Abstract
Background
The C-X-C motif chemokine ligand 13 (CXCL13) and its receptor CXCR5 play an important role in the homing of B-lymphocytes. As a biomarker in the cerebrospinal fluid (CSF), CXCL13 has increasingly been used for the diagnosis of neuroborreliosis (NB). We evaluated the diagnostic and prognostic potential of CXCL13 for NB and other neuroinflammatory diseases in an unselected cohort, paying attention to those patients particularly who might benefit from newly emerging CXCL13-directed therapies.
Methods
We report the CSF CXCL13 concentrations and other relevant baseline characteristics for an unselected cohort of 459 patients. We compare different diagnostic groups and analyse the sensitivity and specificity of CSF CXCL13 as a marker of NB. The course of the CXCL13 concentrations is reported in a subgroup of 19 patients.
Results
We confirm the high diagnostic yield of CXCL13 for NB in this unselected cohort. The optimal cut-off for the reliable diagnosis of NB was 93.83 pg/ml, resulting in a sensitivity and specificity of 95 and 97%, respectively (positive predictive value 55.9%, negative predictive value 99.8%), surpassing the sensitivity of both serological testing and PCR. CSF CXCL13 concentration showed a swift response to therapy. Non-NB patients with high CSF CXCL13 concentrations suffered from meningeosis neoplastica or infectious encephalitis.
Conclusions
CXCL13 is a valuable tool for the diagnosis and assessment of therapeutic response in NB. Furthermore, our data point towards an emerging role of CXCL13 in the diagnosis and prognosis of viral encephalitis and meningeosis neoplastica. These results are of particular interest in the light of recently developed approaches to CXCL13-directed therapeutic interventions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The C-X-C motif chemokine ligand 13 (CXCL13) is a chemokine produced by antigen-presenting cells such as follicular dendritic cells and macrophages. Via its receptor—CXCR5—it serves as a chemoattractant, homing B cells into secondary lymphoid organs [1,2,3].
Cerebrospinal fluid (CSF) CXCL13 was suggested to be a highly specific and sensitive diagnostic marker for neuroborreliosis (NB; [4, 5]), but is also raised in other neuroinfectious and inflammatory disorders [6, 7]. Recently, an increasing focus has been on CXCL13 as a potential therapeutic target in these diseases [8].
We aimed to investigate the CXCL13 concentration in the CSF of an unselected group of patients, intending to improve our understanding of this chemokines diagnostic and prognostic properties. We will discuss our findings for the subgroup of patients diagnosed with NB, but also those for other diagnostic groups. We will particularly emphasize those nosological entities exhibiting extraordinarily high CSF CXCL13 levels, as these might be susceptible to CXCL13-directed therapeutic interventions.
Methods
Patients
We included all patients in whom CSF analysis for CXCL13 was performed between May 2015 and November 2016 and whose documentation on clinical symptoms and diagnosis at discharge was available (n = 459). The results for CXCL13 as well as all other blood and CSF parameters and the patients’ clinical information were retrospectively extracted from the patients’ electronic clinical records. NB was diagnosed and diagnostic certainty was graded according to Kaiser [9].
Laboratory analyses
For the detection of CXCL13 in the CSF, the Euroimmun CXCL13-ELISA kit was used. 50 µl of native CSF were placed in the Euroimmun Analyser. The concentration was calculated by the software using a standard curve. When the titers exceeded the highest value of the calibrator (usually 550 pg/ml), the CSF probe was diluted 1:10 and the diluted was reanalysed as described above. When no further titration was possible, the cut-off was used for statistical calculations. Routine laboratory assays were used for all other CSF, serum and peripheral blood assessment.
Statistical analysis
Excel and MedCalc statistical software were used for evaluation of patient data and for creation of figures. We calculated absolute frequencies and percentages for categorical variables and the median or mean and range or 95% confidence intervals for continuous variables. Correlation was calculated using Spearman’s rank coefficient. For sensitivity and specificity analyses, a receiver operating characteristic (ROC) curve analysis was performed. Groups were compared using the Mann–Whitney U test and the Chi-square test. Statistical significance was assumed for p < 0.05.
Results
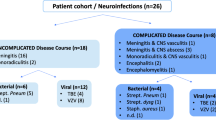
608 patients at our department received a spinal tap between May 2015 and November 2016. In 459, CSF CXCL13 was determined, irrespective of the diagnostic hypothesis. 19 patients received two or more spinal taps with analysis of CXCL13. In NB patients, the first tap was performed before initiation of treatment. 255 of all patients were male. Median age was 57 years (range 15–87). The list of final diagnoses is displayed in Table 1. Baseline characteristics of different diagnostic groups are shown in Table 2.
CSF CXCL13 concentration
The median CSF CXCL13 concentration in all patients was 10 pg/ml (range 0–6548 pg/ml), and 900 pg/ml (range 10–6500 pg/ml) in NB patients. Duration of symptoms in NB and CSF CXCL13 concentration did not show a significant correlation. Neither was there a significant difference between patients with the diagnoses of likely and confirmed NB. At the optimal cut-off point of 93.83 pg/ml, sensitivity and specificity for the diagnosis of NB were 95 and 97%, respectively (Fig. 1; positive predictive value 55.9%, negative predictive value 99.8%). Of 15 non-NB patients surpassing this threshold, two suffered from meningeosis neoplastica due to systemic neoplastic disease (cervical carcinoma, Waldenstrom’s macroglobulinemia), five from neuroimmunological disease (multiple sclerosis (MS), neuromyelitis optica spectrum disorders, CNS vasculitis), three from viral encephalitis (HIV, varicella zoster, tick-borne encephalitis), three from bacterial meningitis, and two from encephalitis of undetermined aetiology (both most probably viral). The highest CXCL13 values in non-NB-patients were seen in HIV-associated encephalitis in a patient naïve to highly active antiretroviral therapy (HAART; 1633 pg/ml), encephalitis of unknown origin (2821 pg/ml), meningeosis neoplastica secondary to Waldenstrom’s macrogammaglobulinemia (6500 pg/ml) and cervical carcinoma (6548 pg/ml). Only two MS patients had a CSF CXCL13 concentration > 93.83 pg/ml, both were seen during acute relapse. The median CXCL13 concentration in the CSF of NB patients and patients from any other of the diagnostic groups were significantly different, except for those with meningeosis secondary to systemic neoplastic disease. This was due to the two extreme outliers in this small cohort (Fig. 2).
Receiver operating characteristics curve (solid line; a) including 95% confidence intervals (CI; dotted lines) for the discrimination between neuroborreliosis patients and all other patients by CSF CXCL13 concentration. The table (b) shows sensitivities and specificities for different criterion values (indicated by the triangles in a). The optimal cut-off value was 93.83 pg/ml
CSF CXCL13 concentrations in different diagnostic groups. The solid horizontal lines indicate medians and 95% CI. The broken line is located at the optimal cut-off value of 93.83 pg/ml NB neuroborreliosis (group 1 in Table 1); NPL CNS involvement in systemic neoplasm (groups 5 and 6); MS multiple sclerosis (group 8); inflamm = neuroinflammatory disease other than MS/CIS/RIS (group 9), others = all other diagnostic groups
The positive correlation of the CSF CXCL13 concentration with the number of CSF lymphocytes (ρ = 0.45; p < 0.0001) and CSF total white cell count (ρ = 0.46; p < 0.0001) was highly significant, yet of moderate strength. There was no significant correlation with CSF total protein, glucose CSF/serum ratio, CSF lactate, serum C-reactive protein, peripheral blood leukocytes and lymphocytes. 47% of patients with an elevated CXCL13 concentration were positive for oligoclonal bands (OCB; determined in 312 patients), while only 13% of patients with CXCL13 levels below the cut-off of 93.83 pg/ml had positive OCB, resulting in a significant difference between these two groups (p < 0.0001).
Other diagnostic tests for NB
Borrelia IgG and/or IgM CSF/serum antibody indices (AI) were tested in a total of 452 patients. 11 non-NB patients had a positive IgG and/or IgM AI (AI > 1.5). Thus, the specificity of these combined parameters was 98%. At 80%, the sensitivity was lower than that of CXCL13, as the four patients classified as likely NB had normal AI. Borrelia burgdorferi PCR was performed for a total of 28 times in 20 NB patients. It was reported as weakly positive at least once in three patients and negative in 17 patients, resulting in a sensitivity of 15%.
CXCL13: course in repeat measurements
The patient cohort with multiple determinations of CXCL13 concentration included seven NB patients, all of whom received iv ceftriaxone 2 g/day. Repeat spinal taps took place after a median of 13 (range 10; 39) days. By then, the CXCL13 concentration had dropped by a mean of 57% (range 0; 99; Fig. 3). In one patient, the lack of CXCL13 decline corresponded to ongoing symptoms. His ceftriaxone dose was increased to 4 g/day, whereupon the symptoms subsided. In two patients with unspecific symptoms attributed to “post-treatment Lyme disease” [10], CXCL13 had normalized from initially high values of > 6500 and 1330 pg/ml, respectively. The Borrelia burgdorferi IgM AI was positive in only one out of seven NB patients with repeat spinal tap (2.17 in the second tap 14 days after the first one, which returned negative for Borrelia IgM). The IgG AI was pathological (i.e. > 1.5) in five patients on the first tap. It increased in five patients between the first and second spinal tap and decreased in two.
Discussion
CXCL13 in neuroborreliosis
The results of the present study confirm the high diagnostic value of CSF CXCL13 in NB, reproducing its high sensitivity and specificity in an unselected group of patients, with a similar specificity but higher sensitivity than Borrelia IgG/IgM-AI and a vastly higher sensitivity than Borrelia burgdorferi PCR. The optimal cut-off value for CXCL13 in the CSF is located at the lower end of the range reported so far (56–1229 pg/ml; [5, 12, 13]). These discrepancies may be due to the different ELISA test kits used [11, 12]. Individual prospective studies for the available kits would be desirable.
The high specificity of serological testing, i.e., CSF/serum Borrelia IgG- and IgM antibody indices, found in our cohort coincides with a recent study reporting values between 97 and 99%. The sensitivity depends on the timing of CSF analysis, increasing with later stages of the disease [13]. Sensitivity of Borrelia burgdorferi PCR in the CSF varies widely, but is generally lower than serology [9]. It too depends on the timing of the spinal tap—being higher in acute cases—as well as on the specific assay used. For an optimal diagnostic outcome in patients with clinical signs of NB considering both sensitivity and specificity, our data suggest a combined approach of serological testing and determination of CXCL13 in the CSF.
The CSF CXCL13 concentration in NB patients proves to be a rapid and sensitive marker to assess therapeutic success, far superior to Borrelia IgG/IgM AI [4]. CXCL13 may thereby help to distinguish patients with acute NB but insufficient therapeutic response from those suffering from unspecific post-treatment Lyme disease symptoms.
Possible misdiagnoses of a raised CSF CXCL13 concentration include other neuroinflammatory disorders, particularly HIV-associated and other viral encephalitis and leptomeningeal spread of systemic neoplastic disease. The association with disorders causing a predominantly lymphocytic invasion of the intrathecal space is unsurprising given the correlation of CXCL13 concentration with the CSF total cell count and CSF total lymphocytes and the fact that CXCL13 is the major determinant for B cell recruitment to the CSF during neuroinflammation [14]. It would be interesting to investigate the CSF of NB patients, patients suffering from the diseases listed above and controls with other neuroinflammatory disorders by flow cytometry to investigate whether there are pathognomonic patterns of the individual lymphocyte subpopulations.
CXCL13 in HIV
One of our patients exhibiting a particularly high CSF CXCL13 concentration was an HIV patient naïve to HAART. His CSF showed inflammatory changes with a moderately increased cell count. Clinically he displayed psychotic symptoms. Relevant pathogens were excluded as were markers of autoimmune disease. Thus, we suspected HIV-associated encephalitis [15,16,17]. Elevated CXCL13 serum and CSF levels have been described in HIV [3, 11]. They have been implicated in impaired B cell trafficking and functioning, thereby probably contributing causally to the immunosuppression in HIV patients [3]. The same authors describe a response of serum CXCL13 levels to HAART therapy. A prospective study would be desirable in these patients to assess the utility of serum CXCL13 as a marker for response (and compliance) to antiretroviral therapy.
CXCL13 in autoimmune disorders
CXCL13 has been implicated in the pathogenesis of autoimmune disease by reducing elimination of autoreactive B cells if overexpressed by follicular dendritic cells [2, 18]. In particular, CXCL13 has repeatedly been described as a diagnostic marker in MS [7, 19]. However, for this indication it proved not be very sensitive in our cohort. Only two patients had CXCL13 concentrations above the 93.83 pg/ml threshold—both were in acute relapse. Interestingly, we found similarly high levels of CXCL13 in our only two patients with suspected neuromyelitis optica (NMO; suggestive lesions, aquaporin-4 antibody negative). This might either reflect a higher activity of the disease at the moment of CXCL13 determination or point towards an increased sensitivity of this marker in NMO spectrum disorders. Although no significant differences of the median CSF CXCL13 levels in NMO and MS have been described, NMO patients display a wider range of CXCL13 levels with very high values in some individuals [7].
CXCL13 in neoplastic disease
The two patients with the highest CSF CXCL13 concentrations of all non-NB patients suffered from meningeal spread of systemic neoplastic disease. This increase of CXCL13 concentration may be an unspecific reflection of inflammation caused by neoplastic cells in the intrathecal space. However, previous reports suggest a specific association of CXCL13 with a subset of haematological and solid tumours. One of our patients had Waldenström’s macrogammaglobulinemia. Increased serum and CSF CXCL13 levels have previously been described in B cell lymphoma [20,21,22]. Given results obtained in patients with primary CNS lymphoma, it has been suggested that CXCL13 may mediate chemotaxis of lymphoma cells, thereby contributing to their CNS tropism [22]. Overexpression of CXCL13 and its receptor CXCR5 as well as increased serum levels of CXCL13 have also been described in patients with B cell chronic lymphocytic leukaemia (CLL), potentially contributing to prolonged survival of CLL cells [20].
The second patient with markedly elevated CSF CXCL13 concentration had carcinomatous meningeosis due to cervical cancer. To our knowledge, this association has not yet been described. However, the CXCL13-CXCR5 axis has been associated with progression and metastasis in different types of cancer, e.g. breast, colon and non-small cell lung carcinoma, probably by inducing epithelial to mesenchymal transition [23,24,25]. Increased CSF CXCL13 concentration may hence reflect the invasive potential of the primary tumour. We suggest further studies of CXCL13 as a tumour marker in the context of cervical carcinoma and other epithelial neoplasms, correlating its parenchymal expression and serum levels with the invasiveness and metastatic potential of the tumour.
In conclusion, our data confirm the significance of CXCL13 for the diagnosis and therapeutic monitoring in NB. Beyond that, our data allude to an important role this chemokine might play in the pathogenesis of viral encephalitis as well as CNS invasion by systemic neoplastic disease. These assumptions should be confirmed in a prospective study design. If this correlation turns out to be positive, CXCL13 might not only serve as an interesting prognostic marker, but also as a potential therapeutic target, e.g. by employing recently developed CXCL13 specific monoclonal antibodies, which are currently being tested in animal models of autoimmune disease [2].
References
van Burgel ND, Bakels F, Kroes ACM, van Dam AP (2011) Discriminating Lyme neuroborreliosis from other neuroinflammatory diseases by levels of CXCL13 in cerebrospinal fluid. J Clin Microbiol 49:2027–2030
Klimatcheva E, Pandina T, Reilly C, Torno S, Bussler H, Scrivens M et al (2015) CXCL13 antibody for the treatment of autoimmune disorders. BMC Immunol 16:6
Widney DP, Breen EC, Boscardin WJ, Kitchen SG, Alcantar JM, Smith JB et al (2005) Serum levels of the homeostatic B cell chemokine, CXCL13, are elevated during HIV infection. J Interferon Cytokine Res Off J Int Soc Interferon Cytokine Res 25:702–706
Senel M, Rupprecht TA, Tumani H, Pfister HW, Ludolph AC, Brettschneider J (2010) The chemokine CXCL13 in acute neuroborreliosis. J Neurol Neurosurg Psychiatry 81:929–933
Schmidt C, Plate A, Angele B, Pfister H-W, Wick M, Koedel U et al (2011) A prospective study on the role of CXCL13 in Lyme neuroborreliosis. Neurology 76:1051–1058
Kothur K, Wienholt L, Brilot F, Dale RC (2016) CSF cytokines/chemokines as biomarkers in neuroinflammatory CNS disorders: a systematic review. Cytokine 77:227–237
Alvarez E, Piccio L, Mikesell RJ, Klawiter EC, Parks BJ, Naismith RT et al (2013) CXCL13 is a biomarker of inflammation in multiple sclerosis, neuromyelitis optica, and other neurological conditions. Mult Scler Houndmills Basingstoke Engl 19:1204–1208
Huber AK, Irani DN (2015) Targeting CXCL13 during neuroinflammation. Adv Neuroimmune Biol 6:1–8
Neuroborreliosis Kaiser R (1998) J Neurol 245:247–255
Kaplan RF, Trevino RP, Johnson GM, Levy L, Dornbush R, Hu LT et al (2003) Cognitive function in post-treatment Lyme disease: do additional antibiotics help? Neurology 60:1916–1922
Bremell D, Mattsson N, Edsbagge M, Blennow K, Andreasson U, Wikkelsö C et al (2013) Cerebrospinal fluid CXCL13 in Lyme neuroborreliosis and asymptomatic HIV infection. BMC Neurol 13:2
Henningsson AJ, Gyllemark P, Lager M, Skogman BH, Tjernberg I (2016) Evaluation of two assays for CXCL13 analysis in cerebrospinal fluid for laboratory diagnosis of Lyme neuroborreliosis. APMIS Acta Pathol Microbiol Immunol Scand 124:985–990
Henningsson AJ, Christiansson M, Tjernberg I, Löfgren S, Matussek A (2014) Laboratory diagnosis of Lyme neuroborreliosis: a comparison of three CSF anti-Borrelia antibody assays. Eur J Clin Microbiol Infect Dis Off Publ Eur Soc Clin Microbiol 33:797–803
Kowarik MC, Cepok S, Sellner J, Grummel V, Weber MS, Korn T et al (2012) CXCL13 is the major determinant for B cell recruitment to the CSF during neuroinflammation. J Neuroinflammation 9:93
Lee EJ, Kim YH, Lee JY, Sunwoo J-S, Park SY, Kim TH (2017) Acute HIV-1 infection presenting with fulminant encephalopathy. Int J STD AIDS 28:1041–1044
Helleberg M, Kirk O (2013) Encephalitis in primary HIV infection: challenges in diagnosis and treatment. Int J STD AIDS 24:489–493
Ferrada MA, Xie Y, Nuermberger E (2015) Primary HIV infection presenting as limbic encephalitis and rhabdomyolysis. Int J STD AIDS 26:835–836
Vinuesa CG, Sanz I, Cook MC (2009) Dysregulation of germinal centres in autoimmune disease. Nat Rev Immunol 9:845–857
Festa ED, Hankiewicz K, Kim S, Skurnick J, Wolansky LJ, Cook SD et al (2009) Serum levels of CXCL13 are elevated in active multiple sclerosis. Mult Scler Houndmills Basingstoke Engl 15:1271–1279
Bürkle A, Niedermeier M, Schmitt-Gräff A, Wierda WG, Keating MJ, Burger JA (2007) Overexpression of the CXCR5 chemokine receptor, and its ligand, CXCL13 in B-cell chronic lymphocytic leukemia. Blood 110:3316–3325
Fischer L, Korfel A, Pfeiffer S, Kiewe P, Volk H-D, Cakiroglu H et al (2009) CXCL13 and CXCL12 in central nervous system lymphoma patients. Clin Cancer Res Off J Am Assoc Cancer 15:5968–5973
Rubenstein JL, Wong VS, Kadoch C, Gao H-X, Barajas R, Chen L et al (2013) CXCL13 plus interleukin 10 is highly specific for the diagnosis of CNS lymphoma. Blood 121:4740–4748
Biswas S, Sengupta S, Roy Chowdhury S, Jana S, Mandal G, Mandal PK et al (2014) CXCL13-CXCR5 co-expression regulates epithelial to mesenchymal transition of breast cancer cells during lymph node metastasis. Breast Cancer Res Treat 143:265–276
Singh R, Gupta P, Kloecker GH, Singh S, Lillard JW (2014) Expression and clinical significance of CXCR5/CXCL13 in human non-small cell lung carcinoma. Int J Oncol 45:2232–2240
Zhu Z, Zhang X, Guo H, Fu L, Pan G, Sun Y (2015) CXCL13-CXCR5 axis promotes the growth and invasion of colon cancer cells via PI3K/AKT pathway. Mol Cell Biochem 400:287–295
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Ethical standard
The study has been approved by the ethics committee of Upper Austria.
Rights and permissions
About this article
Cite this article
Wagner, J.N., Weis, S., Kubasta, C. et al. CXCL13 as a diagnostic marker of neuroborreliosis and other neuroinflammatory disorders in an unselected group of patients. J Neurol 265, 74–81 (2018). https://doi.org/10.1007/s00415-017-8669-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-017-8669-7