos, Manson, Amazon, Smashwords, Books4Patients, and Cambridge (movement disorders books). He is an associate editor for New England Journal of Medicine Journal Watch Neurology. He has participated in CME and educational activities on movement disorders (in the last 36) months sponsored by PeerView, Prime, QuantiaMD, WebMD, Medicus, MedNet, Henry Stewart, and by Vanderbilt University. The institution and not Dr. Okun receives grants from Medtronic, Abbvie, Allergan, and ANS/St. Jude, and the PI has no financial interest in these grants. He has participated as a site PI and/or co-I for several NIH, foundation, and industry sponsored trials over the years but has not received honoraria. Dr. Espay has received grant support from the NIH, Great Lakes Neurotechnologies, and the Michael J Fox Foundation; personal compensation as a consultant/scientific advisory board member for Abbvie, TEVA, Impax, Acadia, Acorda, Cynapsus/Sunovion, Lundbeck, and USWorldMeds; publishing royalties from Lippincott Williams & Wilkins, Cambridge University Press, and Springer; and honoraria from Abbvie, UCB, USWorldMeds, Lundbeck, Acadia, the American Academy of Neurology, and the Movement Disorders Society. He serves on the editorial boards of the Journal of Parkinson’s Disease and Parkinsonism and Related Disorders. Dr. Merola is supported by NIH (KL2 TR001426) and has received speaker honoraria from CSL Behring and Cynapsus Therapeutics. He has received grant support from Lundbeck.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Vizcarra, J.A., Situ-Kcomt, M., Artusi, C.A. et al. Subthalamic deep brain stimulation and levodopa in Parkinson’s disease: a meta-analysis of combined effects. J Neurol 266, 289–297 (2019). https://doi.org/10.1007/s00415-018-8936-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-018-8936-2