Abstract
Objective
Crossed aphasia (CA), usually referred to as an acquired language disturbance, is caused by a lesion in the cerebral hemisphere ipsilateral to the dominant hand, and the exact mechanism is not clear. The development of handedness is influenced by education and training and the impact of habitualization, while language is more susceptible to the impact of speech habits, and it is not absolutely accurate to judge cerebral language dominance by the degree of hand preference.
Methods
We describe a case of CA after right hemispheric stroke in a right-handed patient with atypical language dominance and attempt to analyze the mechanism of CA based on functional imaging methods, including arterial spin labeling (ASL) and positron emission tomography/magnetic resonance imaging (PET-MRI).
Results
Brain MRI at 24 h after admission showed a large cerebral infarction in the right cerebral hemisphere, including the posteroinferior part of Broca’s area in the right frontal lobe, the right temporal lobe, and the right occipital lobe. The patient exhibited a non-fluent aphasia on a standard language test (the Aphasia Battery of Chinese [ABC]) performed on the 7th day after onset. Thus, atypical language dominance was suspected. One week after admission, ASL imaging showed high perfusion in the infarct core zone and low perfusion in the left cerebellar hemisphere. Two months later, PET/MRI demonstrated low metabolism in the posterior frontal lobe, temporal lobe, temporal occipital junction area, and the right basal ganglia.
Conclusion
The findings suggest that the patient has right-sided cerebral language dominance, or that both hemispheres have linguistic functions. Not all patients show linguistic capabilities on the side opposite hand preference. The language dominance should be predicted by a combination of clinical manifestations and functional imaging techniques.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The prevailing view that there is some link between hand preference and language dominance comports with the finding that approximately 95% of right-handed and 80% of left-handed or ambidextrous individuals exhibit left hemisphere language dominance [1,2,3]. Crossed aphasia (CA) is an acquired language disturbance that is associated with lesions affecting the cerebral hemisphere ipsilateral to the dominant hand, and aphasia that occurs after damage to the right hemisphere in a right-handed patient is now commonly referred to as CA. CA in right-handed patients accounts for 0.38–3% of all aphasia syndromes [4, 5]. In addition to the typical clinical symptoms, the diagnosis of CA requires the exclusion of potential structural damage in the left hemisphere, including childhood brain damage that may have caused lateralization of speech function. Because handedness is influenced by education and training and may be acquired by habitualization, while language is more susceptible to the impact of speech habits, it is not absolutely accurate to judge cerebral language dominance by the degree of hand preference. There is still debate about whether CA should be excluded when there is a family history of left-handedness ambidexterity [6], and the definite mechanism of CA is not yet fully understood. At present, the most widely accepted hypotheses include: (1) activation of dormant or occult lesions in the left hemisphere following onset of a new lesion in the right hemisphere; (2) control of hand preference by the ipsilateral cerebral hemisphere; (3) presence of linguistic functions in both hemispheres; and (4) cessation of lateralization of language function after a certain stage of development [7].
We describe a case of CA after right hemispheric stroke in a right-handed patient with atypical language dominance and attempt to analyze the mechanism of CA based on functional imaging methods.
Case description
A 66-year-old right-handed man without history of diabetes, coronary heart disease, or arrhythmia, was admitted to our hospital after sudden loss of consciousness and left limb paralysis following an episode of nausea and vomiting. No seizure or incontinence was reported. The patient did not have a history of alcohol or tobacco use. His blood pressure had been found to be slightly elevated a month previously.
The neurological examination at admission was notable for confusion, restlessness, aphasia, and eyes staring right. The left side was completely paralyzed, and there was a positive Babinski sign. The National Institutes of Health Stroke Score (NIHSS) was 18.
Emergent non-contrast computed tomography (CT) at admission showed a right hyperdense middle cerebral artery sign (HMCAS) (Fig. 1a). The admitting electrocardiogram (ECG) showed sinus rhythm, and no arrhythmias were detected on cardiac rhythm monitoring during hospitalization. The patient was hypertensive at admission, with a blood pressure of 150/90. Complete blood count, chemistry, and coagulation profiles at admission were normal. A large-area cerebral embolism in the right hemisphere was the presumptive diagnosis, and intravenous thrombolytic therapy with alteplase 58.5 mg (weight 65 kg) was initiated. The patient’s mental status gradually improved, and within 24 h the left upper limb muscle strength was 2/5 and left lower limb strength was 3/5, but aphasia persisted, with an NIHSS of 12 points. Brain MRI at 24 h after admission confirmed a large cerebral infarction in the right hemisphere, with no apparent structural damage within the left hemisphere. Carotid ultrasound showed hyper-echoic plaques at the origins of both internal carotid arteries (ICAs) with 20–30% local stenosis of the right ICA and 5% stenosis of the left ICA. Doppler echocardiography was essentially normal, showing no atrial enlargement or valve abnormality. We gave argatroban because of the unknown etiology of the embolism. The patient recovered full limb strength after 1 week and the NIHSS dropped to 3 points, but the obvious aphasia persisted. Transesophageal echocardiography (TEE) showed no definite thrombus in any cardiac chamber, and the left atrial appendage function was normal. There was an arrhythmia detected during the TEE, and cardiac embolism, therefore, was not ruled out as a possible etiology.
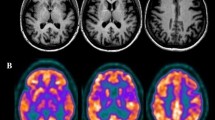
a Unenhanced computed tomography at admission showed a right hyperdense middle cerebral artery sign (HMCAS) (white thin arrow). b Diffusion-weighted magnetic resonance imaging (DW-MRI) at 24 h after admission showed multiple high signals near the posteroinferior portion of Broca’s area in the right frontal lobe (circled in white), and in the right temporal (white thick arrow) and occipital (red thick arrow) distributions of the lower trunk of the middle cerebral artery (MCA) and the posterior cerebral artery (PCA). c Magnetic resonance angiography indicated right MCA recanalization (thick arrow) after thrombolysis and showed that the right PCA blood supply was coming from the ipsilateral carotid artery (thin arrow). Possible sources of emboli included the proximal end of the right internal carotid artery and the heart. d Arterial spin labeling (ASL) imaging demonstrated high perfusion in the infarct core zone (oval marker) indicated blood flow recovery of the right cerebral infarction, and low perfusion in the left cerebellar hemisphere, suggesting a crossed cerebellar diaschisis (CCD) (red triangles marker). e Two months later, positron emission tomography (PET)/MRI indicated low metabolism in the posterior frontal lobe (oval marker) and temporal lobe (big white triangle marker), the temporal–occipital junction area (purple triangles marker), and the right basal ganglia (little white triangle marker), consistent with local neuronal necrosis after infarction, while the low metabolism in the left cerebellar hemisphere (red triangle marker) indicated possibly irreversible cerebellar damage
Further evaluation of the patient’s aphasia included the Edinburgh handedness inventory, which indicated that the patient was strongly right handed, with laterality quotient = + 80. The results of a standard language test for aphasia by the Aphasia Battery of Chinese (ABC) on the 7th day after onset are shown in Fig. 2. The patient had a non-fluent aphasia, difficulties in finding words, and dysprosody, accompanied by reading and writing dyslexia, partial loss of structural and visuospatial ability, without neglect, and marked decrease in attention span, short-term memory, and calculation skills. However, the patient retained almost normal auditory comprehension, orientation ability, and executive function.
Because neither CT nor MRI can exclude functional impairments to the contralateral hemisphere after infarct, we performed arterial spin labeling (ASL) and positron emission tomography (PET)/MRI to more thoroughly assess regional cerebral blood flow and metabolism. ASL performed 1 week after admission indicated high perfusion in the right hemispheric infarction area with low perfusion in the left cerebellar hemisphere, and no abnormal perfusion reduction in the left cerebral hemisphere (Fig. 1d). Two months later, speech had improved but remained problematic. However, there was no improvement in writing and reading comprehension. PET/MRI suggested a reduced metabolic rate in the area of the right cerebral infarct and in the left cerebellar hemisphere (Fig. 1e) that indicated possibly irreversible structural damage. A flowchart shown in Fig. 3 was attached below to help to better follow this case.
Discussion
Language lateralization and hand preference show inter-individual variation in the degree of lateralization to either side, but their relationship is not fully understood. Most researchers favor the theory that a combination of genetic factors and education and training plays a role in the formation of handedness. Studies have shown that left-hemisphere lesions are present in more than 90% of cases of right-handed aphasia, and that even in cases of left-handed aphasia, more than two-thirds of lesions are still located in the left hemisphere [1,2,3]. It is thought that the left hemisphere is the dominant hemisphere in most people. A study by Zhang et al. found that there were 7 cases of aphasia among 148 right-handed patients with right hemisphere lesions, 11 cases of aphasia among 19 non-right-handed patients with left hemisphere lesions, and only 2 cases of aphasia among 15 patients with right hemisphere lesions [8]. Thus, it is not always accurate to determine language lateralization according to hand preference. In patients with pedigrees showing multi-generational left-handedness, the degree of hand preference did not mirror degree of language lateralization. Instead, the prevalence of right-hemispheric and bilateral language lateralization increased with increasing degree of left-handedness, which demonstrated that, in this group, the degree of hand preference did not predict degree of language lateralization [9]. The findings in our case, which involved a right-handed patient with a right hemisphere infarction, support the above viewpoint. While Coppens et al. have reported that a genetic background of familial left-handedness was not associated with CA, they reported that male patients with CA were significantly more likely than female patients with CA to have a positive history of familial sinistrality, which might imply that male language lateralization is more susceptible to the genetic influence on handedness [6]. Our patient’s sister and mother were left handed, which might further support a genetic contribution to right cerebral language dominance, although it does not explain why, based on the large temporal lobe infarction, our patient did not exhibit any of the typical clinical manifestations of Wernicke’s aphasia. We believe that this could only be because he has atypical cerebral language dominance, i.e., that both hemispheres have linguistic functions, or because he has a left-sided Wernicke’s area.
Crossed diaschisis, or remote effect, might also be a mechanism of CA in a right-handed patient with a right hemisphere infarction. Ishizaki et al. reported a case of CA after infarction of the right corpus callosum in a patient who also showed low perfusion in the left temporal and occipital regions, including Broca’s and Wernicke areas, on single-photon emission CT (SPECT) examination, indicating crossed diaschisis as a reasonable explanation for the CA [10]. Cappa et al. have reported results of PET imaging in 2 patients with CA, both showing low perfusion in the left hemisphere, indicative of abnormal function, in the absence of structural abnormalities in the acute phase, a remote effect that may have been due to a crossed diaschisis [11]. In contrast, in our patient, the ASL did not detect a perfusion abnormality in the left hemisphere, the patient’s language function had improved but remained problematic, and 2 months later, PET/MRI showed an area of low metabolism in the right brain corresponding to the area of the infarction with no obvious metabolic abnormality in the left brain. These findings do not support crossed diaschisis as the cause of our patient’s CA. Rather, it seems to further support right language dominance or language function in both hemispheres. The findings of both reduced perfusion (ASL) and reduced metabolism (PET/MRI) in the left cerebellar hemisphere may also be indicative of a crossed cerebellar diaschisis (CCD). CCD is a neuron-suppressed state caused by loss of connection to damaged neural structures far from the cerebellum. The inferior frontal gyrus, as well as other sites, such as the lower part of the precentral gyrus, which may have functional and anatomical connections with the cerebellum, has been reported as important sites of lesions in patients with Broca’s aphasia, suggesting that the cerebellum may also contribute to language function [12]. In our case, this, combined with imaging findings typical of CCD, supports a contribution of cerebellar damage.
Unlike aphasia following left hemisphere lesions, there may be some association between right basal ganglia lesions and CA [13]. Kim et al. found using brain MRI for lesion mapping that the right lentiform nucleus, along with other portions of basal ganglia, was the area most involved in CA, and this implies that there is a wide range of functional connections between this region and the prefrontal, temporal, and parietal cortices [14]. However, the pathophysiology explaining the involvement of these regions is not fully understood, and it could be that CCD was the cause of CA. Our patient’s lesion also involved the right lentiform nucleus, and the PET/MRI examination showed a low metabolic state of the entire right basal ganglia, which is consistent with the commonly reported lesions associated with CA, and which supports the correlation between the incidence of CA and lesions of the right basal ganglia.
In conclusion, although language dominance is most frequently found in the left cerebral hemisphere, language lateralization cannot simply be predicted according to degree of hand preference, especially in patients with pedigrees for multi-generational left-handedness, for whom this judgment is often not accurate enough. Although the Wada test is the gold standard for lateralization of language dominance for neurosurgery, its application is constrained due to the risks and discomforts brought by this invasive test. There has been a significant endeavor to replace Wada testing with noninvasive functional neuroimaging techniques, including those mentioned herein, as well as functional MRI, magnetoencephalography, diffusion tensor imaging (DTI) [15,16,17], or functional transcranial Doppler ultrasound (fTCD) [9]. It may be more accurate to predict language dominance by a combination of clinical manifestations and functional imaging techniques mentioned above. At the same time, it should be remembered that crossed diaschisis may be an important cause of CA.
References
Pujol J, Deus J, Losilla JM, Capdevila A (1999) Cerebral lateralization of language in normal left-handed people studied by functional MRI. Neurology 52(5):1038–1043
Knecht S, Drager B, Deppe M, Bobe L, Lohmann H, Floel A et al (2000) Handedness and hemispheric language dominance in healthy humans. Brain 123(Pt 12):2512–2518
Szaflarski JP, Binder JR, Possing ET, McKiernan KA, Ward BD, Hammeke TA (2002) Language lateralization in left-handed and ambidextrous people: fMRI data. Neurology 59(2):238–244
Mariën P, Paghera B, De Deyn P, Vignolo L (2004) Adult crossed aphasia in dextrals revisited. Cortex 40:41–74
Vassal M, Le Bars E, Moritz-Gasser S, Menjot N, Duffau H (2010) Crossed aphasia elicited by intraoperative cortical and subcortical stimulation in awake patients. J Neurosurg 113:1251–1258
Coppens P, Hungerford S, Yamaguchi S et al (2002) Brain Lang 83:425–463
Bakar M, Kirshner HS, Wertz RT (1996) Crossed aphasia: functional brain imaging with PET or SPECT. Arch Neurol 53:1026–1032
Yumei Z, Yongjun W, Ruihua M et al (2005) Clinical Research on the relationship between the hand-preference and language dominant hemisphere. Chin J Rehabil Med 20(4):281–282
Somers M, Aukes MF, Ophoff RA et al (2015) On the relationship between degree of hand-preference and degree of language lateralization. Brain Language 144:10–15
Ishizaki M, Ueyama H, Nishida Y et al (2012) Crossed aphasia following an infarction in the right corpus callosum. Clin Neurol Neurosurg 114(2):161–165
Cappa SF, Perani D, Bressi S et al (1993) Crossed aphasia: a PET follow up study of two cases. J Neurol Neurosurg Psychiatry 56(6):665–671
Abe K, Ukita H, Yorifuji S et al (1997) Crossed cerebellar diaschisis in chronic Broca’s aphasi. Neuroradiology 39:624–626
Habib M, Joanette Y, Ali-Cherif A, Poncet M (1983) Crossed aphasia in dextrals: a case report with special reference to site of lesion. Neuropsychologia 21:413–418
Kim WJ et al (2013) Neural substrate responsible for crossed aphasia. J Korean Med Sci 28:1529–1533
Baxendale S (2009) The Wada test. Curr Opin Neurol 22(2):185–189
Abou-Khalil B (2007) An update on determination of language dominance in screening for epilepsy surgery: the Wada test and newer noninvasive alternatives. Epilepsia 48(3):442–455
De Witte E, Van Hecke W, Dua G et al (2014) Atypical cerebral language dominance in a right-handed patient: an anatomoclinical study. Clin Neurol Neurosurg 117:12–21
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Ethical standards
This study was approved by the local Institutional Review Board and performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Informed consent
Informed consent was obtained from the patient.
Rights and permissions
About this article
Cite this article
Tan, X., Guo, Y., Dun, S. et al. Crossed aphasia following cerebral infarction in a right-handed patient with atypical cerebral language dominance. J Neurol 265, 1671–1675 (2018). https://doi.org/10.1007/s00415-018-8901-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-018-8901-0