Abstract
Background
D-dimer levels are used in several clinical settings, such as in predicting venous thrombosis, cardioembolic stroke and cancer status. In the present study, we investigated the associations between plasma D-dimer levels at admission, clinical characteristics and mortality at discharge in cryptogenic stroke patients. We also investigated whether D-dimer levels can predict long-term outcomes in those patients, including those with and without right-to-left shunt (RLS).
Methods
Acute cryptogenic stroke patients (n = 295, 72 ± 13 years old) were consecutively enrolled and retrospectively analyzed. We defined the cryptogenic stroke as an undetermined etiology according to the Trial of Org 10172 in Acute Stroke Treatment criteria. Plasma D-dimer levels at admission were evaluated. Assessments for RLS were performed using saline contrast-transcranial Doppler ultrasonography or contrast-transesophageal echography. Survivors (at discharge) underwent follow-up for up to 3 years after stroke onset.
Results
Of the total enrolled cohort, 17 patients died at discharge. D-dimer levels correlated with initial National Institutes of Health Stroke Scale (NIHSS) score (r = 0.391, P < 0.001) and were associated with mortality at discharge [odds ratio 1.04; 95% confidence interval (CI) 1.00–1.08, P = 0.049] after adjusting for age, sex and initial NIHSS score. Of the 278 survivors at discharge, 266 patients were evaluated to assess RLS during hospitalization, and 62 patients (23.3%) exhibited RLS. According to the median plasma D-dimer levels at admission (0.7 µg/ml), the patients were divided into a low D-dimer group (n = 136, < median) and a high D-dimer group (n = 130, ≥ median). Patients in the high D-dimer group were older, more frequently female, had a lower BMI, had a higher prevalence of cancer and had greater initial neurological severity compared to the patients in the low D-dimer group. During the follow-up period (median, 1093 days), 31 patients developed recurrent stroke and 33 patients died. High D-dimer levels at admission were independently associated with recurrent stroke and all-cause mortality [hazard ratio (HR) 3.76; 95% CI 1.21–14.1, P = 0.021) in patients with RLS, but not in those without RLS (HR 1.35; 95% CI 0.74–2.50, P = 0.335).
Conclusions
Increased D-dimer levels at admission were associated with mortality at discharge in cryptogenic stroke patients. In addition, high D-dimer levels were also associated with long-term outcomes in cryptogenic stroke patients with RLS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plasma D-dimer, which is a byproduct of fibrin degradation, provides useful information in clinical settings for acute and chronic stroke patients. Several studies have shown that plasma D-dimer levels in patients experiencing cardioembolic stroke are higher than those in other stoke subtypes [2, 18, 22]. In addition, increased D-dimer levels have been associated with infarction volume and short-term functional outcome in ischemic stroke patients with atrial fibrillation (AF) [21]. In the general population, high D-dimer concentration is also a risk marker for ischemic stroke, especially cardioembolic stroke [5].
Cryptogenic stroke, defined as stroke with undetermined etiology, accounts for 20–40% of all ischemic strokes [7, 20]. Paroxysmal AF is thought to be the most frequent potential mechanism in patients with cryptogenic stroke. Some of these patients are also thought to have experienced paradoxical embolism. Paradoxical embolism refers to the embolic entry of a venous thrombus into the systemic circulation through a right-to-left shunt (RLS) [19]. In addition, cancer-related stroke (including occult cancer) was also one possible cause of cryptogenic stroke. We hypothesized that D-dimer levels might be useful for predicting the degrees of clinical conditions that indicate the presence of AF, venous thrombus and cancer in patients experiencing cryptogenic stroke. There is little evidence regarding whether D-dimer levels at admission can predict the short-term and long-term clinical outcomes of these patients; hence, we investigated the associations between plasma D-dimer levels at admission, clinical characteristics and mortality at discharge in this patient population. We also investigated whether D-dimer levels can predict long-term outcomes in patients with cryptogenic stroke, including those with and without RLS.
Methods
Subjects
This was a retrospective, observational study. This study complied with the Declaration of Helsinki for investigations involving humans, and the study protocol was approved by the Ethics Committee of the Kawasaki Medical School Hospital. We enrolled consecutive, acute ischemic stroke patients who were admitted to the Kawasaki Medical School Hospital within 7 days of stroke onset between April 2008 and March 2012. A total of 1338 acute ischemic stroke patients were classified according to the Trial of Org 10172 in Acute Stroke Treatment (TOAST) criteria [1]. Among these patients, we excluded patients with small vessel occlusion (n = 221), large artery atherosclerosis (n = 137) and cardioembolism (n = 412). Of the remaining 568 patients, we diagnosed 122 patients with stroke due to other determined etiologies and 446 patients with stroke due to undetermined etiologies. Among the 446 patients with undetermined etiologies, we excluded 151 patients with more than one possible cause. Finally, 295 patients with cryptogenic stroke were analyzed in the present study (Fig. 1).
Clinical information during hospitalization
Patient data, including age, sex, body mass index, known vascular risk factors (e.g., hypertension, diabetes mellitus and dyslipidemia) and past disease history (e.g., ischemic stroke, intracerebral hemorrhage, coronary artery disease and cancer), were collected by attending physicians. Physicians also assessed neurological deficits and scored patients according to the National Institutes of Health Stroke Scale (NIHSS) upon admission and the modified Rankin Scale (mRS) prior to discharge. Plasma D-dimer levels were assessed for all patients upon admission and measured using the same technique during the study period. Secondary prevention methods (e.g., antiplatelet use, anticoagulant use or none) were comprehensively determined by the attending physicians.
Assessments for RLS were performed using saline contrast-transcranial Doppler ultrasonography (c-TCD) and/or contrast-transesophageal echography (TEE). RLS was diagnosed if more than 1 microembolic signal was detected in any of the four c-TCD tests (one test without and three tests with the Valsalva method). In c-TEE analyses, RLS was diagnosed if microbubbles were visualized in transition from the right atrium to the left atrium after performing the Valsalva method. These methods were performed as previously described [10, 11, 27]. The occurrence of RLS was determined by the attending physicians following an examination of the c-TCD and/or c-TEE results. We collected information from medical records to assess RLS among the patients.
Follow-up and long-term outcomes
Patient follow-up was performed at 3, 6, 12 months, 2 and 3 years after stroke onset. Trained research assistants were blinded to the detailed clinical data and used standardized questionnaires to contact the patients or their caregivers by telephone or postal mail. The following endpoints were considered: “recurrent stroke”, “all-cause mortality” and the combination of “recurrent stroke and all-cause mortality”. Recurrent stroke was defined as brain infarction (including transient ischemic attack) or intracranial hemorrhage that was diagnosed at the hospital. Additionally, detailed information for stroke type (i.e., ischemic stroke subtype) and causes of mortality were assessed using multiple approaches, including reviewing hospital and outpatient medical records and contacting patients or their caregivers.
Statistical analysis
Statistical analyses were performed using JMP 10.0 statistical software (SAS Institute Inc., USA). For continuous variables, data are expressed either as the means ± standard deviations (SD) or medians (25th and 75th percentiles). Discrete variables are expressed as frequencies and percentages. The statistical significance of inter-group differences was assessed by χ2, unpaired t and Mann–Whitney U tests, as appropriate. The relationship between initial NIHSS score and plasma D-dimer levels was examined using Spearman’s correlation. Multiple logistic regression analysis was performed to estimate the risk of mortality at discharge using several factors, including age, sex, initial NIHSS score and D-dimer levels. Next, survivors at discharge in whom RLS could be assessed were analyzed as follows. The patients were divided into two categories (high D-dimer group vs. low D-dimer group) according to their median plasma D-dimer levels at admission. Event-free survival analysis was performed using Kaplan–Meier plots and the log-rank test to compare patients with high D-dimer levels to those with low D-dimer levels. The Cox proportional-hazards model was used to estimate the association between long-term outcomes and high D-dimer levels compared to low D-dimer levels after adjustments for age and sex. Statistical significance was established at P < 0.05.
Results
Associations between D-dimer levels and stroke mortality at discharge
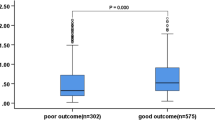
A total of 295 patients with cryptogenic stroke (143 women, 72 ± 13 years old) were enrolled. Of these patients, 17 (5.8%) died at discharge: eight from stroke, four from pneumonia, three from disseminated intravascular coagulation due to various causes and two from cancer. Old age, initial neurological severity (high NIHSS score) and high D-dimer levels were associated with mortality at discharge (Table 1). Increased D-dimer levels correlated with initial NIHSS score (r = 0.391, P < 0.001). Multivariate logistic analysis revealed that both increased NIHSS (per 1 points) and increased D-dimer levels (per 1 µg/ml) were associated with stroke mortality at discharge [odds ratio (OR), 1.16; 95% confidence interval (CI) 1.09–1.25, P < 0.001 and OR 1.04; 95% CI 1.00–1.08, P = 0.049, respectively].
Associations between D-dimer levels and long-term stroke outcomes
Among the survivors at discharge (n = 278), 266 patients (95.7%) were assessed for RLS (Fig. 1). Of these patients, 62 (23.3%) exhibited RLS. According to the median plasma D-dimer levels at admission (0.7 µg/ml), the patients were divided into a low D-dimer group (n = 136, < median) and a high D-dimer group (n = 130, ≥ median). Patients in the high D-dimer group were older, more frequently female, had a lower BMI, had a higher prevalence of cancer and had greater initial neurological severity (NIHSS score at admission) compared to the patients in the low D-dimer group (Table 2).
During the follow-up period [median (interquartile range) 1093 (374–1106) days], 31 patients (11.7%) had recurrent stroke and 33 patients (12.4%) died. Among the 31 patients with recurrent stroke, 18 were affected by ischemic stroke (12 patients were cryptogenic, 3 patients had large artery atherosclerosis, 1 patient had cardioembolism, and 2 patients had transient ischemic attack), 7 developed intracerebral hemorrhage, and 6 patients had an unknown stroke subtype. Among the 33 patients who died, 4 died from cancer, 5 from pneumonia, 2 from stroke, 5 from heart disease, 2 from liver failure, and 15 from unknown causes (including sudden death or aging).
Figure 2 shows the Kaplan–Meier curves for each endpoint. Kaplan–Meier curve analysis showed that the patients in the high D-dimer group had a higher risk for the combined endpoint (recurrent stoke and all-cause mortality) and all-cause mortality than the patients in the low D-dimer group (log-rank test, P = 0.012 or P < 0.001, respectively; Fig. 2a, c), although there was no significant difference for the association between D-dimer levels and recurrent stroke (log-rank test, P = 0.858, Fig. 2b). The multivariate Cox proportional-hazards model showed that high D-dimer levels were independently associated with all-cause mortality [hazard ratio (HR) 3.34; 95% CI 1.49–8.53, P = 0.003] after adjusting for age and sex (Fig. 3).
Of the 266 patients assessed for RLS, 224 used antithrombotics for secondary prevention (205 patients used antiplatelet drugs and 19 patients used anticoagulants). The 42 patients (15.8%) who did not use antithrombotic agents frequently exhibited diabetes mellitus, had higher D-dimer levels, and had lower rates of independence at discharge than those who used antithrombotic agents (Supplemental Table I). Independence at discharge (mRS 0–2) was also significantly different between patients with low D-dimer levels and those with high D-dimer levels. After adjusting for age, sex, use of antithrombotic agents and independence at discharge, high D-dimer levels were also independently associated with all-cause mortality (HR 3.19; 95% CI 1.44–8.06, P = 0.003).
Association between D-dimer levels and long-term stroke outcomes for patients with or without right-to-left shunt
There was no significant difference in baseline characteristics between the patients with RLS and those without (Supplemental Table II). High D-dimer levels in patients without RLS (n = 204) were associated with all-cause mortality (log-rank test, P < 0.001), however, no significant associations with the combined endpoint or stroke recurrence were noted (Fig. 4). High D-dimer levels were also independently associated with all-cause mortality after adjusting for age and sex (HR 3.49; 95% CI 1.35–10.9, P = 0.009) among patients without RLS (Fig. 3).
Among the patients with RLS (n = 62), Kaplan–Meier curve analysis revealed that patients with high D-dimer levels had a higher risk of the combined endpoint and of stroke recurrence than patients with low D-dimer levels (log-rank test, P = 0.013 or P = 0.044), although there was no significant difference in all-cause mortality (log-rank test, P = 0.071) (Fig. 5). High D-dimer levels were independently associated with the combined endpoint [hazard ratio (HR) 3.76; 95% CI 1.21–14.1, P = 0.021] in patients with RLS after adjusting for age and sex. In addition, high D-dimer levels were slightly associated with stroke recurrence in this group (HR 4.37; 95% CI 0.98-30.3, P = 0.053) (Fig. 3).
Discussion
In the present study, increased D-dimer levels at admission correlated to initial NIHSS score and were also independently associated with mortality at discharge after adjusting for stroke severity in patients with cryptogenic stroke. For long-term stroke outcomes, we found an independent association between high D-dimer levels and the combined endpoint (stroke recurrence and all-cause mortality) in patients with cryptogenic stroke with RLS.
Several studies have shown that plasma D-dimer levels in patients with cardioembolic stroke are higher than those in patients with other stoke subtypes because D-dimer levels could reflect thrombus formation activity in the left atrium or in the left ventricle [2, 18, 22]. In addition, initial stroke severity and initial infarct volume correlated with D-dimer levels in patients with cardioembolic stroke with AF, which could be a result of increased D-dimer levels produced by a large thrombus. In this study, plasma D-dimer levels at admission correlated with initial NIHSS score in patients with cryptogenic stroke. Most of these patients are likely to experience stroke from an embolic mechanism, such as a cardioembolism. Our results indicate that some patients with cryptogenic stroke had occult AF. D-dimer also activates inflammatory cytokines and causes advanced blood coagulation or progression of stroke status [3, 23]. These factors might contribute to the association between high D-dimer levels and short-term mortality independently of initial severity. In addition, increased D-dimer levels have also been associated with advanced cancer [16]. Indeed, in the present study, two patients died of cancer at discharge and were diagnosed with Trousseau syndrome.
Although recent studies have reported an association between D-dimer levels in acute ischemic stroke patients and short-term outcomes [28, 30], whether D-dimer levels are associated with long-term outcomes remains unknown [8, 26]. In addition, few studies have evaluated the association between D-dimer levels and long-term outcomes in patients with cryptogenic stroke. For these patients, D-dimer levels could provide useful information for several occult statuses, such as venous thrombus, atrial fibrillation and cancer. In the present study, high D-dimer levels were associated with all-cause mortality in all patients with cryptogenic stroke. A prior study also reported that high D-dimer levels serve as an independent predictor of poor survival in cancer patients with cryptogenic stroke [25]. Additionally, high D-dimer levels may be used to predict occult cancer in patients with cryptogenic stroke. Therefore, one explanation for the association between high D-dimer levels and mortality was the presence of known or occult cancer in this study. Among the 33 patients who died during follow-up period, 4 died from cancer. Notably, several studies have shown an association between increased D-dimer levels and all-cause mortality in the general population [4, 17, 29]. Therefore, the associations observed here might not be specific to patients with cryptogenic stroke. Although D-dimer is thought to stimulate inflammatory mediators, D-dimer levels have also been associated with all-cause mortality independent of inflammation in prior studies. D-dimer levels might also reflect the occult AF, as previously mentioned. A meta-analysis demonstrated that new AF was detected in 15.9% of 425 cryptogenic stroke patients [15]. Recently, two randomized trials of prolonged monitoring to detect new paroxysmal AF after ischemic stroke were conducted among patients with cryptogenic stroke [6, 24]. These clinical trials also found that 10–20% of cryptogenic stroke patients have underlying paroxysmal AF. In our study, the work-protocols for the detection of AF were not unified for long-term follow-up periods, and no cryptogenic patients received the implantable cardiac monitoring. Therefore, data regarding the number of cryptogenic stroke patients who experienced news AF during the study period were not available, although one patient had recurrent stroke caused by cardioembolism. Further studies assessing whether D-dimer levels might be useful for the detection of paroxysmal AF in cryptogenic stroke patients over long-term periods are needed.
Notably, our results showed that the association between D-dimer levels and stroke recurrence differed between cryptogenic patients with RLS and those without. These differences in stoke recurrence might be explained by the etiology of paradoxical embolism. Of the 62 patients with RLS, 59 (95.2%) were evaluated for deep venous thrombosis (DVT) by ultrasonography or angiography, and none were found to have DVT. However, the timing of the DVT evaluations was different among individuals. A previous report investigated whether D-dimer levels can predict long-term stroke outcomes in patients with cryptogenic stroke with patent foramen ovale (PFO) [14]. Our results provide additional evidence for the usefulness of D-dimer levels in predicting recurrent stroke in patients with cryptogenic stroke with RLS. There are insufficient data to establish that anticoagulation is equivalent or superior to antiplatelet therapy in patients with PFO, hence, antiplatelet therapy is generally recommended in these patients if there are no findings of DVT [12]. D-dimer levels might be useful for predicting recurrent stroke events, especially those assumed to result from paradoxical embolism, in this patient population. Anticoagulation therapy might be useful for patients with RLS and high D-dimer levels, even with no evidence of DVT.
The current study has several limitations. First, the retrospective study design limited our analyses, and the single-center study design led to potential selection bias. Second, RLS data were collected from medical records; therefore, RLS diagnoses were determined by attending physicians. The present study showed that 62 (23.3%) of 266 patients with cryptogenic stroke exhibited RLS. This prevalence is lower than that reported in previous cryptogenic stroke studies (43 and 61%) [9, 13]. We were also unable to evaluate the detailed causes of RLS, such as PFO. Third, we could not evaluate the presence of DVT in all patients. Indeed, patients with an incomplete negative investigation based on TOAST criteria were included in this study. However, D-dimer levels provided useful information regarding these patients even when there was insufficient work-up to detect the embolic source of a stroke.
In conclusion, increased D-dimer levels at admission were associated with mortality at discharge in patients with cryptogenic stroke. Additionally, high D-dimer levels were associated with long-term stroke outcomes in these patients, especially those with RLS. Large, prospective studies are needed to determine whether anticoagulation therapy could be useful for ameliorating recurrent stroke in patients with cryptogenic stroke with RLS and high D-dimer levels.
References
Adams HP, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, Marsh EE (1993) Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 24:35–41
Ageno W, Finazzi S, Steidl L, Biotti MG, Mera V, Melzi D’Eril G, Venco A (2002) Plasma measurement of D-dimer levels for the early diagnosis of ischemic stroke subtypes. Arch Intern Med 162:2589–2593
Castellanos M, Castillo J, García MM, Leira R, Serena J, Chamorro A, Dávalos A (2002) Inflammation-mediated damage in progressing lacunar infarctions: a potential therapeutic target. Stroke 33:982–987
Di Castelnuovo A, de Curtis A, Costanzo S, Persichillo M, Olivieri M, Zito F, Donati MB, de Gaetano G, Iacoviello L, Investigators M-SP (2013) Association of D-dimer levels with all-cause mortality in a healthy adult population: findings from the MOLI-SANI study. Haematologica 98:1476–1480
Folsom AR, Gottesman RF, Appiah D, Shahar E, Mosley TH (2016) Plasma d-Dimer and incident ischemic stroke and coronary heart disease: the atherosclerosis risk in communities study. Stroke 47:18–23
Gladstone DJ, Spring M, Dorian P, Panzov V, Thorpe KE, Hall J, Vaid H, O’Donnell M, Laupacis A, Côté R, Sharma M, Blakely JA, Shuaib A, Hachinski V, Coutts SB, Sahlas DJ, Teal P, Yip S, Spence JD, Buck B, Verreault S, Casaubon LK, Penn A, Selchen D, Jin A, Howse D, Mehdiratta M, Boyle K, Aviv R, Kapral MK, Mamdani M, Coordinators EIa (2014) Atrial fibrillation in patients with cryptogenic stroke. N Engl J Med 370:2467–2477
Grau AJ, Weimar C, Buggle F, Heinrich A, Goertler M, Neumaier S, Glahn J, Brandt T, Hacke W, Diener HC (2001) Risk factors, outcome, and treatment in subtypes of ischemic stroke: the German stroke data bank. Stroke 32:2559–2566
Haapaniemi E, Tatlisumak T (2009) Is D-dimer helpful in evaluating stroke patients? A systematic review. Acta Neurol Scand 119:141–150
Huang YY, Shao B, Ni XD, Li JC (2014) Differential lesion patterns on T2-weighted magnetic resonance imaging and fluid-attenuated inversion recovery sequences in cryptogenic stroke patients with patent foramen ovale. J Stroke Cerebrovasc Dis 23:1690–1695
Iguchi Y, Kimura K, Kobayashi K, Aoki J, Sakai K, Terasawa Y, Uemura J, Shibazaki K (2010) Detection of right-to-left shunts may be associated with body size. J Neuroimaging 20:130–133
Iguchi Y, Kimura K, Kobayashi K, Ueno Y, Inoue T (2006) Ischaemic stroke with malignancy may often be caused by paradoxical embolism. J Neurol Neurosurg Psychiatry 77:1336–1339
Kernan WN, Ovbiagele B, Black HR, Bravata DM, Chimowitz MI, Ezekowitz MD, Fang MC, Fisher M, Furie KL, Heck DV, Johnston SC, Kasner SE, Kittner SJ, Mitchell PH, Rich MW, Richardson D, Schwamm LH, Wilson JA, American Heart Association Stroke Council CoCaSN, Council on Clinical Cardiology, and Council on Peripheral Vascular Disease (2014) Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 45:2160–2236
Kim JW, Kim SJ, Yoon CW, Park CH, Kang KW, Kim SK, Kim YH, Bang OY (2013) Association between the amount of right-to-left shunt and infarct patterns in patients with cryptogenic embolic stroke: a transcranial Doppler study. Int J Stroke 8:657–662
Kim YD, Song D, Nam HS, Lee K, Yoo J, Hong GR, Lee HS, Nam CM, Heo JH (2015) D-dimer for prediction of long-term outcome in cryptogenic stroke patients with patent foramen ovale. Thromb Haemost 114:614–622
Kishore A, Vail A, Majid A, Dawson J, Lees KR, Tyrrell PJ, Smith CJ (2014) Detection of atrial fibrillation after ischemic stroke or transient ischemic attack: a systematic review and meta-analysis. Stroke 45:520–526
Kono T, Ohtsuki T, Hosomi N, Takeda I, Aoki S, Sueda Y, Ishihara K, Nakamura T, Yamawaki T, Matsumoto M (2012) Cancer-associated ischemic stroke is associated with elevated D-dimer and fibrin degradation product levels in acute ischemic stroke with advanced cancer. Geriatr Gerontol Int 12:468–474
Lee JK, Bettencourt R, Brenner D, Le TA, Barrett-Connor E, Loomba R (2012) Association between serum interleukin-6 concentrations and mortality in older adults: the Rancho Bernardo study. PLoS One 7:e34218
Liu LB, Li M, Zhuo WY, Zhang YS, Xu AD (2015) The role of hs-CRP, D-dimer and fibrinogen in differentiating etiological subtypes of ischemic stroke. PLoS One 10:e0118301
Loscalzo J (1986) Paradoxical embolism: clinical presentation, diagnostic strategies, and therapeutic options. Am Heart J 112:141–145
Marnane M, Duggan CA, Sheehan OC, Merwick A, Hannon N, Curtin D, Harris D, Williams EB, Horgan G, Kyne L, McCormack PM, Duggan J, Moore A, Crispino-O’Connell G, Kelly PJ (2010) Stroke subtype classification to mechanism-specific and undetermined categories by TOAST, A-S-C-O, and causative classification system: direct comparison in the North Dublin population stroke study. Stroke 41:1579–1586
Matsumoto M, Sakaguchi M, Okazaki S, Furukado S, Tagaya M, Etani H, Shimazu T, Yoshimine T, Mochizuki H, Kitagawa K (2013) Relationship between plasma (D)-dimer level and cerebral infarction volume in patients with nonvalvular atrial fibrillation. Cerebrovasc Dis 35:64–72
Montaner J, Perea-Gainza M, Delgado P, Ribó M, Chacón P, Rosell A, Quintana M, Palacios ME, Molina CA, Alvarez-Sabín J (2008) Etiologic diagnosis of ischemic stroke subtypes with plasma biomarkers. Stroke 39:2280–2287
Robson SC, Shephard EG, Kirsch RE (1994) Fibrin degradation product D-dimer induces the synthesis and release of biologically active IL-1 beta, IL-6 and plasminogen activator inhibitors from monocytes in vitro. Br J Haematol 86:322–326
Sanna T, Diener HC, Passman RS, Di Lazzaro V, Bernstein RA, Morillo CA, Rymer MM, Thijs V, Rogers T, Beckers F, Lindborg K, Brachmann J, Investigators CA (2014) Cryptogenic stroke and underlying atrial fibrillation. N Engl J Med 370:2478–2486
Shin YW, Lee ST, Jung KH, Kim DY, Park CK, Kim TM, Choi SH, Chu K, Lee SK (2016) Predictors of survival for patients with cancer after cryptogenic stroke. J Neurooncol 128:277–284
Squizzato A, Ageno W, Finazzi S, Mera V, Romualdi E, Bossi A, Venco A (2006) D-dimer is not a long-term prognostic marker following acute cerebral ischemia. Blood Coagul Fibrinolysis 17:303–306
Ueno Y, Iguchi Y, Inoue T, Shibazaki K, Urabe T, Kimura K (2007) Paradoxical brain embolism may not be uncommon-prospective study in acute ischemic stroke. J Neurol 254:763–766
Wang J, Ning R, Wang Y (2016) Plasma D-dimer Level, the Promising Prognostic Biomarker for the Acute Cerebral Infarction Patients. J Stroke Cerebrovasc Dis 25:2011–2015
Wannamethee SG, Whincup PH, Lennon L, Papacosta O, Lowe GD (2014) Associations between fibrin D-dimer, markers of inflammation, incident self-reported mobility limitation, and all-cause mortality in older men. J Am Geriatr Soc 62:2357–2362
Yang XY, Gao S, Ding J, Chen Y, Zhou XS, Wang JE (2014) Plasma D-dimer predicts short-term poor outcome after acute ischemic stroke. PLoS One 9:e89756
Acknowledgements
This study was supported in part by research grants from JSPS KAKENHI (Grant No. 17K17907) and the Japan Heart Foundation.
Author information
Authors and Affiliations
Contributions
Study concept and design by Nezu and Yagita; acquisition of data by Nezu, Kitano, Kubo, Uemura, Yamashita, Iwanaga, Inoue; analysis and interpretation of data by Nezu and Yagita; manuscript drafting by Nezu; critical revision of the manuscript for important intellectual content by all the coauthors; study supervision by Kimura and Yagita.
Corresponding author
Ethics declarations
Conflicts of interest
Naohisa Hosomi reports an honorarium from Mochida Pharmaceutical Co., Ltd which is outside the submitted work. Hirofumi Maruyama reports grants from Daiichi Sankyo Co., Ltd which is outside the submitted work. Masayasu Matsumoto reports grants from Takeda Pharmaceutical Co., LTD., Sanofi K.K., Mochida Pharmaceutical Co., LTD., Otsuka Pharmaceutical, and Daiichi Sankyo Co., LTD. and honoraria from Sanofi K.K., Bayer Health Care, and Daiichi Sankyo Co., LTD, which is outside the submitted work. Yoshiki Yagita reports an honorarium from Daiichi Sankyo Co., Ltd which is outside the submitted work. Other authors declare that there is no conflict of interest.
Ethical standards
This study complied with the Declaration of Helsinki for investigations involving humans, and the study protocol was approved by the Ethics Committee of the Kawasaki Medical School Hospital (#2334).
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Nezu, T., Kitano, T., Kubo, S. et al. Impact of D-dimer levels for short-term or long-term outcomes in cryptogenic stroke patients. J Neurol 265, 628–636 (2018). https://doi.org/10.1007/s00415-018-8742-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-018-8742-x