Abstract
Depression is common in patients with Parkinson disease and causes suffering and increased caregiver burden. A better understanding of depressive symptoms in Parkinson disease, their progression, and risk factors may, therefore, benefit management of these patients. The present study included 187 drug-naïve patients with incident PD and 166 controls from the population-based Norwegian ParkWest project. Depressive symptoms were examined with the Montgomery and Aasberg Depression Rating Scale (MADRS) at time of diagnosis and inclusion in the study and after 1, 3, 5, and 7 years of follow-up. Associations between MADRS scores and risk factors were assessed using generalized estimating equations (GEE). The mean MADRS score from all 823 examinations during the study period was 4.2 in patients and 1.3 in 732 examinations among controls. Among controls, the occurrence of depressive symptoms was also lower and rather stable during follow-up, while in patients, we observed a decrease from time of diagnosis and until the 1-year visit, followed by a steady increase in these symptoms over time. Factors associated with higher MADRS score in the multivariable model were female sex, being dependent, higher pain score, higher Unified PD Rating Scale (UPDRS) motor score, and lower Mini-Mental State Examination (MMSE) score. The results from this study underscore the importance and frequency of depressive symptoms in patients with early PD. Furthermore, risk factors that may be considered PD-nonspecific are associated with depressive symptoms as are factors that reflect the progression of PD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Depression is perhaps the most frequent cause of emotional suffering in later life in the general population and significantly reduces quality of life in older adults and their caregivers [1]. Major depression occurs in 1–3% of the general elderly population, and an additional 8–16% have clinically significant depressive symptoms [2]. In patients with Parkinson’s disease (PD), the prevalence of depression has been found to be substantially higher [3].
The available information on occurrence, profile of the depressive syndromes, and risk factors of depressive disturbances in PD (dPD) is primarily based on data from cross-sectional studies. Only few studies have examined the course of dPD over several years and usually with just two measurements [4,5,6]. Studies of depression over time in prospectively followed cohorts of patients with PD and comparable controls could supplement our knowledge on several essential issues related to depression in patients with PD. First, data from longitudinal studies could improve our understanding of the natural history of depression in PD [7]. Second, to study the development of subtypes of dPD such as dysphoria, retardation, and vegetative symptoms [8] may clarify the relationship between depression and the symptomatology of PD. Third, a longitudinal study design also represents a more comprehensive approach to examine the importance of PD-specific and PD-nonspecific risk factors in the etiology of dPD [9].
The aim of the present study was, therefore, to examine these issues in The Norwegian ParkWest study. Patients and controls had longitudinal follow-up of depressive symptoms from time of diagnosis and until 7 years later.
Methods
Study design
The Norwegian ParkWest project is a prospective longitudinal cohort study of unselected patients with incident PD and non-PD control subjects designed to examine the progression of motor and non-motor problems over time in PD. In the current study, we describe the development and risk factors of dPD from the time of diagnosis and until 7 years of follow-up.
Patients and controls
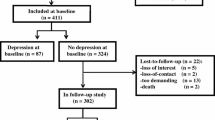
Patients diagnosed with PD and non-PD controls were recruited between November 1, 2004 and August 31, 2006. The recruitment strategies have been described previously in detail [10]. Of 212 patients with PD in the original cohort, 207 were drug naïve at baseline. During follow-up, 20 patients have been rediagnosed to not having idiopathic PD. The remaining 187 patients fulfilled both the United Kingdom Brain Bank [11] and Gelb [12] diagnostic criteria at their latest follow-up visit. A total of 205 non-PD controls were recruited from friends and spouses of patients with PD, or from social clubs for elderly. After matching for age and sex, 166 controls were eligible for the present study.
During the 7-year follow-up period, 43 patients and 42 controls had dropped out of the study, see Fig. 1. Thirty-four patients and 14 controls had died and nine patients and 28 controls had withdrawn for other reasons. Average (total) follow-up time was 6.0 (1130) years among patients and 5.9 (983) years among controls.
Examinations
Standardized examinations of patients and controls were performed by members of the ParkWest study group at baseline and after 1, 3, 5, and 7 years of follow-up. Participants unable to attend the clinic were examined at their homes or in nursing homes. Information regarding demographic variables, medical history, and medications, including antidepressants, was obtained during a semi-structured interview. Information was also collected from the patients’ caregivers.
A neurological examination was performed by a study neurologist experienced in movement disorders. Severity of parkinsonism and problems with functioning were assessed using the motor and activity of daily living sections of the Unified PD Rating Scale (UPDRS-M and UPDRS-ADL) [13]. Pattern of dominating motor symptoms was categorized as tremor dominant, postural instability and gait difficulties (PIGD) or indeterminate motor phenotypes [14]. As a proxy for symptoms of pain, we applied item 17 of the UPDRS (0 = none sensory complaint related to parkinsonism; 4 = excruciating pain). This measure of pain may include sensory deficits due to polyneuropathy. Cognitive functioning was evaluated by the Mini-Mental State Examination (MMSE) [15]. Study data also include daily levodopa equivalent dose (LED) [16] and Charlson comorbidity index [17], which has been shown to be a valid and reliable [18] comorbidity index in both patients and controls. The Schwab and England activities of daily living scale [19] were used as a measure of dependence among the patients with PD at each study visit, with scores below 80% considered to be indicative of a dependent state.
Assessment of depressive symptoms
Severity of depressive symptoms was evaluated by the Montgomery and Aasberg Depression Rating Scale (MADRS) [20]. The MADRS is a ten-item observer-rated scale that has been recommended by a Movement Disorder Society task force to examine dPD in epidemiological studies [21]. Although the MADRS is not developed as a diagnostic tool, cutoffs may be applied to describe diagnostic categories of depression. The study participants were defined to be not depressed (scores 0–6) [22], to have minor depression (scores 7–14), or to have major depression (scores above 14) [23] at the different visits during follow-up. In total, 1555 MADRS examinations were performed. In addition, 18 single visits had incomplete or missing registrations of MADRS. Based on data from the neuropsychiatric inventory (NPI) [24] and item 3 (depression) from the UPDRS, we found that for nine of these 18 visits, there were no depressive symptoms. The MADRS examination was only missing from one patient observation with indication of major depression.
We also applied a three-factor model of MADRS to measure possible subdimensions or subgroups of depressive symptoms [8]. Subdimensions included dysphoria (reported sadness, pessimistic thoughts, and suicidal thoughts), retardation (apparent sadness, concentration difficulties, lassitude, and anhedonia), and vegetative symptoms (reduced sleep, reduced appetite, and inner tension).
Statistical analysis
The selection of controls included in this study for comparison with PD patients was based on frequency matching within 5-year age groups and sex. Descriptive statistics were presented as means and standard deviations (SD) and/or medians and interquartile ranges (IQR) for continuous variables and as counts and percentages for categories. Furthermore, for variables where a ceiling or floor effect was observed, the counts and percentages with maximum/minimum score were indicated. Associations between MADRS scores and risk factors among patients were assessed using generalized estimating equations (GEE) with an independent working correlation matrix and with robust estimation of standard errors [25]. Due to highly skewed distributions of MADRS scores, including scores of zero, a negative binomial regression model was chosen; thus, effects are given as ratios of means (RM). The dispersion parameter was estimated by maximum likelihood. We assumed linear effects of all continuous factors including time since baseline, thus ignoring the initial drop in mean MADRS scores during the first year of follow-up. All statistical analyses were performed in IBM SPSS Statistics v. 23. Charts were created in SPSS and Excel.
Results
In total, 187 patients with PD and 166 non-PD controls were included in this study. Table 1 shows baseline characteristics of the participants. Patients were similar to controls regarding comorbidity, but had more pain and depressive symptoms at baseline.
Depressive symptoms during follow-up for patients and controls
The mean MADRS score from all 823 examinations during the study period was 4.2 (median 2; IQR 0–6) in patients and 1.3 (median 0; IQR 0–2) in 732 examinations among controls, indicating a tenfold higher depressive load in patients as compared with controls.
Figure 2 shows mean observed MADRS scores for all available observations from baseline and until the 7-year visit for patients and controls. The corresponding results for participants that completed all five visits during the study period were near identical (data not shown). Among controls, the occurrence of depressive symptoms was low and stable during follow-up, while in patients, we observed decreased dPD from baseline and until the 1-year visit when 87% were on dopaminergic medication. Thereafter, we found a steady increase in these symptoms. The patients had significantly more depressive symptoms than the controls during the whole study period.
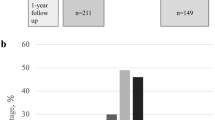
In Fig. 3, we present all recorded relative frequencies of minor and major depressions at each study visit for the participants. Again, the patients have clearly more of both major and mild depressions compared to the controls. The increase over time is most prominent for major depression that increases from 3 to 4% at baseline and the 1-year visit and up to about 10% after 7 years of follow-up.
Among the 187 patients with PD in this study, 87 (47%) never experienced mild or major depression. Thirty-four patients (18%) had a least one observation with MADRS score above 14. Among these, 12 had remissions and seven a chronic course, ten patients had major depression only at the last 7-year visit, and five had one such observation and died before the next visit. One patient had major depression at baseline and then withdrew from the study. Among the 66 patients (35%) with at least one observation with mild depression but never major depression, 39 had minor depression as an intermittent complaint, 15 patients had this only at the baseline examination, and 12 had chronic, mild depressive symptoms during the study period.
We also examined the longitudinal development of dysphoria, retardation, and vegetative depressive symptoms in the patients with PD and in the controls. Figure 4 shows that all three subdimensions of depression were rather stable or slowly increasing over time in controls during follow-up and less prominent than in patients with PD. Among the patients, all subdimensions improved during the first year after diagnosis, but less marked for vegetative symptoms. These symptoms and retardation showed higher scores than dysphoria throughout the study period and with a near identical development over time for all subdimensions.
Mean scores of vegetative symptoms (solid lines, diamonds), dysphoria (dotted lines, squares), and retardation (dashed lines, triangles) as subdimensions of depressive symptoms during the 7-year follow-up period for patients (black; filled symbols) and controls (grey; open symbols). Data shown for available observations at each study visit
At baseline, 12% of the patients with PD and 2% of the controls were using antidepressant medication. During follow-up, the proportions of patients (11–16%) and controls (2–7%) using antidepressants were rather stable.
Possible risk factors associated with depressive symptoms for PD patients
Table 2 shows the univariable and multivariable associations between possible risk factors and higher levels of depressive symptoms during 7 years of follow-up for the PD patients. In the univariable analysis, we found significant relations between MADRS score and each of PIGD status, dependence status, pain score, UPDRS-M score, UPDRS-ADL score, and MMSE score. The mean levels of MADRS scores increased annually by an estimated 4% (RM 1.04; 95% CI 1.01–1.07).
UPDRS-ADL was excluded from the multivariable model due to multicollinearity issues. We found no effect of duration of follow-up (ageing) on mean MADRS scores when controlling for all other covariates. The factors that were statistically significant in the adjusted analysis were sex, dependence status, pain score, and UPDRS-M score. With the exception of sex, the estimated effects for all risk factors decreased substantially when adjusting for the other covariates.
The unadjusted effect of LED on MADRS score was negligible (RM per 100 mg: 1.01, 95% CI 0.99–1.04, p = 0.31) and entering LED into the multivariable model in Table 2 did not substantially alter the results for the other predictors, nor did it contribute significantly to the model (RM per 100 mg: 1.01, 95% CI 0.97–1.06, p = 0.56).
Discussion
This study shows that patients with PD had both at time of diagnosis and during 7 years of follow-up threefold more depressive symptoms than a carefully matched control group. We observed a decrease in dPD between baseline and 1-year follow-up, followed by a steady increase in symptoms during the remaining study period. At the 7-year visit, about 10% of the patients had major depression. In the majority of patients, the presence of dPD was inconsistent, with frequent remissions. The increase in depressive symptoms was not caused solely by increasing vegetative symptoms that may overlap with symptoms of PD itself, but also by an equally important and steady increase of dysphoria and retardation. The severity of parkinsonism seemed to have an influence on dPD. However, PD-nonspecific factors, i.e., sex, dependency, and pain, were also found to be independently associated with these symptoms. The results from this study underscore the importance and frequency of dPD in patients with early PD. Furthermore, our findings indicate that also depressive symptoms that do not overlap with symptoms of PD are important for the observed deterioration of mood in these patients.
This is the first study to explore the natural history of depressive symptoms in an unselected long-term cohort study of patients with incident PD and an age- and sex-matched control group. A systematic review [3] of cross-sectional studies has shown that 17% of PD patients have major depression and even more patients have less pronounced dPD. Of note, these high figures seem to be caused partly by studying selected patient populations. The previous studies of depression in early PD have also been based on such cohorts and show higher frequencies than those found in this population-based approach [4, 6]. Still, we found that depressive symptoms were about threefold higher in patients with early PD than in comparable controls.
The longitudinal development of depressive symptoms showed a marked improvement in patients from the time of diagnosis and until the 1-year follow-up visit when the majority of them were treated with dopaminergic medications. This may have been caused by an improvement of parkinsonism and physical functioning or by social–psychological factors related to having received a diagnosis or a reassuring management support from the study group. Still, the improvement was rather strong and could, therefore, support the notion that dopaminergic drugs also improve depression at least in early PD, although there is no clear evidence from controlled class I trials of such effects [26]. However, D2 agonists seem to be proven as antidepressants in some PD patients [27].
A few studies have examined the long-term longitudinal development of dPD, but only with examinations of depression at the first and last observation in the studies [5, 28]. In this study, we found that the improvement of dPD during the first year after diagnosis was followed by a steady increase of such symptoms during the next 6 years of follow-up. In addition, both major depression and less pronounced depressive symptoms were increasingly present, and after 7 years, about 30% had dPD. This was clearly higher than at the 1-year visit, but not different from the findings at baseline. In contrast, the frequency of more severe depressive symptoms showed a threefold increase from baseline until the 7-year visit, at which 10% of the patients had a MADRS score compatible with the presence of major depression. This observation is in line with the view that depression in PD is increasing with either more severe brain pathology or more dependence as the disease progresses.
The potential overlap between symptoms of mood disorders and PD may represent a diagnostic challenge with the possibility of both over- and underdiagnosing depression in PD patients [29]. In addition, the somatic or vegetative symptoms of PD may bias the results from studies using depression rating scales like MADRS to examine and follow dPD over time in cohort studies. To investigate this possible bias, we also examined the levels and development of the three subdimensions such as dysphoria, retardation, and vegetative symptoms [8]. We found almost identical development over time for all subdimensions and that vegetative symptoms were also important in controls. This indicates that the confounding effect by somatic symptoms does not seem to be an important problem when using total scores from rating scales to evaluate depression in at least patients with early PD.
The main findings from this study were related to the natural history of PD on a group level. Still, we have some information regarding its development in individual patients. Our data show that the intensity of dPD varies over time and that these symptoms can be reversed. This is also in line with observations of new depressive episodes and remissions in the general elderly population [30]. This finding should encourage an optimistic approach to management of depression in patients with PD.
The increased frequency of depression in patients with PD as compared to the general elderly population may be caused by the biology of PD itself or by living with a chronic disease that causes several distressing symptoms and various degrees of dependence [7]. It has been postulated that PD-nonspecific risk factors for depression may be more prominent markers of depression in patients with PD than PD-specific factors [31]. We have, therefore, examined the influence of different PD-specific and nonspecific factors on the level of MADRS scores during this longitudinal cohort study. We found in the adjusted multivariable model that higher scores were found in patients with more severe parkinsonism. In addition, female sex, presence of dependence, and higher pain score were independently associated with higher MADRS scores. The interpretation of these findings is not straightforward as only sex is not directly or indirectly influenced by the severity of the disease process of PD. Still, the levels of pain and functional impairment seem to be important for depression as well as the development of PD itself, and actions that relieve pain and dependence may also reduce depressive symptoms.
Dropout among patients was dominated by deaths, with 18% in total over 7 years, whereas elective dropout summed to 5%. Dropouts are handled by applying statistical models that relate to the dynamic cohort of participants [25], which for patients approximates to the dynamic cohort of survivors. There were more elective dropouts among the controls, 17% over the 7 years. Elective dropouts were approached for their reasons for opting out of the study, which ranged from having a busy life or moving, to increased burden as caregiver and other health conditions. When comparing the distributions of depressive symptoms at each visit for those dropping out before the next visit (for any reason, including death) vs. those staying in the study, we found that they were, overall, quite similar. This may be an indication that the presented results are representative of the study population.
The major limitation of this study is the use of a depression rating scale and not diagnostic interviews to determine level and type of dPD and presence or not of clinically significant depressive symptoms. We have, however, examined the severity of depressive symptoms at more than 1500 study visits, and the MADRS has been recommended for such purposes in PD by an international group of movement disorder experts [21]. We also believe that our findings from the subdimensions of MADRS show that the presented results are valid and thus bring new and important information on the natural history of depressive symptoms in PD. Strengths of this study include the use of a large long-term cohort study of unselected and carefully examined patients with PD and a well-matched control group over 7 years. Participants were followed prospectively with a well-designed study program and repetitive measurements of depression, and great efforts have also been invested to keep attrition of participants to a minimum.
Depression is correlated with poor quality of life in patients with PD and increased caregiver burden [32]. This study shows that depressive symptoms are a complaint of a substantial proportion of patients already at time of diagnosis and more severe symptoms are increasingly present during the first 7 years after diagnosis. These distressing depressive symptoms include both dysphoria and retardation in addition to vegetative symptoms that may be confused with parkinsonian symptoms. Furthermore, general predicting factors for depression such as female sex, dependence, and pain may be as important for the increased depressive symptoms as the disease itself. These findings on the natural history of depressive symptoms in early PD should be confirmed in other long-term cohort studies and also extended into the more advanced stages of the disease.
References
Blazer DG (2003) Depression in late life: review and commentary. J Gerontol A Biol Sci Med Sci 58:249–265
Cole M, Dendukuri N (2003) Risk factors for depression among elderly community subjects: a systematic review and meta-analysis. Am J Psychiatry 160:1147–1156
Reijnders JS, Ehrt U, Weber WE, Aarsland D, Leentjens AF (2008) A systematic review of prevalence studies of depression in Parkinson’s disease. Mov Disord 23:183–189
Ravina B, Camicioli R, Como PG et al (2007) The impact of depressive symptoms in early Parkinson disease. Neurology 69:342–347
Vu TC, Nutt JG, Holford NH (2012) Progression of motor and nonmotor features of Parkinson’s disease and their response to treatment. Br J Clin Pharmacol 74:267–283
Bega D, Luo S, Fernandez H et al (2015) Impact of depression on progression of impairment and disability in early Parkinson’s disease. Mov Disord Clin Pract 2:371–378
Aarsland D, Påhlhagen S, Ballard CG, Ehrt U, Svenningsson P (2011) Depression in Parkinson disease—epidemiology, mechanisms and management. Nat Rev Neurol 8:35–47
Suzuki A, Aoshima T, Fukasawa T et al (2005) A three-factor model of the MADRS in major depressive disorder. Depress Anxiety 21:95–97
Leentjens AF, Moonen AJ, Dujardin K et al (2013) Modeling depression in Parkinson disease: disease-specific and nonspecific risk factors. Neurology 81:1036–1043
Alves G, Muller B, Herlofson K et al (2009) Incidence of Parkinson’s disease in Norway: the Norwegian ParkWest study. J Neurol Neurosurg Psychiatry 80:851–857
Hughes AJ, Ben-Shlomo Y, Daniel SE, Lees AJ (1992) What features improve the accuracy of clinical diagnosis in Parkinson’s disease: a clinicopathologic study. Neurology 42:1142–1146
Gelb DJ, Oliver E, Gilman S (1999) Diagnostic criteria for Parkinson disease. Arch Neurol 56:33–39
Fahn S, Elton RL, Development Committee UPDRS (1987) Unified Parkinson’s Disease Rating Scale. In: Fahn S, Marsden CD, Calne DB, Goldstein M (eds) Recent developments in Parkinson’s disease. Macmillan, Florham Park, pp 153–163
Jankovic J, McDermott M, Carter J et al (1990) Variable expression of Parkinson’s disease: a base-line analysis of the DATATOP cohort. The Parkinson Study Group. Neurology 40:1529–1534
Folstein MF, Folstein SE, McHugh PR (1975) “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12:189–198
Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, Clarke CE (2010) Systematic review of levodopa dose equivalency reporting in Parkinson’s disease. Mov Disord 25:2649–2653
Charlson ME, Pompei P, Ales KL, MacKenzie CR (1987) A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 40:373–383
de Groot V, Beckerman H, Lankhorst GJ, Bouter LM (2003) How to measure comorbidity. A critical review of available methods. J Clin Epidemiol 56:221–229
Schwab JF, England AC (1969) Projection technique for evaluating surgery in Parkinson’s disease. In: Gillingham FJ, Donaldson IML (eds) Third symposium on Parkinson’s disease. E & S Livingston, Edinburgh, pp 152–157
Montgomery SA, Asberg M (1979) A new depression scale designed to be sensitive to change. Br J Psychiatry 134:382–389
Schrag A, Barone P, Brown RG et al (2007) Depression rating scales in Parkinson’s disease: critique and recommendations. Mov Disord 22:1077–1092
Snaith RP, Harrop FM, Newby PA, Teale C (1986) Grade scores of the Montgomery-Asberg depression and the clinical anxiety scales. Br J Psychiatry 148:599–601
Leentjens AF, Verhey FR, Lousberg R, Spitsbergen H, Wilmink FW (2000) The validity of the Hamilton and Montgomery-Asberg depression rating scales as screening and diagnostic tools for depression in Parkinson’s disease. Int J Geriatr Psychiatry 15:644–649
Cummings JL, Mega M, Gray K et al (1994) The neuropsychiatric inventory: comprehensive assessment of psychopathology in dementia. Neurology 44:2308–2314
Kurland BF, Heagerty PJ (2005) Directly parameterized regression conditioning on being alive: analysis of longitudinal data truncated by deaths. Biostatistics 6:241–258
Aarsland D, Marsh L, Schrag A (2009) Neuropsychiatric symptoms in Parkinson’s disease. Mov Disord 24:2175–2186
Barone P, Poewe W, Albrecht S et al (2010) Pramipexole for the treatment of depressive symptoms in patients with Parkinson’s disease: a randomised, double-blind, placebo-controlled trial. Lancet Neurol 9:573–580
Xu YY, Kuo SH, Liang Z et al (2015) The natural history of depression in Parkinson’s disease within 30-month follow-up. Parkinsons Dis 2015:362892
Marsh L, McDonald WM, Cummings J, Ravina B (2006) Provisional diagnostic criteria for depression in Parkinson’s disease: report of an NINDS/NIMH Work Group. Mov Disord 21:148–158
Stek ML, Vinkers DJ, Gussekloo J, van der Mast RC, Beekman AT, Westendorp RG (2006) Natural history of depression in the oldest old: population-based prospective study. Br J Psychiatry 188:65–69
Leentjens AF, Moonen AJ, Dujardin K et al (2013) Modeling depression in Parkinson disease: disease-specific and nonspecific risk factors. Neurology 81:1036–1043
Chen JJ, Marsh L (2013) Depression in Parkinson’s disease: identification and management. Pharmacotherapy 33:972–983
Acknowledgements
The authors are grateful to all participants and the personnel involved in planning and conducting the Norwegian ParkWest study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical standards
The study was approved by the Regional Committee for Medical and Health Research Ethics, Western Norway. Signed written consent was obtained from all participants.
Conflicts of interest
OBT has been invited speaker for GSK, Orion Pharma, Pfizer, UCB, Novartis and Lundbeck and has participated in an advisory board for Lundbeck. JPL, ID, and KFP declare that they have no conflict of interest.
Funding
The Norwegian ParkWest study was supported by Grant #9111218 from the Western Norway Regional Health Authority, Grant #177966 from the Research Council of Norway, and the Norwegian Parkinson’s Disease Association.
Rights and permissions
About this article
Cite this article
Larsen, J.P., Dalen, I., Pedersen, K.F. et al. The natural history of depressive symptoms in patients with incident Parkinson’s disease: a prospective cohort study. J Neurol 264, 2401–2408 (2017). https://doi.org/10.1007/s00415-017-8638-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-017-8638-1