Abstract
Patients with bilateral vestibulopathy (BVP) suffer from persistent imbalance during standing and walking as well as an impaired gaze stabilization during head movements. Disabilities associated with BVP severely compromise patients’ daily activities and are often linked to an increased risk of falls. Currently, the only established treatment option in BVP is physical therapy. However, treatment effects of physical therapy in BVP are most often limited and many patients do not adequately recover performance. Therefore, a number of technical therapeutic approaches are being explored that either try to substitute lost vestibular sensation with a congruent stimulation of other sense modalities or to artificially mimic vestibular function by means of an implantable vestibular prosthesis. Besides, attempts have recently been made to augment and optimize residual vestibular function in patients with BVP using an imperceptible noisy galvanic vestibular stimulation (nGVS). This approach is based on the natural phenomenon of stochastic resonance, wherein the signal processing in sensory systems can be improved by adding an appropriate level of noise to the system. Promising first study outcomes of nGVS treatment in patients with BVP indicate the feasibility of a future non-invasive sensory prosthetic device for BVP rehabilitation. This paper gives an overview about recent research on nGVS treatment in patients with BVP and discusses future research perspectives in this field.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Complete or partial loss of bilateral vestibular function leads to impaired vestibulospinal and vestibulo-ocular reflexes, which are significantly involved in maintaining stable posture and gaze. Patients with bilateral vestibulopathy (BVP) experience chronic disequilibrium, spatial disorientation, and postural imbalance during standing and walking, as well as oscillopsia, i.e., illusionary movements of the visual scene during head movements. Stance and gait unsteadiness in BVP is linked to a higher risk of falls, in particular in the presence of a concomitant peripheral neuropathy [30]. BVP is a heterogeneous disease, most commonly caused by ototoxic aminoglycosides, Ménière’s disease, or meningitis, although in approximately 50% of patients, the etiology remains unclear [37]. Despite BVP, decrements of vestibular function also occur in advanced age due to an age-related decrease in vestibular cell counts. Although a majority of patients retain residual vestibular functionality, the general prognosis of BVP is poor in that more than 80% of patients do not show any significant improvements in vestibular function, regardless of etiology, sex, or age at the first manifestation [1, 37]. Treatment strategies must, therefore, either cope with residual vestibular function (in the case of partial loss) or establish alternative means to provide information to the brain that is normally sensed by the intact vestibular system.
The only established symptomatic treatment option in BVP so far is physical therapy that aims to recruit visual and proprioceptive cues to compensate for the lack of vestibular function. Active vestibular rehabilitation programs have been shown to improve dynamic gait stability and dynamic visual acuity in several but not all patients with BVP [14, 19]. However, the percentage of patients who benefit from vestibular rehabilitation varies considerably depending upon the specific outcome measures used [10, 13, 14]. Furthermore, the benefits of this intervention are mostly apparent for active and predictable head movements and only moderate for rapid and unpredictable movements. Therefore, several technical approaches for rehabilitation in BVP are currently being explored: (1) one attempt is to develop sensory substitutive devices that supplant lost vestibular sensation by a congruent stimulation of other sense modalities; (2) furthermore, efforts are being made to engineer artificial vestibular implants that may adequately mimic vestibular sensation; (3) finally, recent attempts examined the potential to augment and optimize the residual vestibular function present in many patients with BVP by an imperceptible noisy galvanic vestibular stimulation (Fig. 1). Importantly, these different approaches should not be understood as exclusive treatment options but may in future be selectively combined to individually optimize rehabilitation solutions for the tailored care of patients with BVP.
Technical approaches for rehabilitation in bilateral vestibulopathy. a Sensory substitutive devices aim to substitute the loss of vestibular feedback by providing congruent tactile or auditory feedback cues. An inertial sensor fixed to the body monitors balance-related information and provides concurrent feedback either by vibrotactile, electrotactile, or auditory stimulation. b Artificial vestibular implants consist of a head-fixed inertial sensor that transforms head motion information into a concurrent pattern of electrical signals, which are then delivered to the motion direction-specific branches of the vestibular nerve by one or more implanted electrodes. c Noisy galvanic vestibular stimulation aims to augment residual vestibular function in patients by a non-invasive imperceptible noise stimulation of the vestibular end organs. This noise stimulation facilitates the processing of weak, subthreshold vestibular signals via the mechanism of stochastic resonance, and can thereby lower vestibular detection thresholds
Sensory substitutive devices
The principle behind sensory substitutive devices is to replace missing feedback from a defective sense modality with cues from other sensory sources. In the context of BVP, biofeedback systems have been developed to substitute for the loss of vestibular sensation by congruent tactile or auditory feedback cues. Patients are being equipped with small inertial sensors that transduce body-motion-related information as would be provided by a functioning vestibular system. The sensor signals are then used to trigger balance-related feedback cues either by vibrotactile stimulation of the torso, auditory stimulation, or electrotactile stimulation on the tongue [5, 32]. The temporal dynamics of balance-related information encoded by these devices are, however, limited compared to the wide-bandwidth information sensed by an intact vestibular system [11]. This bandwidth mismatch likely precludes the intended substitution for natural vestibular information and suggests that substitutive devices might rather act by a mechanism of sensory addition. Nevertheless, several studies indicate that sensory substitutive devices can at least partially recover balance capacities in patients with BVP but are unlikely to present a comprehensive treatment for other symptoms associated with BVP (Fig. 1a).
Artificial vestibular implants
Inspired by the successful application of cochlear implants in the rehabilitation of patients with hearing loss, the feasibility of an artificial vestibular implant is currently being explored [12, 20]. The premise of a vestibular implant is to deliver adequate vestibular feedback through a specific electrical activation of the vestibular nerves. Prototypes of a vestibular prosthesis consist of (1) external inertial sensors fixed on the head that track head movements in space, (2) a processing unit that transforms the sensor signals into a consistent pattern of electrical signals, and (3) implanted electrodes that selectively stimulate motion direction-specific branches of the vestibular nerve. Up to now, the application of these implants has been limited to the restoration of semicircular canal function, since the highly complex arrangement of motion-sensitive hair cells on the surface of the otolith organs imposes greater difficulties for a targeted external stimulation [20]. Results, mainly derived from animal experiments but also from the first trials of vestibular implants in patients, demonstrate the feasibility to at least partially restore vestibulo-ocular-reflex function in BVP. Whether and to what extent artificial vestibular implants might also restore motion perception and postural stability in patients with BVP remains to be determined. Moreover, since the required surgical procedures for electrode implantation are highly invasive and to date involve a non-negligible risk of causing a permanent hearing loss, this approach might not be an appropriate solution for all patients with BVP (Fig. 1b).
Stochastic resonance and noise-enhanced sensory feedback
Only a small proportion of patients with BVP suffer from a complete loss of vestibular sensation, whereas a majority retains residual vestibular functionality [37]. Recent efforts have, therefore, been made to boost and optimize residual vestibular function in these patients by a non-invasive imperceptible noise stimulation of the vestibular end organs. Usually, the presence of noise in sensory systems is thought to have detrimental effects on the system’s ability to detect signals and process incoming information flow. There is, however, growing evidence that an appropriate amount of noise can likewise enhance the detection and transmission of weak input signals in nonlinear systems [21, 23]. The rationale behind this phenomenon is a mechanism known as stochastic resonance (SR) wherein the response of a nonlinear system to weak input signals can be optimized by the presence of a particular non-zero level of stochastic interference, i.e., noise [3]. SR-type dynamics have been demonstrated experimentally in human psychophysical studies on tactile sensation, auditory, and visual perception [23]. In all of these systems, external application of a particular amount of noise facilitates the processing of weak, subthreshold stimuli, and thereby effectively lowers the system’s detection threshold. Diminished sensitivity of human sensory systems due to elevated detection thresholds is a common consequence of aging or disease. In this context, SR is of particular clinical interest and already inspires new generations of sensory prostheses [28] (Fig. 1c).
The presence of SR-type dynamics in the vestibular system is supported by experimental evidence from animal studies in the bullfrog and chicken showing that the mechano-electrical transduction of inner ear hair cells can be enhanced by intermediate levels of noisy motion of the hair bundles [17]. Stochastic motion of the inner ear hair bundles is also thought to occur naturally, both passively due to Brownian motion of surrounding fluid molecules as well as actively via feedback mechanisms that self-tune hair cells to an oscillatory instability thereby enabling them to actively amplify signals [17, 26].
Noisy galvanic vestibular stimulation
Recent attempts to transfer the beneficial effects of SR to vestibular rehabilitation in patients with BVP have used galvanic vestibular stimulation (GVS) to deliver an imperceptible electrical noise to the vestibular end organs (nGVS). GVS is a simple and save method to induce neuronal activity in both the semicircular canals and the otolith organs of the peripheral vestibular system. In the past, it has widely been used to study the role of vestibular signals in spatial orientation, gaze, posture, and locomotion control [6]. While it is commonly believed that GVS exclusively acts on the primary afferents of the vestibular apparatus, recent evidence suggests that also a direct activation of vestibular hair cells contributes to the GVS-induced vestibular responses [9]. Moreover, repeated long-term exposure to GVS in patients has been shown to be well tolerable and safe [33].
The previous studies that examined the effects of nGVS on posture and locomotion used a bipolar GVS configuration with electrodes placed bilaterally on the mastoid processes behind the ears. Using a portable constant-current stimulator, stochastic stimuli consisting of a zero-mean current noise were applied within a broad frequency range from 0 to up to 30 Hz that covers the natural frequency range of the human vestibular system [29]. One study in healthy subjects compared the effects of nGVS application on balance within a narrow (i.e., 1–2 Hz) versus a broad (0–30 Hz) frequency range and did not reveal significant differences between the two stimuli [24]. Optimal intensities for nGVS application were determined in several studies by comparing the nGVS effects on stance and gait balance at different stimulation intensities. Individual optimal nGVS responses in healthy subjects and patients with BVP were predominantly found at peak amplitudes within a range of 100–500 µA, consistent with the SR phenomenon [8, 16, 24, 25]. To avoid placebo effects, other studies used a fixed imperceptible nGVS intensity at a predefined level below the individual cutaneous sensory threshold for GVS [18, 34, 35].
Effects of noisy galvanic vestibular stimulation on balance
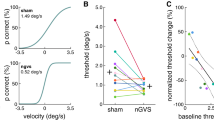
During standing, the vestibular system provides a stable gravito-inertial reference frame for balance control. Patients with BVP are usually able to maintain a stable posture in good lighting conditions. However, when additional sensory feedback sources cease to exist or the support surface becomes unstable, falls are more likely to occur [22]. The elevated fall risk presented by unstable support surfaces and challenging sensory conditions imposes an increased risk of falls and fall-related injuries during patients’ daily routine [30]. Iwasaki et al. were the first to examine the effects of nGVS on balance control in patients with BVP while standing with eyes closed [16]. In 90% of patients, they found an optimal nGVS intensity at which postural stability considerably improved with respect to the range, area, and velocity of body sway. Furthermore, all patients responsive to nGVS reported a perceived improvement of their postural balance during stimulation. The observed noise-enhanced balance regulation in patients with BVP is consistent with the earlier reports on nGVS effects on posture in healthy individuals during challenging balance conditions [24]. In a subsequent study, it was further demonstrated that nGVS exhibits strong after-effects on vestibular balance control and can induce a sustained, up to several hours lasting improvement in postural stability [8].
Effects of noisy galvanic vestibular stimulation on locomotion
Vestibular feedback during locomotion is required to regulate head movements and ensure gaze stability as well as for accurate spatial orientation during navigational tasks [7, 27]. Furthermore, vestibular feedback essentially contributes to the maintenance of dynamic gait stability by fine-tuning the timing and magnitude of foot placements [4]. Consequently, the gait disorder in patients with BVP is characterized by an increased spatiotemporal gait variability that is linked to an increased risk of falls [30]. Furthermore, gait unsteadiness in BVP predominantly manifests at slow-to-moderate walking speeds where stability control most critically relies on adequate vestibular feedback [2]. In a recent study, we examined the effects of nGVS on dynamic gait stability in patients with BVP during walking at different speeds on a treadmill [34]. nGVS was found to be effective in improving impaired gait stability of these patients predominantly during slow-to-moderate walking speeds. Noise-induced alterations in walking performance compared to sham stimulation primarily consisted of a reduced spatiotemporal gait variability and a more regular bilateral walking coordination, hence an optimization of locomotor aspects that are closely linked to dynamic stability control [36]. The objective gait improvements in patients were further accompanied by a perceived improvement of balance during the stimulation. Furthermore, the noise-induced improvements in walking performance of patients with BVP are consistent with previously reported nGVS effects on gait stability in healthy individuals during challenging walking conditions [25, 35].
Open questions
Noisy galvanic vestibular stimulation treatment in patients with BVP has revealed positive effects on balance control during standing and walking. The mechanism underlying these improvements is thought to be SR, by which the external noise stimulation facilitates vestibulospinal reflex responses to weak input signals required for adequate postural adjustments [16, 34]. However, direct evidence for SR-like dynamics in human vestibular perception and reflex function is so far missing. Moreover, it is currently not known whether the ameliorating effects of nGVS on balance that were exclusively observed during preassigned laboratory conditions can be transferred to relevant off-laboratory settings. Further studies will, therefore, be necessary to examine the impact of nGVS treatment on daily mobility, incidence of falls, and quality of life in patients with BVP. Finally, while there is first evidence that nGVS can also have a positive impact on vestibulo-ocular function [15, 31], it so far remains unclear whether other BVP-related symptoms like impaired gaze stabilization (i.e., oscillopsia) and spatial orientation deficits might benefit from nGVS treatment.
Conclusions
Noisy galvanic vestibular stimulation might present a future non-invasive treatment option for the considerable proportion of patients with BVP that retain residual vestibular functionality. Future research is required to examine the effects of nGVS treatment on the wide spectrum of BVP-related symptoms in relevant daily life situations.
References
Brandt T, Huppert T, Hufner K, Zingler VC, Dieterich M, Strupp M (2010) Long-term course and relapses of vestibular and balance disorders. Restor Neurol Neurosci 28:69–82
Brandt T, Strupp M, Benson J (1999) You are better off running than walking with acute vestibulopathy. Lancet 354:746
Collins J, Chow CC, Imhoff TT (1995) Stochastic resonance without tuning. Nature 376:236–238
Dakin CJ, Inglis JT, Chua R, Blouin JS (2013) Muscle-specific modulation of vestibular reflexes with increased locomotor velocity and cadence. J Neurophysiol 110:86–94
Dozza M, Chiari L, Horak FB (2005) Audio-biofeedback improves balance in patients with bilateral vestibular loss. Arch Phys Med Rehabil 86:1401–1403
Fitzpatrick RC, Day BL (2004) Probing the human vestibular system with galvanic stimulation. J Appl Physiol (1985) 96:2301–2316
Fitzpatrick RC, Wardman DL, Taylor JL (1999) Effects of galvanic vestibular stimulation during human walking. J Physiol 517(Pt 3):931–939
Fujimoto C, Yamamoto Y, Kamogashira T, Kinoshita M, Egami N, Uemura Y, Togo F, Yamasoba T, Iwasaki S (2016) Noisy galvanic vestibular stimulation induces a sustained improvement in body balance in elderly adults. Sci Rep 6:37575
Gensberger KD, Kaufmann AK, Dietrich H, Branoner F, Banchi R, Chagnaud BP, Straka H (2016) Galvanic vestibular stimulation: cellular substrates and response patterns of neurons in the vestibulo-ocular network. J Neurosci 36:9097–9110
Gillespie MB, Minor LB (1999) Prognosis in bilateral vestibular hypofunction. Laryngoscope 109:35–41
Goodworth AD, Wall C 3rd, Peterka RJ (2009) Influence of feedback parameters on performance of a vibrotactile balance prosthesis. IEEE Trans Neural Syst Rehabil Eng 17:397–408
Guyot JP, Perez Fornos A, Guinand N, van de Berg R, Stokroos R, Kingma H (2016) Vestibular assistance systems: promises and challenges. J Neurol 263(Suppl 1):S30–S35
Herdman SJ, Hall CD, Maloney B, Knight S, Ebert M, Lowe J (2015) Variables associated with outcome in patients with bilateral vestibular hypofunction: preliminary study. J Vestib Res 25:185–194
Herdman SJ, Hall CD, Schubert MC, Das VE, Tusa RJ (2007) Recovery of dynamic visual acuity in bilateral vestibular hypofunction. Arch Otolaryngol Head Neck Surg 133:383–389
Iwasaki S, Karino S, Kamogashira T, Togo F, Fujimoto C, Yamamoto Y, Yamasoba T (2017) Effect of noisy galvanic vestibular stimulation on ocular vestibular-evoked myogenic potentials to bone-conducted vibration. Front Neurol 8:26
Iwasaki S, Yamamoto Y, Togo F, Kinoshita M, Yoshifuji Y, Fujimoto C, Yamasoba T (2014) Noisy vestibular stimulation improves body balance in bilateral vestibulopathy. Neurology 82:969–975
Jaramillo F, Wiesenfeld K (1998) Mechanoelectrical transduction assisted by Brownian motion: a role for noise in the auditory system. Nat Neurosci 1:384–388
Kim DJ, Yogendrakumar V, Chiang J, Ty E, Wang ZJ, McKeown MJ (2013) Noisy galvanic vestibular stimulation modulates the amplitude of EEG synchrony patterns. PLoS One 8:e69055
Krebs DE, Gill-Body KM, Riley PO, Parker SW (1993) Double-blind, placebo-controlled trial of rehabilitation for bilateral vestibular hypofunction: preliminary report. Otolaryngol Head Neck Surg 109:735–741
Lewis RF (2016) Vestibular implants studied in animal models: clinical and scientific implications. J Neurophysiol 116:2777–2788
McDonnell MD, Ward LM (2011) The benefits of noise in neural systems: bridging theory and experiment. Nat Rev Neurosci 12:415–426
Mergner T, Schweigart G, Fennell L, Maurer C (2009) Posture control in vestibular-loss patients. Ann N Y Acad Sci 1164:206–215
Moss F, Ward LM, Sannita WG (2004) Stochastic resonance and sensory information processing: a tutorial and review of application. Clin Neurophysiol 115:267–281
Mulavara AP, Fiedler MJ, Kofman IS, Wood SJ, Serrador JM, Peters B, Cohen HS, Reschke MF, Bloomberg JJ (2011) Improving balance function using vestibular stochastic resonance: optimizing stimulus characteristics. Exp Brain Res 210:303–312
Mulavara AP, Kofman IS, De Dios YE, Miller C, Peters BT, Goel R, Galvan-Garza R, Bloomberg JJ (2015) Using low levels of stochastic vestibular stimulation to improve locomotor stability. Front Syst Neurosci 9:117
Nadrowski B, Martin P, Julicher F (2004) Active hair-bundle motility harnesses noise to operate near an optimum of mechanosensitivity. Proc Natl Acad Sci USA 101:12195–12200
Pozzo T, Berthoz A, Vitte E, Lefort L (1991) Head stabilization during locomotion. Perturbations induced by vestibular disorders. Acta Otolaryngol Suppl 481:322–327
Priplata AA, Niemi JB, Harry JD, Lipsitz LA, Collins JJ (2003) Vibrating insoles and balance control in elderly people. Lancet 362:1123–1124
Sadeghi SG, Chacron MJ, Taylor MC, Cullen KE (2007) Neural variability, detection thresholds, and information transmission in the vestibular system. J Neurosci 27:771–781
Schniepp R, Schlick C, Schenkel F, Pradhan C, Jahn K, Brandt T, Wuehr M (2017) Clinical and neurophysiological risk factors for falls in patients with bilateral vestibulopathy. J Neurol 264:277–283
Serrador JM, Geraghty MC, Deegan BM, Wood SJ (2011) Enhancing vestibular function by imperceptible electrical stimulation. J Vestib Res 21:101–103
Wall C 3rd, Weinberg MS, Schmidt PB, Krebs DE (2001) Balance prosthesis based on micromechanical sensors using vibrotactile feedback of tilt. IEEE Trans Biomed Eng 48:1153–1161
Wilkinson D, Zubko O, Sakel M (2009) Safety of repeated sessions of galvanic vestibular stimulation following stroke: a single-case study. Brain Inj 23:841–845
Wuehr M, Nusser E, Decker J, Krafczyk S, Straube A, Brandt T, Jahn K, Schniepp R (2016) Noisy vestibular stimulation improves dynamic walking stability in bilateral vestibulopathy. Neurology 86:2196–2202
Wuehr M, Nusser E, Krafczyk S, Straube A, Brandt T, Jahn K, Schniepp R (2016) Noise-enhanced vestibular input improves dynamic walking stability in healthy subjects. Brain Stimul 9:109–116
Wuehr M, Pradhan C, Brandt T, Jahn K, Schniepp R (2014) Patterns of optimization in single- and inter-leg gait dynamics. Gait Posture 39:733–738
Zingler VC, Weintz E, Jahn K, Mike A, Huppert D, Rettinger N, Brandt T, Strupp M (2008) Follow-up of vestibular function in bilateral vestibulopathy. J Neurol Neurosurg Psychiatry 79:284–288
Acknowledgements
The authors thank Haike Dietrich for copy-editing the manuscript. The work was supported by the Federal Ministry for Education and Science (BMBF, Nr. 80121000-49) of Germany.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
Max Wuehr, Julian Decker, and Roman Schniepp declare that there are no financial disclosures or conflicts of interest.
Ethical standards
The content of this review complied with all ethical standards.
Additional information
This manuscript is part of a supplement sponsored by the German Federal Ministry of Education and Research within the funding initiative for integrated research and treatment centers.
Rights and permissions
About this article
Cite this article
Wuehr, M., Decker, J. & Schniepp, R. Noisy galvanic vestibular stimulation: an emerging treatment option for bilateral vestibulopathy. J Neurol 264 (Suppl 1), 81–86 (2017). https://doi.org/10.1007/s00415-017-8481-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-017-8481-4