Abstract
Background
Autologous hematopoietic stem cell transplantation (aHSCT) is used in aggressive relapsing and progressive multiple sclerosis (MS). The multicentre studies and case series reported have relatively short follow-up.
Aim
To evaluate long-term effect and safety of HSCT in MS.
Materials and methods
Patients referred to the MS centre of Cagliari and undergoing HSCT were included. Variations in relapses and EDSS before and after HSCT were evaluated by Wilcoxon test. A descriptive analysis was made for other clinical data.
Results
Nine patients (female 6, males 3; 5 relapsing–remitting, 2 secondary progressive, 1 primary progressive, and 1 progressive relapsing) performed HSCT (1999–2006). The median follow-up was 11 years (11–18). Eight patients underwent aHSCT, seven using a low intensity conditioning regimen, and one an intermediate intensity. The primary progressive underwent allogeneic HSCT, due to onco hematological disease. The relapses number decreased in the 2 years following the procedure compared to the two preceding years (p = 0.041). New relapses or disease progressions were observed after a range of 7 (low intensity regimen)–118 (intermediate intensity) months. At last follow-up, the EDSS was stable in two patients, improved in two, and worse in five (maximum 2 EDSS in one patient). Six patients showed new lesions, and seven gadolinium-enhancing on brain MRI after a mean of 23.3 and 19.8 months, respectively. Two serious adverse events were reported: melanoma, and progressive multifocal leukoencephalopathy.
Conclusions and discussion
Our results confirm in a long follow-up the efficacy of HSCT in reducing relapses and disability progression. The risk/benefit profile is better for intermediate intensity regimens.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The approved therapies for MS are efficacious in controlling clinical and radiological inflammation in the relapsing forms of the disease [1, 2]. However, no treatments are able to prevent disability progression. Moreover, the course of MS could be very aggressive and could display refractoriness to conventional disease-modifying drugs (DMD). In such cases, autologous hematopoietic stem cell transplantation (aHSCT) may be a valid therapeutic option [3].
aHSCT is an ‘old’ treatment used for the first time in progressive MS patients in the 1990s [4]. Since then, the procedure has been used worldwide, with approximately 800 MS subjects being treated in this manner to date [5]. Initially, mainly secondary progressive (SP) patients were enrolled. In recent years, however, the proportion of relapsing–remitting (RR) has seen a large increase [6]. The procedure consists of two phases: the mobilization of hematopoietic stem cells; and immunoablation using conditioning regimens of different intensities [7]. The immunological result is a diminished self-reactive immunity, whereby the ‘new’ re-engrafted stem cells are able to generate a new self-tolerant immune cell repertoire [7]. Thus, aHSCT acts both by immunosuppression and immunomodulation, resetting the immune system [7].
The efficacy of aHSCT has been clearly demonstrated in the suppression of clinical and neuroradiological inflammation assessed by magnetic resonance imaging (MRI) [8,9,10]. However, there is less evidence regarding the reduction in disability progression [6, 11, 12]. It is important to note that the great majority of studies reporting multicentre experience are open-label, single-arm, and observational, with relatively short follow-up [6, 12]. Conversely, the studies with longer follow-up involve small case series of patients, with a maximum median follow-up of 11 years [9, 13, 14]. At present, only one phase II randomized clinical trial has been conducted in MS subjects. This study compared aHSCT with mitoxantrone in aggressive RR and SP patients [10], using mainly intermediate and high intensity regimens [6].
One of the limitations in the wide use of the procedure is the possibility of adverse events (AEs), such as infections, secondary malignancies and infertility [15], and the risk of transplant-related mortality (TRM), which is estimated to occur in 2.1% of cases [6].
The aim of the present study is to evaluate the long-term efficacy and safety of HSCT in the clinical practice of a single centre.
Materials and methods
The study was retrospectively conducted in the MS centre of the University of Cagliari between August 1999 and June 2017, and the last follow-up was June 2017 for the totality of subjects. We enrolled patients with MS according to diagnostic criteria evolving over time [16,17,18]. These were referred to the MS centre of the University of Cagliari for the entire follow-up, and underwent HSCT. All the subjects signed written informed consent for the use of their data at the moment of MS diagnosis or their first visit to the MS centre. The data were collected from the medical records by an MS neurologist. The ethics committee of the University of Cagliari approved the study.
The following data were recorded: year of birth; gender; age at onset; clinical course at the time of HSCT; type of mobilization and conditioning regimen; DMD administered before and after the procedure including the relative time of exposure; relapses in the 2 years before and after HSCT; time to first relapse after HSCT; disability measured by the expanded disability status scale (EDSS) at the time of HSCT, after 1 and 2 years, and at the last follow-up.
When available, the following brain MRI data were collected: the last brain MRI with new and/or enhancing lesions before HSCT; the first brain MRI with new and/or enhancing lesions after HSCT.
To assess long-term safety, all the AEs occurring from the time of HSCT until the last visit were recorded. AE grades were recorded in accordance with World Health Organization classification criteria.
The Wilcoxon test was used to study the difference in the number of relapses between the 2 years preceding and the 2 years following HSCT. The same test was also used to evaluate the difference between EDSS scores at the time of transplant and after 1 year.
In all cases, HSCT was performed at the Bone Marrow Transplantation Centre of the University of Cagliari. The following three HSCT modalities were used: HSCT with intermediate intensity; aHSCT with low intensity; and allogeneic HSCT. The first two differ in terms of their conditioning regimens. The intermediate intensity consists in the association of BCNU, etoposide, Ara-C, and melphalan (BEAM). In the low intensity regimen, cyclophosphamide and rabbit anti-thymocyte globulin (ATG) were administered. For the single patient who had also an onco hematological disease, and undergoing allogeneic HSCT [19], conditioning was carried out using fludarabine, busulfan, and cyclophosphamide, while a prophylaxis with cyclosporine and methotrexate was administered to reduce the risk of graft versus host disease (GVHD). The stem cells were derived from a sex-matched brother of the patient.
Results
Of the nine MS patients enrolled, six were female and three male (2:1). The clinical course was RR in five patients (55.5%), primary progressive (PP) in 1 (11.1%), progressive relapsing (PR) in 1 (11.1%), and SP in 2 (22.2%). All the HSCTs were performed from 1999 until 2006, and the mean and median follow-up were 13 and 11 years, respectively (minimum 11 and maximum 18 years) (SD ± 3). At the time of the procedure, the mean age was 38 years (SD ± 11.4), and the mean duration of the disease was 10 years (SD ± 8). Eight subjects underwent aHSCT. The intensity of conditioning regimen was low in seven patients and intermediate in one. In all but two patients, the indication for the aHSCT was the aggressive course of MS, defined as two or more relapses in the previous year. In the other two subjects, the aHSCT was performed due to a diagnosis of iatrogenic acute promyelocytic leukaemia, as a result of previous therapy with mitoxantrone. Only the PP patient underwent an allogeneic HSCT, due to a concomitant diagnosis of large granular lymphocytic leukaemia. At the time of HSCT, the mean EDSS was 5.3 (SD ± 1.7), and the number of relapses in the 2 years before HSCT was 2.9 (SD ± 5.1).
The clinical and demographic features are summarized in the Tables 1 and 2.
Comparing the 2 years preceding HSCT with the 2 years after, the number of relapses was significantly reduced (p = 0.041). All the patients showed a reactivation of the disease in terms of relapse or disability progression. The shortest reactivation time was 7 months in a patient who underwent a low intensity conditioning regimen, while the longest was 118 months in a patient receiving intermediate intensity conditioning regimen.
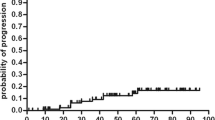
No significant difference was found evaluating the EDSS score before HSCT and 1 year after HSCT (p = 0.4). Comparing the EDSS score before the procedure with the score at last follow-up, it remained stable in two patients, improved in two, and worsened in the other five (Table 3, Fig. 1).
The collection of brain MRI data after the transplant showed new lesions in six patients after a minimum of 11 and a maximum of 120 months, and gadolinium-enhancing lesions in seven subjects after a minimum of 8 and a maximum of 120 months.
A new DMD was started in five patients a mean of 5 years after HSCT (SD ± 4.2). Specifically, N1 started natalizumab 18 months after the procedure, and the disease is stable until now. N2 started natalizumab 30 months after HSCT, the therapy was interrupted in December 2016 due to the high risk of progressive multifocal leukoencephalopathy (PML), and alemtuzumab was administered in April 2017. N3 started natalizumab 8 years after HSCT and the treatment was interrupted after 3 years, in January 2017, due to diagnosis of PML. N4 started natalizumab 11 years after HSCT, which was interrupted after 6 months due to subjective inefficacy; he subsequently took fingolimod over 9 months, which was again suspended due to subjective inefficacy. N9 started natalizumab 2 years after HSCT, and took the personal decision to interrupt the therapy after 4 years.
No TRM was reported and all the patients are currently alive.
During the procedure, one patient reported an infection due to Candida albicans, and one displayed the following AEs: fever, anaemia, asthenia, nausea (allergic reaction to ATG). After HSCT, the following AEs were recorded: benign cutaneous neoplasm 30 months after transplantation (AE grade 1); cutaneous melanoma 3 years after transplant and 6 months after the temporary interruption of natalizumab due to pregnancy (AE grade 4); a case of progressive multifocal leukoencephalopathy (PML) 12 years after transplant, during natalizumab treatment (AE grade 4).
The patient who underwent allogeneic HSCT reported the following AEs: acute cutaneous GVHD 16 days after the procedure (AE grade 2); cytomegalovirus infection 3 months after HSCT (AE grade 2); chronic GVHD 12 months after HSCT (AE grade 2); and insulin-dependent diabetes 5 years after HSCT (AE grade 3).
Discussion
HSCT is a procedure that has been widely investigated in MS patients, in particular by open-label studies and one randomized-controlled study. However, due to the small number of patients included in most of these, their different clinical characteristics and the diversity of regimens used, no clear conclusions have yet been drawn about the efficacy of HSCT and its associated risks [9].
Patient selection is very important for the success of the procedure. A recent meta-analysis showed that the largest benefit/risk profile could be obtained in RR patients with aggressive MS who have not yet acquired a high level of disability [6]. In another study, the factors associated with better outcomes were younger age, RR course, fewer prior immunotherapies, and lower EDSS score at the time of HSCT [12].
Our study confirmed the efficacy of HSCT on MS relapses in the context of a long-term follow-up. After the procedure, the number of the relapses was considerably decreased in all but one patient who had experienced relapses previously. Moreover, the great majority of patients (4/5) did not experience relapses for more than 2 years. The efficacy of the procedure was also evident in terms of EDSS stabilization, with the score improved in 3/9 patients, stabilized in 5/9, and worse in only 1 after 2 years. Furthermore, at the last follow-up (performed after a minimum of 11 years and a maximum of 18 years), the EDSS score was stable in two patients, improved in two, and worse in the remaining five. As shown in Table 3, the worsening was modest, with the EDSS score two points higher in one case and less than two points higher in the remaining four. Other studies which have evaluated the disability progression after nonmyeloablative regimens have shown discordant results. In particular, Burt et al. [14] found an improvement in EDSS score in 50% of patients after 2 years, and in 64% after 4 years, while another study recorded a worsening of EDSS score after 5 years of follow-up [20]. Recently, a large multicentre study with predominantly progressive MS patients showed that almost half of the subjects survived free from progression for 5 years after aHSCT [12]. In our study, the longest efficacy was in an SP male patient who underwent aHSCT with intermediate conditioning regimen (BEAM). He was free from relapse and progression for 118 months. This is in line with the consolidated efficacy of the myelo-ablative BEAM regimen in the induction of disease remission in RR patients [21], and in the reduction of progression disability in progressive patients, for both SP and PP [22]. The relapsing patients who performed HSCT with low intensity regimen had a more rapid disease reactivation within 12 months of transplantation in two cases. In particular, the subjects who underwent aHSCT due to the aggressive course of MS had new relapses after 9, 29, and 86 months. The shorter efficacy of the low intensity lympho-ablative regimen is probably due to the lack of certain reconstitution kinetics inducted by myeloablation, such as the thymus-dependent repopulation of the CD4+ population [7].
In our cohort, the risk profile of aHSCT was acceptable. During the procedure, only two patients experienced some light AEs. In the follow-up, three AEs were recorded, two of which were serious. None of these were clearly associated to aHSCT, but the procedure may have increased the risk for the patients. In particular, the development of PML in natalizumab-treated patients is clearly associated to prolonged exposition to the monoclonal antibody [23]. It is worth noting that the risk of PML during natalizumab therapy is highly increased by previous immunosuppressive drugs, such as those administered during the HSCT [24]. Also, in the case of melanoma, an increased risk after immunosuppressive drugs could not be excluded. However, melanoma could also be associated with natalizumab therapy, which was administered until 6 months before the diagnosis of the neoplasm [25]. A notable point emerged from our study is the importance of a long and continuous follow-up, in particular if other DMD have been administered after HSCT. Indeed, some patients of our study, including N2 who were diagnosed with melanoma, have been previously described as not reporting serious AEs in a shorter follow-up [26].
Regarding the AEs observed in our study, we did not find differences in relation to the different intensities of the regimens. Thus, no advantages in terms of risk were found in the nonmyeloablative regimen, while its efficacy was largely lower than that of the myeloablative procedure.
In our experience, all the regimens have shown efficacy regarding relapses and disability progression in both the short and long term, for both RR and progressive patients. The risk/benefit profile is better for the intermediate intensity-conditioning regimen than for its low intensity equivalent.
Given its intrinsic toxicity and the risk of TRM, HSCT must be considered in highly selected cases. This is especially so given the large number of efficacious DMD currently available for patients suffering from aggressive MS.
Significant attention may also be given to the prolonged immunosuppressive action, which can increase the risk of infectious and malignant diseases, in particular in aggressive MS subjects, who, over the course of their lives, undergo drug regimens with different mechanisms of action.
The limitations of our study are the small number of patients and the variability of their MS courses, as is true for the majority of case series reported [27,28,29]. However, we believe that our research is somewhat robust. The follow-up is longer than that reported in literature. The great majority of other studies have a median follow-up of less than 8 years, while ours is 11 years [6, 12].
References
Sorensen PS (2014) New management algorithms in multiple sclerosis. Curr Opin Neurol 27(3):246–259
Fenu G, Lorefice L, Frau F, Coghe GC, Marrosu MG, Cocco E (2015) Induction and escalation therapies in multiple sclerosis. Antiinflamm Antiallergy Agents Med Chem. 14(1):26–34 (review)
Reston JT, Uhl S, Treadwell JR, Nash RA, Schoelles K (2011) Autologous hematopoietic cell transplantation for multiple sclerosis: a systematic review. Mult Scler 17(2):204–213
Fassas A, Anagnostopoulos A, Kazis A, Kapinas K, Sakellari I, Kimiskidis V, Tsompanakou A (1997) Peripheral blood stem cell transplantation in the treatment of progressive multiple sclerosis: first results of a pilot study. Bone Marrow Transplant 20(8):631–638
Bell SM, Sharrack B, Snowden JA (2017) Autologous hematopoietic cell transplantation in multiple sclerosis. Expert Opin Biol Ther 17(1):77–86
Sormani MP, Muraro PA, Schiavetti I, Signori A, Laroni A, Saccardi R, Mancardi GL (2017) Autologous hematopoietic stem cell transplantation in multiple sclerosis: a meta-analysis. Neurology 88(22):2115–2122
Collins F, Kazmi M, Muraro PA (2017) Progress and prospects for the use and the understanding of the mode of action of autologous hematopoietic stem cell transplantation in the treatment of multiple sclerosis. Expert Rev Clin Immunol 13(6):611–622
Nash RA, Hutton GJ, Racke MK, Popat U, Devine SM, Griffith LM, Muraro PA, Openshaw H, Sayre PH, Stüve O, Arnold DL, Spychala ME, McConville KC, Harris KM, Phippard D, Georges GE, Wundes A, Kraft GH, Bowen JD (2015) High-dose immunosuppressive therapy and autologous hematopoietic cell transplantation for relapsing–remitting multiple sclerosis (HALT-MS): a 3-year interim report. JAMA Neurol 72(2):159–169
Burman J, Iacobaeus E, Svenningsson A, Lycke J, Gunnarsson M, Nilsson P, Vrethem M, Fredrikson S, Martin C, Sandstedt A, Uggla B, Lenhoff S, Johansson JE, Isaksson C, Hägglund H, Carlson K, Fagius J (2014) Autologous haematopoietic stem cell transplantation for aggressive multiple sclerosis: the Swedish experience. J Neurol Neurosurg Psychiatry 85(10):1116–1121
Mancardi GL, Sormani MP, Gualandi F, Saiz A, Carreras E, Merelli E, Donelli A, Lugaresi A, Di Bartolomeo P, Rottoli MR, Rambaldi A, Amato MP, Massacesi L, Di Gioia M, Vuolo L, Currò D, Roccatagliata L, Filippi M, Aguglia U, Iacopino P, Farge D, Saccardi R, ASTIMS Haemato-Neurological Collaborative Group, On behalf of the Autoimmune Disease Working Party (ADWP) of the European Group for Blood and Marrow Transplantation (EBMT); ASTIMS Haemato-Neurological Collaborative Group On behalf of the Autoimmune Disease Working Party ADWP of the European Group for Blood and Marrow Transplantation EBMT (2015) Autologous hematopoietic stem cell transplantation in multiple sclerosis: a phase II trial. Neurology 84(10):981–988
Casanova B, Jarque I, Gascón F, Hernández-Boluda JC, Pérez-Miralles F, de la Rubia J, Alcalá C, Sanz J, Mallada J, Cervelló A, Navarré A, Carcelén-Gadea M, Boscá I, Gil-Perotin S, Solano C, Sanz MA, Coret F (2017) Autologous hematopoietic stem cell transplantation in relapsing–remitting multiple sclerosis: comparison with secondary progressive multiple sclerosis. Neurol Sci 38(7):1213–1221
Muraro PA, Pasquini M, Atkins HL, Bowen JD, Farge D, Fassas A, Freedman MS, Georges GE, Gualandi F, Hamerschlak N, Havrdova E, Kimiskidis VK, Kozak T, Mancardi GL, Massacesi L, Moraes DA, Nash RA, Pavletic S, Ouyang J, Rovira M, Saiz A, Simoes B, Trnený M, Zhu L, Badoglio M, Zhong X, Sormani MP, Saccardi R, Multiple Sclerosis-Autologous Hematopoietic Stem Cell Transplantation (MS-AHSCT) Long-term Outcomes Study Group (2017) Long-term outcomes after autologous hematopoietic stem cell transplantation for multiple sclerosis. JAMA Neurol. 74(4):459–469
Mancardi GL, Sormani MP, Di Gioia M, Vuolo L, Gualandi F, Amato MP, Capello E, Currò D, Uccelli A, Bertolotto A, Gasperini C, Lugaresi A, Merelli E, Meucci G, Motti L, Tola MR, Scarpini E, Repice AM, Massacesi L, Saccardi R, BMT Italian Study Group (2012) Autologous haematopoietic stem cell transplantation with an intermediate intensity conditioning regimen in multiple sclerosis: the Italian multi-centre experience. Mult Scler 18(6):835–842
Burt RK, Balabanov R, Han X, Sharrack B, Morgan A, Quigley K, Yaung K, Helenowski IB, Jovanovic B, Spahovic D, Arnautovic I, Lee DC, Benefield BC, Futterer S, Oliveira MC, Burman J (2015) Association of nonmyeloablative hematopoietic stem cell transplantation with neurological disability in patients with relapsing-remitting multiple sclerosis. JAMA 313(3):275–284
Currò D, Mancardi G (2016) Autologous hematopoietic stem cell transplantation in multiple sclerosis: 20 years of experience. Neurol Sci 37(6):857–865
Poser CM, Paty DW, Scheinberg L, McDonald WI, Davis FA, Ebers GC, Johnson KP, Sibley WA, Silberberg DH, Tourtellotte WW (1983) New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Ann Neurol 13(3):227–231
McDonald WI, Compston A, Edan G, Goodkin D, Hartung HP, Lublin FD, McFarland HF, Paty DW, Polman CH, Reingold SC, Sandberg-Wollheim M, Sibley W, Thompson A, van den Noort S, Weinshenker BY, Wolinsky JS (2001) Recommended diagnostic criteria for multiple sclerosis: guidelines from the international panel on the diagnosis of multiple sclerosis. Ann Neurol 50(1):121–127
Polman CH, Reingold SC, Edan G, Filippi M, Hartung HP, Kappos L, Lublin FD, Metz LM, McFarland HF, O’Connor PW, Sandberg-Wollheim M, Thompson AJ, Weinshenker BG, Wolinsky JS (2005) Diagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald Criteria”. Ann Neurol. 58(6):840–846 (review)
La Nasa G, Littera R, Cocco E, Battistini L, Marrosu MG, Contu L (2004) Allogeneic hematopoietic stem cell transplantation in a patient affected by large granular lymphocyte leukemia and multiple sclerosis. Ann Hematol 83(6):403–405 (epub 2003 Nov 26)
Curro’ D, Vuolo L, Gualandi F, Bacigalupo A, Roccatagliata L, Capello E, Uccelli A, Saccardi R, Sormani MP, Mancardi G (2015) Low intensity lympho-ablative regimen followed by autologous hematopoietic stem cell transplantation in severe forms of multiple sclerosis: a MRI-based clinical study. Mult Scler 21(11):1423–1430
Nash RA, Hutton GJ, Racke MK, Popat U, Devine SM, Steinmiller KC, Griffith LM, Muraro PA, Openshaw H, Sayre PH, Stuve O, Arnold DL, Wener MH, Georges GE, Wundes A, Kraft GH, Bowen JD (2017) High-dose immunosuppressive therapy and autologous HCT for relapsing–remitting MS. Neurology 88(9):842–852
Fassas A, Anagnostopoulos A, Kazis A, Kapinas K, Sakellari I, Kimiskidis V, Smias C, Eleftheriadis N, Tsimourtou V (2000) Autologous stem cell transplantation in progressive multiple sclerosis—an interim analysis of efficacy. J Clin Immunol 20(1):24–30
Berger JR (2017) Classifying PML risk with disease modifying therapies. Mult Scler Relat Disord 12:59–63
Berger JR, Fox RJ (2016) Reassessing the risk of natalizumab-associated PML. J Neurovirol 22(4):533–535
Bergamaschi R, Montomoli C (2009) Melanoma in multiple sclerosis treated with natalizumab: causal association or coincidence? Mult Scler 15(12):1532–1533
Capobianco M, Motuzova Y, Frau J, Cocco E, Mamusa E, Marrosu MG, Bertolotto A (2012) Natalizumab in aggressive multiple sclerosis after haematopoietic stem cell transplantation. Neurol Sci 33(4):863–867
Krasulová E, Trneny M, Kozák T, Vacková B, Pohlreich D, Kemlink D, Kobylka P, Kovárová I, Lhotáková P, Havrdová E (2010) High-dose immunoablation with autologous haematopoietic stem cell transplantation in aggressive multiple sclerosis: a single centre 10-year experience. Mult Scler 16(6):685–693
Fassas A, Kimiskidis VK, Sakellari I, Kapinas K, Anagnostopoulos A, Tsimourtou V, Sotirakoglou K, Kazis A (2011) Long-term results of stem cell transplantation for MS: a single-center experience. Neurology 76(12):1066–1070
Bowen JD, Kraft GH, Wundes A, Guan Q, Maravilla KR, Gooley TA, McSweeney PA, Pavletic SZ, Openshaw H, Storb R, Wener M, McLaughlin BA, Henstorf GR, Nash RA (2012) Autologous hematopoietic cell transplantation following high-dose immunosuppressive therapy for advanced multiple sclerosis: long-term results. Bone Marrow Transplant 47(7):946–951
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical standards
All the subjects signed written informed consent for the use of their data at the moment of MS diagnosis or their first visit to the MS centre. The data were collected from the medical records by an MS neurologist. The ethics committee of the University of Cagliari approved the study.
Rights and permissions
About this article
Cite this article
Frau, J., Carai, M., Coghe, G. et al. Long-term follow-up more than 10 years after HSCT: a monocentric experience. J Neurol 265, 410–416 (2018). https://doi.org/10.1007/s00415-017-8718-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-017-8718-2