Abstract
It has been challenging to identify clinical cognitive markers that can differentiate patients with Alzheimer’s disease (AD) from those with behavioral variant frontotemporal dementia (bvFTD). The short-term memory binding (STMB) test assesses the ability to integrate colors and shapes into unified representations and to hold them temporarily during online performance. The objective of this study is to investigate whether free recall deficits during short-term memory binding (STMB) test can differentiate patients with AD from those with bvFTD and controls. Participants were 32 cognitively intact adults, 35 individuals with AD and 18 with bvFTD. All patients were in the mild dementia stage. Receiver-operating characteristic (ROC) analyses were used to examine the diagnostic accuracy of the STMB. The results showed that AD patients performed significantly worse than controls and bvFTD patients in the STMB test, while the latter groups showed equivalent performance. The bound condition of the STMB test showed an AUC of 0.853, with 84.4% of sensitivity and 80% of specificity to discriminate AD from controls and an AUC of 0.794, with 72.2% of sensitivity and 80% of specificity to differentiate AD from bvFTD. Binding deficits seem specific to AD. The free recall version of the STMB test can be used for clinical purposes and may aid in the differential diagnosis of AD. Findings support the view that the STMB may be a suitable cognitive marker for AD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It has been challenging to identify clinical cognitive markers that can differentiate patients with AD from those with behavioral variant frontotemporal dementia (bvFTD). There is considerable overlap in cognitive scores between these two conditions [1] and recent studies failed to find the expected executive function (bvFTD) and episodic memory (AD) asymmetry between these two dementia sub-types [2, 3], especially in the mild dementia stages [4]. Therefore, a cognitive test that could contribute to the differential diagnosis between AD and bvFTD would be valuable.
The short-term memory binding (STMB) test assesses the ability to integrate colors and shapes into unified representations and hold them temporarily during online performance [5]. Previous studies have shown that STMB is not affected by normal ageing. Relative to young adults, healthy older adults have shown no additional cost when remembering bindings as compared to remembering single features [5,6,7,8]. Moreover, STMB seems to be insensitive to the educational level of the individual [9]. Besides, the STMB is not affected by repeated testing or practice [10]. Finally, STMB has been shown to capture a specific deficit in AD patients. The test differentiated pre-clinical familial AD from controls [11], AD dementia from chronic depression in the elderly [12], and AD from non-AD dementias [13]. This evidence has led to the suggestion that the STMB may be a suitable cognitive marker for AD or pre-clinical AD [14].
There are different STMB paradigms and in clinical settings two versions have been used. One uses the change detection paradigm [6], in which participants are asked to recognize changes in colors, shapes or their combination across two consecutive screens. The other is a free recall version of the STMB test [13, 15] in which participants are required to verbally recall objects and colors individually or in combinations. The present study relied on the free recall version of the STMB test.
Parra and colleagues [15] demonstrated that, when compared with controls, AD patients showed a specific deficit in holding integrated features in verbal short-term memory. Della Sala et al. [13] reported that only AD patients showed significant deficits in recalling object-color bindings when compared to patients that suffered from other types of dementias. In these two previous studies, controls and patients performed tasks with different set sizes. This procedure was aimed at titrating the difficulty of the task to keep performance level on baseline conditions (i.e., single features) similar across groups. This procedure, however, may not be suitable to be used in clinical settings. Therefore, it remains to be investigated whether the free recall STMB test differentiates AD from controls and other dementias, when the same difficulty level is used for all groups.
The present study investigated whether free recall deficits during STMB differentiate patients with AD from patients with the bvFTD. Based on a previous study [13], we predicted worse scores among AD patients and that the free recall STMB would show high accuracy to differentiate AD from controls and bvFTD.
Methods
Participants
Patients were recruited from neurology outpatient units from the University of São Paulo (USP) and the Federal University of Minas Gerais (UFMG). We recruited 42 patients who met criteria for dementia due to probable AD based on the NIA-AA (National Institute on Aging/Alzheimer’s Association) [16]. Of these, seven were excluded: three presented moderate dementia (CDR = 2.0), one had visual deficits, one had object-naming problems, one was unable to complete the free recall test, and one received a diagnosis of Parkinson’s disease. For the bvFTD group, we recruited 30 patients who met the international diagnostic criteria for this type of dementia [17]. Of these, 11 were excluded: 8 presented moderate dementia (CDR = 2.0), 2 due to object-naming problems, and 1 patient was unable to complete the free recall test. For the control group, we recruited 39 older adults from senior centers and University of Third Age programs (10 from USP Ribeirão Preto; 22 from USP São Paulo; and 7 from the Paulista Institute of Geriatrics and Gerontology). Of these, seven were excluded: five due to low performance on cognitive tests, one participant was not fluent in Portuguese and one was using psychoactive medication with no stable doses. The final sample consisted of 35 AD patients, 18 patients with bvFTD, and 32 cognitively healthy older adults (controls). Control participants and caregivers of patients with dementia signed the informed consent form which was approved by the Ethics Committee from USP (protocol number 16627413.0.0000.0068) and UFMG (protocol number CAA 17850513.2.0000.5149).
Instruments and procedures
All patients were assessed by a neurologist and a neuropsychologist. In neurological care, patients underwent a clinical evaluation and screening tests for dementia (MMSE) [18, 19] and laboratory and neuroimaging exams. Patients completed a neuropsychological battery to assist in the dementia diagnosis. The diagnosis was made by neurologists involved in the project. After the diagnosis, patients were referred to perform the assessment with the STMB test. Controls completed the neuropsychological battery to ascertain normal cognitive status, and, in the same session, they were assessed with the STMB test.
Short-term memory binding
Of the free recall paradigm previously used to assess memory binding [5, 13] we selected two conditions, the unbound and bound features conditions. The rationale behind this selection was that the unbound condition represents a better baseline against which the binding cost could be assessed, than conditions assessing STMB for single features (i.e., color or object only). This is because the only difference between the unbound and bound condition is the need to remember the features together in the latter, that is, the binding. At the beginning of the task, participants were presented with two separate arrays—one consisting of 20 colors and the other consisting of 20 objects. These arrays consisted of the 11 colors and 11 objects used in the experiment and other 9 colors and 9 objects intermixed within the arrays as distractors. Participants were requested to name colors and objects to ensure that they had no problems naming the items used in the experiment (see section Participants above for the outcomes of this screening test).
Unbound features
In this condition, the study array consisted of three colors and three objects presented as separate features. Half of the items were colored squares and the other half were line drawings of common objects. The study array was presented for 9 s (1.5 s per feature). Participants were given the following instructions: ‘Now we will test your memory for colors and objects. You will see three colors and three objects on the screen. You should try to remember as many colors and objects as you can. After these colors and objects disappear, you will have to say aloud all the colors and objects that you have just seen’. The experimenter recorded responses using a scoring sheet.
Bound features
In this condition, the study array consisted of three objects filled with a different color each (i.e., colored objects), and was also presented for 9 s. These colored objects were constructed by randomly combining objects with colors from the two sets in a way that avoided prototypical color-object associations (e.g., red apple). During this condition, participants were asked to try to remember ‘as many colored objects as possible, that is, remember each object together with the color in which it was presented’. The participants should memorize the combination of colors and objects, for instance: “red-bed”, or “gray-shoe”. A correct response was considered only when the two features (color and object) were recalled together.
Each condition (bound and unbound) consisted of six trials with six features each (three colors and three objects). The bound and unbound conditions were counterbalanced. Figure 1 presents an illustration of this task.
Statistical analyses
To assess normality in the distribution of the data, the Shapiro–Wilk test was used. Only age followed a normal distribution in all groups. Thus, descriptive analyses comparing the clinical groups were carried out using the ANOVA test to compare age and the Kruskal–Wallis test was used for the other variables. To evaluate the effect of group, condition and their interaction, a 3 × 2 mixed model with a between-subject factor diagnostic Group (controls, AD and bvFTD) and a within-subject factor Condition (unbound versus bound) was used, and to this aim, we relied on the adjusted rank transform test [20], for nonparametric data. The effect size, as informed by partial eta-squared (ƞ 2), and power by Beta (β), were calculated in these mixed models as well. In addition, the binding cost was calculated as the percentage of loss in performance observed in the bound condition compared to the unbound condition [binding cost = 100 – 100 × (bound/unbound)]. Receiver-operating characteristic (ROC) analyses were used to examine the diagnostic accuracy of the bound STMB and binding cost measures to differentiate between the clinical groups. The area under the curve (AUC), specificity and sensitivity values were calculated. Bivariate correlations were calculated for STMB (bound condition) with age, education and MMSE variables. Significance level was set at 0.05.
Results
Sample characteristics and cognitive profiles are presented in Table 1. Comparisons showed that the three groups were equivalent in age and years of formal education. AD and bvFTD patients were in similar stages of dementia as informed by CDR. AD patients had worse cognitive performance when compared with controls (MMSE, unbound STMB, bound STMB and binding cost). Patients with bvFTD differed from controls in the unbound STMB. AD patients differed from bvFTD in the bound STMB and binding cost.
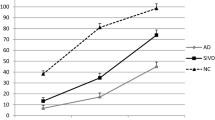
The results of the adjusted rank transform test showed no significant main effect of test condition [F(1,82) = 0.403, p = 0.527, ƞ 2 = 0.005, β = 0.096] but there was a significant main effect of diagnostic group [F(1,82) = 27.867, p < 0.001, ƞ 2 = 0.405, β = 1.000]. In addition, there was a significant interaction between condition and group [F(1,82) = 3.366, p = 0.039, ƞ 2 = 0.076, β = 0.620]. When the three groups were compared (Fig. 2), there was a significant difference between controls and both dementia groups in the unbound condition. In the bound condition, however, there was a significant difference between controls and AD, bvFTD and AD, but no significant difference between controls and bvFTD patients.
The results of the binding cost analyses indicated that the AD group showed a significantly higher percentage drop (26.23%) than the other groups (controls = 11.66% and bvFTD = 7.44%). There was a significant difference between controls and AD (p = 0.011) and bvFTD and AD (p = 0.009), but no difference between controls and bvFTD (p = 1.000).
ROC analyses using the bound STMB (Table 2) indicated that the highest diagnostic accuracy was obtained when the test contrasted the controls and the AD groups. Moderate accuracy was observed when the two dementia groups were contrasted. Low accuracy was observed when the STMB was used to differentiate controls from bvFTD. ROC analyses using the cost of binding variable indicated that the highest diagnostic accuracy was observed when the test contrasted AD and bvFTD groups, followed closely by the contrast of controls and AD, and it showed low accuracy when contrasting controls and bvFTD.
The STMB test (bound condition) showed no significant correlation with age (p = 0.541) or education (p = 0.098), and showed a significant correlation coefficient of 0.454 (p < 0.001) with the MMSE, indicating it maintains a moderate association with general cognition.
Discussion
In this study, we aimed to compare cognitively healthy controls, patients with AD and bvFTD on the free recall modality of the STMB test. For the unbound condition, there was a significant difference between controls and both dementia groups (controls > bvFTD = AD). However, in the bound condition, AD patients showed significantly lower performance compared to bvFTD and controls, and there was no difference between controls and bvFTD (controls = bvFTD > AD). ROC analyses confirmed that the bound condition of the STMB test can be helpful in the differential diagnosis between AD and bvFTD. When we compared the groups in the binding cost (relative percentage drop in performance from the unbound to the bound condition), the results showed that the AD group presented the highest percentage drop when compared with the other groups. In ROC analyses, the binding cost yielded lower accuracy to distinguish the clinical groups when compared with the bound condition. Therefore, present results suggest that the condition of the SMB test with best diagnostic accuracy is that assessing free recall of bound features.
To discuss our results, we would like to consider these in the light of previous findings [13, 15]. Such earlier evidence may provide valuable insights to best interpret our current data. In Table 3 below the results from the current study were contrasted with those previously reported. These earlier studies used an easier version of the task, whereby AD patients were presented with screens of four features, whereas in the present study their screens presented six features. In addition, controls were exposed to a larger number features on the screen, in an attempt to equate task difficulty among groups. Of note, controls and AD patients in the earlier studies were similar in age to participants of the present study but they had fewer years of education. Despite methodological differences, present results are largely consistent with previous findings.
Compared to Parra et al. [15], our results were similar for both clinical groups, even with differences in education and with patients performing a task with more items. Compared to Della Sala et al. [13], the present study showed a smaller performance drop in AD patients from the unbound to the bound condition. This may be due to the higher difficulty of the present task and to the fact that Della Sala et al. [13] included patients in the moderate stage of AD dementia, while the present sample included only mild cases (CDR 0.5 or 1.0). Regarding FTD patients, Della Sala et al. [13] reported a performance of approximately 65% in the unbound condition and 80% in the bound condition, and, in present study, this clinical group performed approximately at 71 and 67%, respectively. That difference might be explained by the fact that Della Sala et al. [13] included the semantic variant of FTD in their group, whereas the current study included solely bvFTD. The semantic variant and bvFTD show different patterns of brain atrophy. While bvFTD patients show atrophy especially in areas of the frontal lobe, anterior cingulate and anterior insula [21, 22], semantic variant patients have anterior and inferior temporal lobe atrophy (in particular, the temporal pole) and perirhinal cortices [23,24,25].
In Della Sala et al. [13] and Parra et al. [15], a smaller set size was used for dementia patients to equate task difficulty across patients and healthy controls. It may be argued that in clinical settings this titration strategy is challenging to implement, as it is impossible to know a priori if someone is a patient or a control. To overcome this barrier, in the present study, the same set size was used for controls and patients, with six features per screen to avoid ceiling effects among controls. Increased task difficulty for patients with dementia may have led to an underestimation of the binding cost, as performance in the unbound condition may have shown a further drop due to the task difficulty, as shown in the comparison between the present study and Parra and colleagues [15]. Therefore, arrays of 4 features might be a more suitable set size if the classical dissociation (performance on unbound > performance on bound) is sought for diagnostic accuracy. The fact that increased task difficulty reduced binding drop (as performance in the unbound condition was already low) may have generated lower scores for the binding cost variable, as observed in Results.
The present findings are also in line with studies that used the change detection paradigm to assess STMB [9, 11]. Taken together, the results from these various studies indicate that short-term conjunctive memory is impaired specifically in AD, even in mild dementia stages, regardless of the nature of the stimuli used (meaningless shapes with non-nameable colors or common objects with common colors) or the retrieval function required (recognition or recall). These results have important clinical implications, as the test could be useful to differentiate AD from bvFTD in the early stages of the disease, which has proven to be quite challenging [3, 4, 26].
We acknowledge that recent studies have pursued similar aims using different memory binding paradigms. One particular type of memory binding, known as relational binding [8], refers to the recall of the association between two different items, for instance, when one recalls a name associated with a face, or information associated with a context, or even the semantic meaning of two words. In the present study, we have used a conjunctive memory binding paradigm, as the recalled feature conjunctions create unique representations (i.e., integrated objects) in memory. Relational and conjunctive memory binding are affected by AD. For instance, the Free and Cued Selective Reminding (FCSR) test [27] showed to be an accurate predictor of AD [28] and mild cognitive impairment (MCI) [29] and possibly fares better in AD and MCI diagnosis than traditional memory tests, such as the Rey Auditory Verbal Learning Test [30]. However, relational binding is affected by age [31] (but see [32]). Conjunctive binding, on the other hand, is not affected by age or education, as the correlation evidence in the present study also suggests, and showed higher diagnostic accuracy for AD when compared with the FCSR test [33]. This may be explained by the fact that relational binding is related to hippocampus activity [34,35,36], whereas conjunctive binding does not seem to be [37, 38]. In addition, hippocampal degeneration does not seem to be an ideal marker to differentiate AD from bvFTD [39] neither seems to be the earliest pathological change causing memory deficits in AD [40].
A few limitations of the study should be noted. Although greater than samples recruited for previous STBM studies, the samples in the current study were not large, restricting the generalization of the outcome. Moreover, we did not have biomarker evidence for the control group making it possible to have included in this group people with normal cognition but in a pre-clinical stage of the disease. This could have decreased the observed discrepancies between controls and the pathological groups.
In conclusion, our results indicate that the free recall version of the STMB test can be used for clinical purposes and may aid the early diagnosis of AD, differentiating this condition from other dementias and validating previous studies with this paradigm. Future studies should continue to explore the specificity of STMB deficits in AD versus other dementias and consider both conjunctive and relational paradigms [32, 41] of temporary binding. Future studies should also address the correlations between performance in STMB tests and biomarkers such as structural, functional or molecular neuroimaging, as well as CSF measures.
References
Hutchinson AD, Mathias JL (2007) Neuropsychological deficits in frontotemporal dementia and Alzheimer’s disease: a meta-analytic review. J Neurol Neurosurg Psychiatry 78:917–928. doi:10.1136/jnnp.2006.100669
Flanagan EC, Wong S, Dutt A, Tu S, Bertoux M, Irish M et al (2016) False recognition in behavioral variant frontotemporal dementia and Alzheimer’s disease—disinhibition or Amnesia? Front Aging Neurosci 8:1–11. doi:10.3389/fnagi.2016.00177
Hornberger M, Piguet O, Graham AJ, Nestor PJ, Hodges JR (2010) How preserved is episodic memory in behavioral variant frontotemporal dementia? Neurology 74:472–479. doi:10.1212/WNL.0b013e3181cef85d
Schubert S, Leyton CE, Hodges JR, Piguet O (2016) Longitudinal memory profiles in behavioral-variant frontotemporal dementia and Alzheimer’s disease. J Alzheimer’s Dis 51:775–782. doi:10.3233/JAD-150802
Parra MA, Abrahams S, Logie RH, Della Sala S (2009) Age and binding within-dimension features in visual short-term memory. Neurosci Lett 449:1–5. doi:10.1016/j.neulet.2008.10.069
Brockmole JR, Parra MA, Della Sala S, Logie RH (2008) Do binding deficits account for age-related decline in visual working memory? Psychon Bull Rev 15:543–547. doi:10.3758/PBR.15.3.543
Rhodes S, Parra M, Logie RH (2016) Ageing and feature binding in visual working memory: the role of presentation time. Q J Exp Psychol 69:654–668. doi:10.1080/17470218.2015.1038571
Isella V, Molteni F, Mapelli C, Ferrarese C (2015) Short term memory for single surface features and bindings in ageing: a replication study. Brain Cogn 96:38–42. doi:10.1016/j.bandc.2015.02.002
Parra MA, Della Sala S, Abrahams S, Logie RH, Méndez LG, Lopera F (2011) Specific deficit of colour–colour short-term memory binding in sporadic and familial Alzheimer’s disease. Neuropsychologia 49:1943–1952. doi:10.1016/j.neuropsychologia.2011.03.022
Logie RRH, Brockmole JRJ, Vandenbroucke AREA (2009) Bound feature combinations in visual short-term memory are fragile but influence long-term learning. Vis cogn 17:160–179. doi:10.1080/13506280802228411
Parra MA, Abrahams S, Logie RH, Méndez LG, Lopera F, Della Sala S et al (2010) Visual short-term memory binding deficits in familial Alzheimer’s disease. Brain 133:2702–2713. doi:10.1093/brain/awq148
Parra MA, Abrahams S, Logie RH, Della Sala S (2010) Visual short-term memory binding in Alzheimer’s disease and depression. J Neurol 257:1160–1169. doi:10.1007/s00415-010-5484-9
Della Sala S, Parra MA, Fabi K, Luzzi S, Abrahams S (2012) Short-term memory binding is impaired in AD but not in non-AD dementias. Neuropsychologia 50:833–840. doi:10.1016/j.neuropsychologia.2012.01.018
Logie RRH, Parra MA, Della Sala S (2015) From cognitive science to dementia assessment. Policy Insights Behav Brain Sci 2:81–91. doi:10.1177/2372732215601370
Parra MA, Abrahams S, Fabi K, Logie R, Luzzi S, Sala Della S (2009) Short-term memory binding deficits in Alzheimer’s disease. Brain 132:1057–1066. doi:10.1093/brain/awp036
McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Kawas CH et al (2011) The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7:263–269. doi:10.1016/j.jalz.2011.03.005
Rascovsky K, Hodges JJR, Knopman D, Mendez MFM, Kramer JH, Neuhaus J et al (2011) Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain 134:2456–2477. doi:10.1093/brain/awr179
Folstein MF, Folstein SE, McHugh PR (1975) Mini-mental state. J Psychiatr Res 12:189–198. doi:10.1016/0022-3956(75)90026-6
Brucki SSMD, Nitrini R, Caramelli P, Bertolucci PHF, Okamoto IH (2003) Sugestões para o uso do mini-exame do estado mental no Brasil. Arq Neuropsiquiatr 61:777–781. doi:10.1590/S0004-282X2003000500014
Leys C, Schumann S (2010) Journal of experimental social psychology a nonparametric method to analyze interactions: the adjusted rank transform test. J Exp Soc Psychol. doi:10.1016/j.jesp.2010.02.007
Seeley WW, Crawford R, Rascovsky K, Kramer JH, Weiner M, Miller BL et al (2008) Frontal paralimbic network atrophy in very mild behavioral variant frontotemporal dementia. Dementia 65:249–256
Schroeter ML, Raczka K, Neumann J, von Cramon DY (2008) Neural networks in frontotemporal dementia—a meta-analysis. Neurobiol Aging 29:418–426. doi:10.1016/j.neurobiolaging.2006.10.023
Collins JA, Montal V, Hochberg D, Quimby M, Mandelli ML, Makris N et al (2016) Focal temporal pole atrophy and network degeneration in semantic variant primary progressive aphasia. Brain 140:457–471. doi:10.1093/brain/aww313
Rohrer JD, Warren JD, Modat M, Ridgway GR, Douiri A, Rossor MN et al (2009) Patterns of cortical thinning in the language variants of frontotemporal lobar degeneration. Neurology 72:1562–1569. doi:10.1212/WNL.0b013e3181a4124e
Hodges JR, Patterson K (2007) Semantic dementia: a unique clinicopathological syndrome. Lancet Neurol 6:1004–1014. doi:10.1016/S1474-4422(07)70266-1
Ramanan S, Bertoux M, Flanagan E, Irish M, Piguet O, Hodges JR et al (2016) Longitudinal executive function and episodic memory profiles in behavioral-variant frontotemporal dementia and Alzheimer’s disease. J Int Neuropsychol Soc 22:1–10. doi:10.1017/S1355617716000837
Buschke H, Einstein A (1984) Cued recall in amnesia. J Clin Exp 6:433–440. doi:10.1080/01688638408401233
Wagner M, Wolf S, Reischies FM, Daerr M, Wolfsgruber S, Hu M (2012) Biomarker validation of a cued recall memory deficit in prodromal Alzheimer disease. Neurology 78:379–386. doi: 10.1212/WNL.0b013e318245f447
Roman F, Iturry M, Rojas G, Barceló E, Buschke H, Allegri RF (2016) Validation of the argentine version of the memory binding test (MBT) for early detection of mild cognitive impairment. Dement Neuropsychol 10:217–226. doi:10.1590/S1980-5764-2016DN1003008
Lemos R, Cunha C, Marôco J, Afonso A, Simões MR, Santana I (2015) Free and cued selective reminding test is superior to the wechsler memory scale in discriminating mild cognitive impairment from Alzheimer’s disease. Geriatr Gerontol Int 15:961–968. doi:10.1111/ggi.12374
Katz M, Sliwinski M, Grober E, Lipton RB, Katz M, Sliwinski M (1998) Demographic influences on free and cued selective reminding performance in older persons. J Clin Exp Neuropsychol (Neuropsychology, Dev Cogn Sect A) 20:221–226. doi:10.1076/jcen.20.2.221.1177
van Geldorp B, Parra MA, Kessels RRPC, van Geldorp B, Parra MA, Kessels RRPC (2015) Cognitive and neuropsychological underpinnings of relational and conjunctive working memory binding across age. Memory 23:1112–1122. doi:10.1080/09658211.2014.953959
Della Sala S, Kozlova I, Stamate A, Parra MA (2016) A transcultural cognitive marker of Alzheimer’s Disease. Int J Geriatr Psychiatry. doi:10.1002/gps.4610
Zammit AR, Ezzati A, Zimmerman ME, Lipton RB, Lipton ML, Katz MJ (2017) Roles of hippocampal subfields in verbal and visual episodic memory. Behav Brain Res 317:157–162. doi:10.1016/j.bbr.2016.09.038
Hannula DE, Ranganath C (2008) Medial temporal lobe activity predicts successful relational memory binding. J Neurosci 28:116–124. doi:10.1523/JNEUROSCI.3086-07.2008
Olson IR (2006) Working memory for conjunctions relies on the medial temporal lobe. J Neurosci 26:4596–4601. doi:10.1523/JNEUROSCI.1923-05.2006
Parra MA, Della Sala S, Logie RH, Morcom AM (2014) Neural correlates of shape–color binding in visual working memory. Neuropsychologia 52:27–36. doi:10.1016/j.neuropsychologia.2013.09.036
Baddeley A, Allen R, Vargha-Khadem F (2010) Is the hippocampus necessary for visual and verbal binding in working memory? Neuropsychologia 48:1089–1095. doi:10.1016/j.neuropsychologia.2009.12.009
De Souza LC, Chupin M, Bertoux M, Lehéricy S, Dubois B, Lamari F et al (2013) Is hippocampal volume a good marker to differentiate Alzheimer’s disease from frontotemporal dementia? J Alzheimer’s Dis 36:57–66. doi:10.3233/JAD-122293
Didic M, Barbeau EJ, Felician O, Tramoni E, Guedj E, Poncet M et al (2011) Which memory system is impaired first in Alzheimer’s disease? J Alzheimer’s Dis 27:11–22. doi:10.3233/JAD-2011-110557
Parra MA, Fabi K, Luzzi S, Cubelli R, Hernandez Valdez M, Della Sala S (2015) Relational and conjunctive binding functions dissociate in short-term memory. Neurocase 21:56–66. doi:10.1080/13554794.2013.860177
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
All authors declare that they have no competing interest.
Ethical standards statement
This research study has been conducted in agreement with international ethical standards for medical research involving human participants, in line with the Declaration of Helsinki.
Funding
We acknowledge the support from FAPESP-UoE (Grant 2014/50203-8); the support from Alzheimer’s Society (Grant AS-SF-14-008) awarded to MAP in collaboration with SDS and MY; the support from the Alzheimer’s Scotland Dementia Research Centre and the Centre for Cognitive Ageing and Cognitive Epidemiology as part of the cross council Lifelong Health and Wellbeing Initiative (MR/K026992/1) both from the University of Edinburgh. MAP work is supported by Alzheimer’s Society in collaboration with SDS [AS-R42303 and AS-SF-14-008].
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Cecchini, M.A., Yassuda, M.S., Bahia, V.S. et al. Recalling feature bindings differentiates Alzheimer’s disease from frontotemporal dementia. J Neurol 264, 2162–2169 (2017). https://doi.org/10.1007/s00415-017-8614-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-017-8614-9