Abstract
Fatigue is one of the most common and debilitating symptoms affecting patients with multiple sclerosis (MS). Sustained cognitive effort induces cognitive fatigue, operationalized as subjective exhaustion and fatigue-related objective alertness decrements with time-on-task. During prolonged cognitive testing, MS patients show increased simple reaction times (RT) accompanied by lower amplitudes and prolonged latencies of the P300 event-related potential. Previous studies suggested a major role of structural and functional abnormalities in the frontal cortex including a frontal hypo-activation in fatigue pathogenesis. In the present study we investigated the neuromodulatory effect of transcranial direct current stimulation (tDCS) over the left dorsolateral prefrontal cortex (DLPFC) on objective measures of fatigue-related decrements in cognitive performance in MS patients. P300 during an auditory oddball task and simple reaction times in an alertness test were recorded at baseline, during and after stimulation. Compared to sham, anodal tDCS caused an increase in P300 amplitude that persisted after the end of stimulation and eliminated the fatigue-related increase in RT over the course of a testing session. Our findings demonstrate that anodal tDCS over the left DLPFC can counteract performance decrements associated with fatigue thereby leading to an improvement in the patient’s ability to cope with sustained cognitive demands. This provides causal evidence for the functional relevance of the left DLPFC in fatigue pathophysiology. The results indicate that tDCS-induced modulations of frontal activity can be an effective therapeutic option for the treatment of fatigue-related declines in cognitive performance in MS patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fatigue is one of the most frequent symptoms in multiple sclerosis (MS) affecting up to 75% of patients [1, 2]. It is characterized by an enhanced perception of effort and a limited endurance of sustained physical and cognitive activities [3]. Fatigue has a substantial negative impact on patient’s quality of life by impairing daily functioning and work capacity. Accordingly, it is a major cause of early retirement for MS patients [4]. Despite its clinical significance, fatigue remains a poorly understood symptom with varying definitions which hampers the development of effective management strategies.
Recently, Kluger et al. [5] proposed a unified taxonomy of fatigue suggesting a differentiation between the subjective perception of fatigue and objective fatigue-related decrements in performance during prolonged activity. Accordingly, fatigue can be studied qualitatively as a subjective phenomenon or quantitatively as an objective phenomenon. Most commonly, fatigue has been assessed subjectively using self-report questionnaires such as the Fatigue Severity Scale or the Modified Fatigue Impact Scale. However, as patients retrospectively rate perceived fatigue symptoms, self-report measures are subject to mood, regression to the mean and recall biases which can reduce their accuracy. Accordingly, available fatigue questionnaires show low correlations among each other and heterogeneous associations to patient’s functional impairment, disease duration or cognitive deficits [6,7,8]. In contrast to these subjective fatigue measures, fatigue-related decline in performance during sustained effort over time, also referred to as fatigability, can be quantified via objective indices [9]. This time-on-task effect has been demonstrated for motor fatigue, reflected by a decrement in muscle strength during exercise, and for cognitive fatigue. Objective cognitive fatigue refers to the decline in processing speed, reaction time (RT) or accuracy during continuous cognitive testing [9]. This fatigue phenomenon can be especially distressing as patients find themselves impaired in their ability to sustain attention and endure cognitive tasks. In the past, objective cognitive fatigue has been operationalized by various measures (for an overview see [9]). Accordingly, inconsistent results were reported with some studies demonstrating performance decreases with stronger subjective fatigue while others either showed no performance decline or decreases only on a subset of administered tests with time-on-task [10,11,12,13,14]. While in most cases findings are based on preliminary data, some studies made good efforts to replicate results with similar fatigue measures such as simple reaction time tests [14,15,16,17,18]. Previous studies demonstrated that subjective cognitive fatigue becomes behaviorally evident specifically in tasks depending on a high level of intrinsic alertness [19]. Bryant et al. [15] showed that alertness performance in MS patients declines earlier in time than in healthy controls independent of the patient’s general cognitive impairment at baseline. This demonstrates that cognitive fatigue and general cognitive impairment seem to operate on different mechanisms. Moreover, after neuropsychological testing inducing high cognitive load, compared to healthy controls MS patients were found to have a stronger increase in simple reaction time while no performance changes were observed for more complex selective and divided attention tests [14, 16, 20]. Accordingly, these findings consistently indicate that simple reaction time tests are a valid objective measure of MS-related cognitive fatigue.
In addition to behavioral markers of cognitive fatigue, measurements of event-related potentials (ERP) provide a further objective and sensitive method for assessing the integrity of information processing. Specifically, the P300 potential has been widely used as an index for cognitive functioning in various psychiatric and neurological disorders [21]. The P300 is an endogenous potential which is commonly elicited in an oddball task when subjects detect rare, target stimuli in a series of standard stimuli. P300 amplitude is proportional to the amount of attentional resources devoted to a task while its latency reflects the speed of stimulus classification, aspects that can be affected in MS due to demyelinating lesions [21]. Previous studies revealed that compared to healthy controls MS patients show diminished P300 amplitudes and prolonged latencies which have been associated with cognitive function in the attention and memory domain and patient’s fatigue level [22,23,24,25]. Recently, Chinnadurai et al. [26] examined the effect of increased cognitive load during an auditory oddball task on P300 in MS patients to investigate the diagnostic value of electrophysiological measures for the assessment of cognitive fatigue. With time-on-task, patients demonstrated a greater decrease in P300 amplitude and a stronger increase in P300 latency compared to healthy controls. Moreover, also healthy subjects demonstrated a decrease in P300 amplitude and an increase in latency after a fatigue-inducing cognitive task [27,28,29]. The diminished amplitude is assumed to reflect the decrease of attention while prolonged latencies are considered to be a measure of delayed information processing. These results suggest that perceived cognitive fatigue can influence higher level cognitive processes. Thus, even without baseline deficits in cognitive functioning, cognitive fatigue can limit the amount of attentional resources devoted to a task leading to measurable changes in P300 [15, 29]. Therefore, P300 changes during sustained cognitive effort have been suggested as a measure for the objective evaluation of neural processes related to cognitive fatigue.
For an effective treatment of fatigue, an understanding of the underlying pathophysiology is essential. For disease-related fatigue in MS, multifactorial mechanisms have been suggested including neuroimmune and neuroendocrine dysregulation as well as morphologic changes within various brain regions, especially in the frontal lobe, basal ganglia and sensorimotor cortex [1, 30, 31]. Account should be taken of the controversial study data with some studies failing to detect a specific fatigue-related pattern of atrophy [32,33,34]. The latter findings may be attributed to the used experimental designs including small sample sizes and short-term follow-up measures [31]. Importantly, structural and functional abnormalities in the frontal lobe were repetitively found to be anatomic brain correlates of fatigue severity. Subjective fatigue scores were associated with white matter lesions in the left frontal cortex and loss of gray matter volume in the left superior frontal gyrus [35, 36] or the left precentral gyrus [37]. In line with these results, fatigued as opposed to non-fatigued MS patients exhibit functional alterations in terms of reduced glucose metabolism in the basal ganglia and frontal lobe at rest [38] and a compensatory brain activation in the frontal, parietal and occipital lobes during task execution that could lead to feelings of fatigue [39,40,41]. These findings suggest that subjective fatigue might be at least partly related to damage in frontal brain regions which overlap with brain networks involved in attentional processing [35, 41].
Transcranial direct current stimulation (tDCS) is a non-invasive technique to modulate cortical excitability in targeted brain regions. The stimulation-induced effects depend on current polarity. Generally, anodal tDCS enhances cortical excitability via depolarization of neuronal membranes, while cathodal tDCS causes a hyperpolarization and a decrease of cortical reactivity in the region under the electrode. Excitability-enhancing effects of anodal tDCS have been successfully demonstrated in the perceptual, cognitive and motor domain [42, 43]. For MS-related fatigue, evidence for treatment effects of tDCS is still sparse. Positive stimulation effects on subjective fatigue assessed with self-report scales were reported after anodal tDCS (1.5 mA for 15 min) targeting the bilateral motor or somatosensory cortex for five consecutive days [44,45,46,47]. Saiote et al. [48] evaluated the effects of five daily sessions of anodal and sham tDCS (1 mA for 20 min) over the left dorsolateral prefrontal cortex (DLPFC). Although results did not show an overall significant improvement in perceived fatigue, the authors found a correlation between response to tDCS and lesion load in the left frontal cortex. By applying anodal tDCS over the left DLPFC with higher intensity (2 mA for 20 min) over a shorter protocol duration of three successive sessions, Ayache et al. [49] did not yield significant effects on subjective fatigue. Recently, Chalah et al. [13] compared the effect of anodal tDCS over the left DLPFC with stimulation over the right posterior parietal cortex over five daily sessions (2 mA for 20 min) showing that only frontal stimulation ameliorated subjective fatigue in MS. Based on these results, first long-term studies applying left frontal tDCS consecutively over 4–6 weeks showed an improvement of subjective fatigue that lasted up to 3 weeks after stimulation [50, 51].
Importantly, most previous studies exclusively showed tDCS effects on subjective fatigue using self-report questionnaires whereas few considered treatment effects on objective fatigue-induced decrements in sustained cognitive performance [52, 53]. Saiote et al. [48] proposed that methodological problems of self-report instruments might have led to an under-estimation of stimulation effects. Only recently, Hanken et al. [54] investigated the modulation of objective fatigue-associated vigilance decrements by tDCS in MS patients. The authors showed that tDCS over the right parietal cortex as part of the fatigue network could counteract the RT decrement during prolonged testing. Supplementary to studies showing mixed tDCS effects on subjective fatigue by using self-report measures, Hanken et al. [54] were the first to demonstrate that objective cognitive fatigue can be modified by tDCS which is an important prerequisite for its application as outcome parameter in intervention research.
The aim of the present study was to investigate the effects of anodal tDCS over the left DLPFC on objective performance-based measures of cognitive fatigue in MS during prolonged testing. Objective cognitive fatigue was conceptualized as a change in simple RT and P300 components with time-on-task. For the assessment of stimulation dependent changes in fatigue severity, cognitive fatigue parameters were assessed before, during, and after tDCS. We assumed that anodal, excitability-enhancing tDCS over the frontal cortex has positive effects on the change in objective cognitive fatigue parameters with time-on-task that persist after the end of stimulation.
Materials and methods
Participants
Fifteen patients diagnosed with clinically definite MS according to the McDonald criteria [55] were enrolled in the study (7 male, age 43.20 ± 14.97 years). 14 patients had relapsing–remitting and one patient secondary progressive MS. The demographic characteristics of the patient group are listed in Table 1. Inclusion criteria were a minimum of 9 points on the cognitive subscale of the Würzburger Fatigue Inventory for MS (WEIMuS), a minimum of 3 months since the last relapse, no severe depression (Beck Depression Inventory (BDI) ≤ 19) and no paresis of the upper limb. Patients did not receive any pharmacological treatment beside their MS therapy which consisted of natalizumab (N = 4), glatiramer acetate (N = 3), interferon beta (N = 3), fingolimod (N = 2), fampridine (N = 2), and dimethyl fumarate (N = 1). Patients were asked for their clinical history and were excluded if they reported current or previous neurological or psychiatric comorbidities. All patients reported having normal hearing and normal or corrected vision. The sample included two left-handed and 13 right-handed patients. All patients were screened and recruited from the outpatient pool of the University Hospital Magdeburg. Ethical approval for all procedures was obtained from the ethics committee of the University of Magdeburg and all participants gave written informed consent before participation.
Experimental procedure
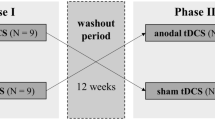
In two separate sessions, cognitive fatigue was assessed. Using a single blind, within-subject design, subjects received either anodal or sham tDCS in a randomized, counterbalanced order (cf. Fig. 1). Each session consisted of three testing blocks, preceded by a short practice trial for the simple reaction time task (SRT; see section “Experimental tasks”). Each testing block started with the SRT followed by P300 recordings during an auditory oddball task (see section “Experimental tasks”) and ended with subjective fatigue measurements by asking the patients for how mentally fit they feel on a 10-point numerical rating scale. By using this compound scale of fatigue we aimed to adequately picture the individually relevant short-term changes in perceived cognitive fatigue. The three blocks differed in terms of stimulation condition. The first block (BL) was performed without tDCS and served as a baseline against which performance in the subsequent two blocks was compared. After the baseline block, tDCS electrodes were attached to the patients scalp during a 5-min break. The second block (T1) measured the online effects of anodal tDCS on SRT, P300, and subjective fatigue scores. For this purpose, each patient received 10 min of tDCS prior to the beginning of the T1 testing block. According to Nitsche et al. [56] this pre-stimulation is necessary to induce stable stimulation effects. Following the 10 min pre-stimulation before T1, stimulation continued until the end of the T1 testing block. After another 5-min break, the last testing block (T2) started measuring post-stimulation effects. Individual sessions were separated by a minimum of 7 days. As fatigue generally underlies diurnal increases in symptom severity [57, 58], the two testing sessions per patient took place at about the same time of day.
Experimental procedure: patients underwent the protocol twice, once under anodal and once under sham stimulation, separated by at least 1 week. In each of the three testing blocks (BL, T1, T2) objective and subjective fatigue parameters were assessed: performance in a simple reaction time task (I), P300 during an auditory oddball task (II) and subjective fatigue measured via a 10-point numerical rating scale (III)
Experimental tasks
RT performance was assessed using a SRT adapted from Woods et al. [59]. Participants responded to the occurrence of a bulls-eye stimulus as quickly as possible by pressing a mouse button with the index finger of their dominant hand. After 15 practice trials, 120 stimuli with a duration of 200 ms were presented. The interstimulus intervals (ISI) ranged from 1000 to 2000 ms in 250 ms steps. The five ISIs were used pseudorandomly with equal probability. During the ISI a fixation cross was shown. The maximum response time was 1000 ms and responses outside this time window were categorized as false positive trials. The bulls-eye had a diameter of 5.72° of visual angle and was presented in black color on a white screen. The duration of the SRT was 4.5 min.
P300 ERPs were measured during an auditory oddball task. Subjects were binaurally presented with a series of 300 tones (10 ms rise/fall, 50 ms plateau) at 70 dB and a constant rate of one tone every 2 s via earphones. Frequent standard tones (1000 Hz, 80%) and rare target tones (2000 Hz, 20%) occurred pseudorandomly with the constraint that two target tones did not appear in succession. Subjects were instructed to press the left mouse button as quickly as possible whenever a target tone was detected. During the 10-min oddball task, subjects were instructed to look at a fixation cross at the center of the screen.
tDCS
For tDCS application, the active electrode (5 × 5 cm) was placed over the left DLPFC corresponding to the F3 electrode of the international 10–20 EEG system. The reference electrode (5 × 7 cm) was placed over the right shoulder. The reference position was chosen to avoid unwanted cephalic hyperpolarization effects under the cathode. The stimulation was delivered by a battery-driven constant current stimulator (NeuroConn GmbH, Ilmenau, Germany) using two rubber electrodes covered with saline-soaked sponges. Direct current was applied with an intensity of 1.5 mA with a 15-s fade in/out time. Impedance of stimulation electrodes was kept below 20 kΩ. After 10 min of pre-stimulation, the second testing block (T1) started while stimulation continued. For anodal stimulation the direct current was applied for 27.29 ± 1.15 min depending on the time of testing for each patient. For sham stimulation, the current was turned off after 30 s with a 15-s fade out time. This procedure ensured that in both stimulation conditions, participants experienced the initial itching sensation that recedes over the first seconds of tDCS. During the debriefing at the end of their final session, patients were asked to rate which of the session was conducted with active or sham tDCS. One patient correctly and two patients incorrectly distinguished between stimulation conditions while all other patients reported to feel no difference between sham and active stimulation.
EEG recording and preprocessing
During the auditory oddball task, EEG was recorded continuously at ten electrode sites (Fz, Cz, Pz, Oz, C1, C2, P3, P4, PO3, PO4 according to the international 10–20 EEG system) using Ag/AgCl-electrodes mounted in an elastic cap. The EEG signal was referenced to the nose with the ground electrode placed at POz. The electrooculogram (EOG) was recorded with two electrodes placed below and approximately 1 cm to the external canthus of the right eye. EEG data were recorded by Brain DC amplifier (Brain Products, Germany) and the corresponding software (Brain Products, Recorder 1.20) sampled at 1000 Hz. Impedances were kept below 10 kΩ. The online filter bandpass was 0.01–250 Hz with a notch filter at 50 Hz.
EEG preprocessing and data analysis were carried out in Analyzer 2.1 (Brain Products, Germany). EEG data were off-line bandpass filtered from 0.01 to 60 Hz. Trials on which the EOG exceeded ± 75 μV were rejected automatically. The EEG recordings were segmented into epochs from − 200 to 700 ms relative to stimulus onset. After baseline correction (− 200 to 0 ms), averages for the standard and target tones were computed separately for each measurement block (BL, T1, T2) and stimulation condition (anodal, sham). A minimum of 20 artifact-free trials were acquired for each combination of tone and measurement block per patient. For P300 analysis, difference waves were computed by subtracting average ERPs of the standard tone from ERPs of the target tone. A peak detection analysis was performed on single-subject difference waves measured at channel Pz using the local maximum between 250 and 450 ms after stimulus onset. Data were statistically analyzed using IBM SPSS software 22.
Statistical analysis
For each subject, values for subjective and objective fatigue (median RT in SRT, P300 amplitude and latency) were assessed. For RT analysis, false positive trials were excluded considering performance in correct trials only. To consider for intra-individual variations in baseline fatigue levels [60], we computed difference scores between baseline values (BL) and the fatigue values obtained during (T1) and after tDCS (T2), separately for the two testing days. Thus, T1BL reflects the change in fatigue scores between testing block T1 and BL, while T2BL reflects the change in fatigue scores between testing block T2 and BL. This procedure resulted in relative performance measures over time-on-task independent from intra-individual daily fatigue variations. These corrected fatigue parameters were entered into separate 2 × 2 repeated measures ANOVAs with the within-subject factors stimulation (anodal, sham) and time-on-task (T1BL, T2BL). For post hoc analyses, paired samples t tests were performed. As previous studies on the interrelationship between subjective and objective fatigue show inconsistent results [9], we further analyzed the correlations between the changes in fatigue measures during sham condition over the course of the testing session (T2BL).
Results
RT performance
The mean changes in cognitive fatigue parameters over time-on-task separately for anodal and sham stimulation sessions are shown in Table 2. The repeated measures ANOVA on RTs revealed no main effect for the factors stimulation [F(1,14) = 0.23, p = 0.637, \(\eta_{\text{p}}^{2}\) = 0.02] nor time-on-task [F(1,14) = 1.21, p = 0.290, \(\eta_{\text{p}}^{2}\) = 0.08], but a significant interaction effect [F(1,14) = 5.00, p = 0.042, \(\eta_{\text{p}}^{2}\) = 0.26] (cf. Fig. 2). The change in RT over time depended on the type of stimulation with an increase in RT from T1BL to T2BL under sham stimulation only [t(14) = − 2.17, p = 0.048]. Under anodal tDCS, the fatigue-related increase in RT was eliminated [t(14) = 1.73, p = 0.106].
ERP data
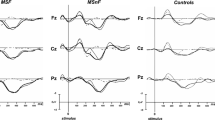
The tDCS-induced changes in electrophysiological data are shown in Fig. 3. For P300 amplitude the ANOVA revealed a significant main effect for the factor stimulation [F(1,14) = 9.13, p = 0.009, \(\eta_{\text{p}}^{2}\) = 0.40]. Compared to sham, anodal tDCS caused an increase in P300 amplitude during stimulation [T1BL: t(14) = 2.16, p = 0.048] which persisted after the end of stimulation [T2BL: t(14) = 2.52, p = 0.025]. There was neither a significant main effect for the factor time-on-task [F(1,14) = 0.38, p = 0.547, \(\eta_{\text{p}}^{2}\) = 0.03] nor a significant interaction effect [F(1,14) = 0.52, p = 0.482, \(\eta_{\text{p}}^{2}\) = 0.04].
Transcranial direct current stimulation induced changes in P300 event-related brain potential (ERP). Upper row: grand average ERPs recorded at channel Pz at baseline (left), during tDCS (middle) and after tDCS (right). The gray-shaded areas delineate the latency window of 250–450 ms used for P300 peak definition. Lower row: changes in P300 amplitude (left) and latency (right) during (T1) and after (T2) stimulation relative to baseline (BL) separately for the anodal and sham stimulation condition (mean ± SEM)
Analysis of P300 latency showed a significant increase in latency with time-on-task [F(1,14) = 5.16, p = 0.039, \(\eta_{\text{p}}^{2}\) = 0.27] reflecting an increase in objective cognitive fatigue during prolonged testing. There was no main effect for the factor stimulation [F(1,14) = 2.38, p = 0.145, \(\eta_{\text{p}}^{2}\) = 0.14] nor an interaction of both factors [F(1,14) = 0.74, p = 0.405, \(\eta_{\text{p}}^{2}\) = 0.05].
Subjective changes of fatigue
Regarding the change in perceived fatigue the ANOVA revealed a significant increase in fatigue with time-on-task [F(1,14) = 18.16, p = 0.001, \(\eta_{\text{p}}^{2}\) = 0.56] indicating that perceived fatigue was successfully induced by the testing protocol. The increase in subjective fatigue was independent of tDCS condition [F(1,14) = 0.00, p = 1.000, \(\eta_{\text{p}}^{2}\) = 0.00] and there was no main effect of stimulation [F(1,14) = 0.34, p = 0.567, \(\eta_{\text{p}}^{2}\) = 0.02]. To investigate the general interrelationship between changes in fatigue self-reports and objective performance we further evaluated the correlation of fatigue parameters during sham stimulation. Data showed no significant correlation between the increase in simple RT and subjective fatigue between baseline and the second testing block (r = − 0.37, p = 0.172). However, analysis revealed a significant negative correlation between the increase in subjective fatigue and the change in P300 amplitude (r = − 0.54, p = 0.040) and a marginal significant positive correlation with the change in P300 latency (r = 0.51, p = 0.051). Thus, the increase in perceived fatigue during the sham session is associated with a decrease in P300 amplitude and an increase in latency.
Discussion
In the present study we demonstrated that a single session of anodal tDCS over the left DLPFC can prevent performance decrements associated with cognitive fatigue in MS patients. Objective cognitive fatigue was operationally defined as the failure to sustain effort over the course of a testing session quantified by changes in simple RT and P300 components with time-on-task. A single dose of less than 30 min tDCS-induced neuromodulation of the frontal cortex caused an increase in P300 amplitude that persisted after the end of stimulation and thus counteracted the fatigue-related decrease in amplitude over time under sham stimulation. Moreover, anodal tDCS attenuated the fatigue-related decrease in RT performance over the course of a testing session. These data provide causal evidence for a functional relevance of the left DLPFC in objective cognitive fatigue and show that modulation of frontal activity by means of tDCS can have positive effects on the patient’s ability to maintain optimal performance during sustained cognitive demand.
The feeling of subjective fatigue has previously been shown to become behaviorally evident in situations which depend on a high level of intrinsic alertness that in turn can be easily distracted by internal factors such as feelings and thoughts [19]. Our study not only demonstrates tDCS-induced behavioral improvements in RT performance but also first electrophysiological evidence for positive stimulation effects on objective cognitive fatigue indexed by P300 ERP. MS-related fatigue has previously been associated with functional alterations in the frontal cortex [31, 38]. Neuroimaging studies showed that P300 originates from a frontal attention network and subsequent temporo-parietal memory operations [61, 62]. The detection of alerting stimuli during oddball tasks is related to neural changes especially in the prefrontal cortex and anterior cingulate structures [62, 63]. MS patients as well as healthy controls demonstrate a decrease in P300 amplitude and an increase in P300 latency with time-on-task [26,27,28] confirming fatigue-related decreases in frontal lobe activity. Our results show that anodal tDCS of the left DLPFC can increase P300 amplitude. The relative depolarization induced by anodal tDCS in the frontal cortex restored neuronal activity in this functionally impaired brain region thereby counteracting the typical fatigue-related amplitude decrease with time-on-task. This provides evidence for a causal relation between DLPFC functioning and the pathogenesis of objective cognitive fatigue. Previous studies showed that tDCS effects can last several minutes to hours after stimulation depending on tDCS parameters, such as stimulation intensity and duration [64]. Our results provide electrophysiological evidence for the effectiveness of the used stimulation protocol to induce lasting improvements in objective cognitive fatigue. In contrast to behavioral RT testing, which confounds cognitive and motor processes, electrophysiological P300 recording enables a direct assessment of fatigue-related changes in neural processing which can be measured despite existing physical disability. Thus, P300 might be a more reliable marker of objective cognitive fatigue and a more sensitive measure of tDCS-induced fatigue improvements that have been found to be subjectively detectable after multiple stimulation sessions [13, 48, 50, 51].
For P300 latency, we observed a significant increase in latency with time-on-task which was independent of tDCS condition. The finding that the stimulation effect, which is reflected by a partial eta-squared value of 0.14, did not reach statistical significance might be the result of an inherent variability in tDCS-induced latency alterations and a subsequent reduction in statistical power. This could point to a differential effectiveness of tDCS depending on varying patient characteristics. In the study by Saiote et al. [48], investigating the effect of five consecutive tDCS sessions on subjective fatigue, patients who responded positively to anodal tDCS of the left DLPFC cortex had higher frontal lesion load compared to non-responders. This finding suggests that the effectiveness of stimulation partly depends on morphological alterations in the targeted brain region. The statistically non-significant stimulation effect on P300 latency in our study might therefore be ascribed to inter-individual differences in frontal alterations across patients. Future studies should examine this relation between regional brain atrophy and the effectiveness of tDCS over the respective target region.
The enhancement of frontal activity, as indexed by P300, is paralleled by positive tDCS effects on behavioral alertness performance. Whereas RT significantly increased with time-on-task during sham stimulation, anodal tDCS attenuated this fatigue-related performance decline. Intrinsic alertness relies on an anterior alerting network that involves frontal, parietal, thalamic, and brain stem structures. Previous neuroimaging studies demonstrated activation in the anterior cingulate gyrus and the dorsolateral frontal cortex during SRT performance [65]. The same structures show more pronounced atrophy and neural dysfunction in MS patients with fatigue [19, 65]. Accordingly, our finding of positive tDCS effects on RT changes with time-on-task can be related to an enhanced frontal excitability which counteracted the fatigue-related performance decrement.
During testing all patients reported an increase in subjective cognitive fatigue. This indicates that the chosen tasks were effective in inducing the feeling of cognitive exhaustion. However, in contrast to positive stimulation effects on performance decrements measured by RT and P300, tDCS did not improve subjective fatigue appraisal. These results are in line with the study by Hanken et al. [54] who showed that single dose tDCS for 20 min over the right parietal cortex counteracts the RT decrement in a vigilance task but not the increase in perceived fatigue. As proposed by Hanken et al. [66], neuromodulation of the frontal cortex by tDCS might have increased cortical excitability within the alerting network without having any effect on subjective fatigue. However, as the frontal cortex is part of the attention network as well as the fatigue circuit [31], frontal neuromodulation might improve attention performance and perceived fatigue severity which in turn could reduce the distraction of attentional resources away from the cognitive process. Importantly, a growing number of studies demonstrated improvements in perceived fatigue after multiple daily sessions of left frontal tDCS [13, 48, 50, 51]. Chalah et al. [13] recently showed that anodal tDCS over the left DLPFC over five consecutive days ameliorated subjective fatigue in MS patients. Moreover, Charvet et al. [50] demonstrated a greater benefit of tDCS when applied with higher stimulation intensity and over a longer treatment period of 4 weeks. Thus, in contrast to the single dose tDCS application in the study by Hanken et al. [54] and our study, repetitive stimulation sessions can lead greater cumulative effects of tDCS [67, 68]. Taking these findings together, one might assume that while objective cognitive fatigue can be effectively modulated by a single dose tDCS treatment, multiple tDCS sessions might be necessary to accumulate stimulation effects to be also subjectively detectable via introspection.
However, the relation between perceived fatigue and objective fatigue-related performance decrements is still controversially discussed. In our study, the increase in subjective fatigue was correlated with the change in P300 component while no significant association was found with the increase in RT. As RT measures can be confounded by motor impairments, greater performance variability might have decreased statistical power. On the contrary, electrophysiological recordings enable a direct assessment of fatigue-related neural processes and might represent a more sensitive correlate of subjective fatigue. In line with these results, Herlofson et al. [69] suggested that the appropriate choice of performance metric may improve the detection of an association between subjective and objective fatigue parameters. Regarding the inconsistency on the relation between the two fatigue phenomena, research on objective fatigue measures should not devalue the use of subjective self-reports but rather run in parallel with the improvement of subjective measures [9]. Self-report scales are an important method for assessing patient’s exhaustion and its perceived impact. However, as subjective fatigue is weakly associated with functional impairment [7, 70] studying objective fatigue is an important complementary approach for the assessment of daily functioning. Differences between subjective and objective fatigue measures might offer additional therapeutic options such as the treatment of underlying dysfunctional coping styles [14]. Therefore, the development of a comprehensive theory of fatigue and its pathophysiology explaining both, the subjective and objective fatigue phenomena, is an important goal in clinical research [9].
An important limitation to the current study that should be addressed in future research refers to tDCS-induced modulations of cortical excitability underlying the changes in cognitive fatigue. Generally, anodal stimulation is associated with an enhancement of the target region which is reflected in the tDCS-induced increase in P300 amplitude in our study. However, due to a lack of tDCS focality it cannot be ruled out that non-selective stimulation of brain regions aside from the DLPFC might also have contributed to the observed effects. Generally, MS fatigue has a multifactorial pathogenesis associated with various functionally connected brain regions. Chalah et al. [31] proposed a complex fatigue network involving frontal, parietal, striatal and thalamic structures. Moreover, MS-related fatigue has been associated with a widespread damage of frontal connections, including fronto-frontal and several fronto-cortical/subcortical white matter tracts [71]. Previous studies in healthy subjects showed that anodal tDCS over the left DLPFC not only affects regional excitability under the electrode but also modulates functional connectivity in frontal and fronto-parietal networks [72, 73]. Accordingly, the beneficial effects on cognitive fatigue observed in our study might be the result of complex modulations within the fatigue network [31]. For the development of optimized stimulation protocols, a combination of neurostimulation and neuroimaging techniques might help to explore tDCS effects in the targeted brain region as well as in connected brain areas. Furthermore, polarity-specific effects on cortical excitability depend on individual baseline activity in the target area [74]. In an unaffected brain state, a further increase or decrease in neural activity will deteriorate neural processing in the target area. However, when neural activity is at a suboptimal level in neurological diseases, excitability-enhancing tDCS may help to restore the optimum level of reactivity. Therefore, a combination of tDCS and direct neurophysiological measures is necessary to examine the mechanism of action of tDCS. Stimulation parameters such as electrode size, position, current density and stimulation duration need to be systematically investigated for the development of efficient treatment protocols. Moreover, we used a single-blind approach with the examiner not being blind to tDCS condition. This could have created a potential interaction between the experimenter and patients, the latter being blind to the type of stimulation. However, as this study was performed as a first-time approach to modulate objective fatigue measures by tDCS, findings should be confirmed by follow-up studies applying multiple stimulation sessions in a double-blind design. Finally, for clinical application the achievement of persistent rather than short-term symptomatic improvements is highly relevant. Based on our findings of positive treatment effects after one single stimulation session, future studies should investigate tDCS effects over additional sessions which might increase its clinical impact on objective and subjective measures of cognitive fatigue [50, 51].
Conclusions
In summary, our results indicate that a single session of anodal tDCS over the left DLPFC can counteract the RT increase and the decrease in P300 amplitude associated with cognitive fatigue in MS patients. To our knowledge this is the first study providing direct electrophysiological evidence for the functional relevance of the left DLPFC in the pathogenesis of objective cognitive fatigue by means of tDCS. As physical disabilities affecting fine motor skills and visual acuity can impede RT testing in MS patients, P300 recordings might enable a more reliable evaluation of treatment effects. In contrast to fatigue assessments via self-report scales, the performance-based approach provides a direct measurement of the patient’s ability to cope with sustained cognitive demands. To conclude, tDCS can be an effective approach for the treatment of fatigue-related declines in cognitive performance in MS patients.
References
Kos D, Kerckhofs E, Nagels G et al (2008) Origin of fatigue in multiple sclerosis: review of the literature. Neurorehabil Neural Repair 22(1):91–100
Lerdal A, Celius EG, Krupp L et al (2007) A prospective study of patterns of fatigue in multiple sclerosis. Eur J Neurol 14(12):1338–1343
Chaudhuri A, Behan PO (2004) Fatigue in neurological disorders. Lancet 363(9413):978–988
Flensner G, Landtblom A-M, Soderhamn O et al (2013) Work capacity and health-related quality of life among individuals with multiple sclerosis reduced by fatigue: a cross-sectional study. BMC Public Health 13:224
Kluger BM, Krupp LB, Enoka RM (2013) Fatigue and fatigability in neurologic illnesses: proposal for a unified taxonomy. Neurology 80(4):409–416
Flachenecker P, Kumpfel T, Kallmann B et al (2002) Fatigue in multiple sclerosis: a comparison of different rating scales and correlation to clinical parameters. Mult Scler 8(6):523–526
Barak Y, Achiron A (2006) Cognitive fatigue in multiple sclerosis: findings from a two-wave screening project. J Neurol Sci 245(1–2):73–76
Lerdal A, Celius EG, Moum T (2003) Fatigue and its association with sociodemographic variables among multiple sclerosis patients. Mult Scler 9(5):509–514
Harrison AM, dasNair R, Moss-Morris R (2016) Operationalising cognitive fatigability in multiple sclerosis: a Gordian knot that can be cut? Mult Scler 23(13):1682–1696
Walker LAS, Berard JA, Berrigan LI et al (2012) Detecting cognitive fatigue in multiple sclerosis: method matters. J Neurol Sci 316(1–2):86–92
Genova HM, Rajagopalan V, DeLuca J et al (2013) Examination of cognitive fatigue in multiple sclerosis using functional magnetic resonance imaging and diffusion tensor imaging. PLoS ONE 8(11):e78811
Aldughmi M, Bruce J, Siengsukon CF (2017) Relationship between fatigability and perceived fatigue measured using the neurological fatigue index in people with multiple sclerosis. Int J MS Care 19(5):232–239
Chalah MA, Riachi N, Ahdab R et al (2017) Effects of left DLPFC versus right PPC tDCS on multiple sclerosis fatigue. J Neurol Sci 372:131–137
Claros-Salinas D, Dittmer N, Neumann M et al (2013) Induction of cognitive fatigue in MS patients through cognitive and physical load. Neuropsychol Rehabil 23(2):182–201
Bryant D, Chiaravalloti ND, DeLuca J (2004) Objective measurement of cognitive fatigue in multiple sclerosis. Rehabil Psychol 49(2):114–122
Neumann M, Sterr A, Claros-Salinas D et al (2014) Modulation of alertness by sustained cognitive demand in MS as surrogate measure of fatigue and fatigability. J Neurol Sci 340(1–2):178–182
Weinges-Evers N, Brandt AU, Bock M et al (2010) Correlation of self-assessed fatigue and alertness in multiple sclerosis. Mult Scler 16(9):1134–1140
Morrow SA, Rosehart H, Johnson AM (2015) Diagnosis and quantification of cognitive fatigue in multiple sclerosis. Cogn Behav Neurol 28(1):27–32
Hanken K, Eling P, Hildebrandt H (2015) Is there a cognitive signature for MS-related fatigue? Mult Scler J 21(4):376–381
Meissner H, Volkert J, König H et al (2007) Fatigue in multiple sclerosis: subjective complaints and intensity of attention. Mult Scler 13:S228
Sur S, Sinha VK (2009) Event-related potential: an overview. Ind Psychiatry J 18(1):70–73
Pokryszko-Dragan A, Zagrajek M, Slotwinski K et al (2016) Event-related potentials and cognitive performance in multiple sclerosis patients with fatigue. Neurol Sci 37:1545–1556
Magnano I, Aiello I, Piras MR (2006) Cognitive impairment and neurophysiological correlates in MS. J Neurol Sci 245(1–2):117–122
Aminoff JC, Goodin DS (2001) Long-latency cerebral event-related potentials in multiple sclerosis. J Clin Neurophysiol 18(4):372–377
Piras MR, Magnano I, Canu ED et al (2003) Longitudinal study of cognitive dysfunction in multiple sclerosis: neuropsychological, neuroradiological, and neurophysiological findings. J Neurol Neurosurg Psychiatry 74(7):878–885
Chinnadurai SA, Venkatesan SA, Shankar G et al (2016) A study of cognitive fatigue in Multiple Sclerosis with novel clinical and electrophysiological parameters utilizing the event related potential P300. Mult Scler Relat Disord 10:1–6
Uetake A, Murata A (2000) Assessment of mental fatigue during VDT task using event-related potential (P300). Robot Hum Commun—Proc IEEE Int Work. https://doi.org/10.1109/ROMAN.2000.892501
Kaseda Y, Jiang C, Kurokawa K et al (1998) Objective evaluation of fatigue by event-related potentials. J Neurol Sci 158(1):96–100
Guo Z, Chen R, Zhang K et al (2016) The impairing effect of mental fatigue on visual sustained attention under monotonous multi-object visual attention task in long durations: an event-related potential based study. PLoS ONE 11(9):e0163360
Heesen C, Nawrath L, Reich C et al (2006) Fatigue in multiple sclerosis: an example of cytokine mediated sickness behaviour? J Neurol Neurosurg Psychiatry 77(1):34–39
Chalah MA, Riachi N, Ahdab R et al (2015) Fatigue in multiple sclerosis: neural correlates and the role of non-invasive brain stimulation. Front Cell Neurosci 9:460
Papadopoulou A, Müller-Lenke N, Naegelin Y et al (2013) Contribution of cortical and white matter lesions to cognitive impairment in multiple sclerosis. Mult Scler 19(10):1290–1296
Gobbi C, Rocca MA, Riccitelli G et al (2014) Influence of the topography of brain damage on depression and fatigue in patients with multiple sclerosis. Mult Scler 20(2):192–201
Codella M, Rocca MA, Colombo B et al (2002) A preliminary study of magnetization transfer and diffusion tensor MRI of multiple sclerosis patients with fatigue. J Neurol 249(5):535–537
Sepulcre J, Masdeu JC, Goni J et al (2009) Fatigue in multiple sclerosis is associated with the disruption of frontal and parietal pathways. Mult Scler 15(3):337–344
Rocca MA, Parisi L, Pagani E et al (2014) Regional but not global brain damage contributes to fatigue in multiple sclerosis. Radiology 273(2):511–520
Riccitelli G, Rocca MA, Forn C et al (2011) Voxelwise assessment of the regional distribution of damage in the brains of patients with multiple sclerosis and fatigue. Am J Neuroradiol 32(5):874–879
Roelcke U, Kappos L, Lechner-Scott J et al (1997) Reduced glucose metabolism in the frontal cortex and basal ganglia of multiple sclerosis patients with fatigue: a 18F-fluorodeoxyglucose positron emission tomography study. Neurology 48(6):1566–1571
de la Cruz HM, Ambrosio A, Valsasina P et al (2017) Abnormal functional connectivity of thalamic sub-regions contributes to fatigue in multiple sclerosis. Mult Scler. https://doi.org/10.1177/1352458517717807
Pravatà E, Zecca C, Sestieri C et al (2016) Hyperconnectivity of the dorsolateral prefrontal cortex following mental effort in multiple sclerosis patients with cognitive fatigue. Mult Scler 22(13):1665–1675
Huolman S, Hämäläinen P, Vorobyev V et al (2011) The effects of rivastigmine on processing speed and brain activation in patients with multiple sclerosis and subjective cognitive fatigue. Mult Scler 17(11):1351–1361
Kuo M-F, Nitsche MA (2012) Effects of transcranial electrical stimulation on cognition. Clin EEG Neurosci 43(3):192–199
Ziemann U, Paulus W, Nitsche MA et al (2008) Consensus: motor cortex plasticity protocols. Brain Stimul 1(3):164–182
Tecchio F, Cancelli A, Cottone C et al (2015) Brain plasticity effects of neuromodulation against multiple sclerosis fatigue. Front Neurol 6:141
Cancelli A, Cottone C, Giordani A et al (2017) Personalized, bilateral whole-body somatosensory cortex stimulation to relieve fatigue in multiple sclerosis. Mult Scler J. https://doi.org/10.1177/1352458517720528
Ferrucci R, Vergari M, Cogiamanian F et al (2014) Transcranial direct current stimulation (tDCS) for fatigue in multiple sclerosis. NeuroRehabilitation 34(1):121–127
Tecchio F, Cancelli A, Cottone C et al (2014) Multiple sclerosis fatigue relief by bilateral somatosensory cortex neuromodulation. J Neurol 261(8):1552–1558
Saiote C, Goldschmidt T, Timaus C et al (2014) Impact of transcranial direct current stimulation on fatigue in multiple sclerosis. Restor Neurol Neurosci 32(3):423–436
Ayache SS, Palm U, Chalah MA et al (2016) Prefrontal tDCS decreases pain in patients with multiple sclerosis. Front Neurosci 10:147
Charvet LE, Dobbs B, Shaw MT et al (2017) Remotely supervised transcranial direct current stimulation for the treatment of fatigue in multiple sclerosis: results from a randomized, sham-controlled trial. Mult Scler J. https://doi.org/10.1177/1352458517732842
Ayache SS, Lefaucheur J-P, Chalah MA (2017) Long term effects of prefrontal tDCS on multiple sclerosis fatigue: a case study. Brain Stimul 10(5):1001–1002
Lefaucheur J-P, Chalah MA, Mhalla A et al (2017) The treatment of fatigue by non-invasive brain stimulation. Clin Neurophysiol 47(2):173–184
Ayache SS, Chalah MA (2017) Fatigue in multiple sclerosis—insights into evaluation and management. Clin Neurophysiol 47(2):139–171
Hanken K, Bosse M, Mohrke K et al (2016) Counteracting fatigue in multiple sclerosis with right parietal anodal transcranial direct current stimulation. Front Neurol 7:154
McDonald WI, Compston A, Edan G et al (2001) Recommended diagnostic criteria for multiple sclerosis: guidelines from the international panel on the diagnosis of multiple sclerosis. Ann Neurol 50(1):121–127
Nitsche MA, Cohen LG, Wassermann EM et al (2008) Transcranial direct current stimulation: state of the art 2008. Brain Stimul 1(3):206–223
Mills RJ, Young CA (2008) A medical definition of fatigue in multiple sclerosis. QJM 101(1):49–60
Comi G, Leocani L, Rossi P et al (2001) Physiopathology and treatment of fatigue in multiple sclerosis. J Neurol 248(3):174–179
Woods DL, Wyma JM, Yund EW et al (2015) The effects of repeated testing, simulated malingering, and traumatic brain injury on high-precision measures of simple visual reaction time. Front Hum Neurosci 9:540
Luck SJ, Kappenman ES (eds) (2012) The Oxford handbook of event-related potential components. Oxford Library of Psychology, Oxford University Press, Oxford
Huang WJ, Chen WW, Zhang X (2015) The neurophysiology of P 300—an integrated review. Eur Rev Med Pharmacol Sci 19(8):1480–1488
Polich J (2007) Updating P300: an integrative theory of P3a and P3b. Clin Neurophysiol 118(10):2128–2148
Kirino E, Belger A, Goldman-Rakic P et al (2000) Prefrontal activation evoked by infrequent target and novel stimuli in a visual target detection task: an event-related functional magnetic resonance imaging study. J Neurosci 20(17):6612–6618
Nitsche MA, Paulus W (2000) Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol 527(3):633–639
Sturm W, deSimone A, Krause BJ et al (1999) Functional anatomy of intrinsic alertness: evidence for a fronto-parietal-thalamic-brainstem network in the right hemisphere. Neuropsychologia 37(7):797–805
Hanken K, Eling P, Hildebrandt H (2014) The representation of inflammatory signals in the brain—a model for subjective fatigue in multiple sclerosis. Front Neurol 5:264
Mattioli F, Bellomi F, Stampatori C et al (2016) Neuroenhancement through cognitive training and anodal tDCS in multiple sclerosis. Mult Scler 22(2):222–230
Alonzo A, Brassil J, Taylor JL et al (2012) Daily transcranial direct current stimulation (tDCS) leads to greater increases in cortical excitability than second daily transcranial direct current stimulation. Brain Stimul 5(3):208–213
Herlofson K, Kluger BM (2017) Fatigue in Parkinson’s disease. J Neurol Sci 374:38–41
Pellicano C, Gallo A, Li X et al (2010) Relationship of cortical atrophy to fatigue in patients with multiple sclerosis. Arch Neurol 67(4):447–453
Bisecco A, Caiazzo G, d’Ambrosio A et al (2016) Fatigue in multiple sclerosis: the contribution of occult white matter damage. Mult Scler 22(13):1676–1684
Park CH, Chang WH, Park JY et al (2013) Transcranial direct current stimulation increases resting state interhemispheric connectivity. Neurosci Lett 539:7–10
Keeser D, Meindl T, Bor J et al (2011) Prefrontal transcranial direct current stimulation changes connectivity of resting-state networks during fMRI. J Neurosci 31(43):15284–15293
Krause B, Marquez-Ruiz J, Cohen KR (2013) The effect of transcranial direct current stimulation: a role for cortical excitation/inhibition balance? Front Hum Neurosci 7:602
Acknowledgements
We are grateful to all patients for their involvement. We wish to thank Julian Moritz Kauk for his help in behavioral data analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Ethical standards
The study was approved by the local ethics committee of the University Hospital Magdeburg.
Informed consent
Informed consent was obtained from all patients prior to their inclusion in this study.
Rights and permissions
About this article
Cite this article
Fiene, M., Rufener, K.S., Kuehne, M. et al. Electrophysiological and behavioral effects of frontal transcranial direct current stimulation on cognitive fatigue in multiple sclerosis. J Neurol 265, 607–617 (2018). https://doi.org/10.1007/s00415-018-8754-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-018-8754-6