Abstract
Human subjects placed in strong magnetic fields such as in an MRI scanner often feel dizzy or vertiginous. Recent studies in humans and animals have shown that these effects arise from stimulation of the labyrinth and are accompanied by nystagmus. Here, we measured the three-dimensional pattern of nystagmus using video eye tracking in five normal human subjects placed in a 7T MRI to infer which semicircular canals are activated by magnetic vestibular stimulation. We found that the nystagmus usually had a torsional as well as a horizontal component. Analysis of the relative velocities of the three eye movement components revealed that the lateral and anterior (superior) canals are the only canals activated, and by a similar amount.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Strong magnetic fields such as those present inside and around MRI scanners can induce transient sensations of vertigo and dizziness [2] and persistent nystagmus [8]. When healthy humans enter the bore of a 7 T MRI scanner in darkness they perceive a rotation that dissipates in minutes. Eye movements recorded in darkness with infrared techniques, however, reveal persistent nystagmus for as long as 90 min (longest measured thus far) [4].
Recent studies support the hypothesis that strong static magnetic fields interact with natural electrical currents within the inner ear endolymph [1, 6]. This interaction produces a Lorentz force in the endolymph that transmits a sustained pressure onto the cupula of the semicircular canals in a manner similar to inertial forces during head accelerations, producing nystagmus. According to this hypothesis [1, 12], the Lorentz force is strongest in the endolymph above the utricle, where current density is high due to the large number of utricular hair cells. The pressure generated by this force propagates through the endolymph to the semicircular canals where it displaces the cupula, bending hair cells and signaling to the brain that one is rotating. Note while the ionic currents responsible for the Lorentz forces are related to activity in the hair cells of the utricle, the Lorentz forces themselves do not excite the utricle but rather the semicircular canals.
There are three semicircular canals in each ear oriented in approximately orthogonal planes: lateral, anterior and posterior. Due to the anatomical proximity of the utricle to the cupulae of the lateral and anterior semicircular canals, we hypothesized that these canals (but not the more distantly located posterior canals) are affected by pressure generated in the endolymph above the utricle (Fig. 1a).
a Hypothesized effect of MVS on the anterior and lateral canals. Yellow arrow represents the direction of the magnetic field, green the direction of the net ionic current and red the direction of the Lorentz force. The orange arrows indicate the direction of the movement of each cupula. If we change the position of the subject from entering into the bore head first to feet first, the direction of the magnetic field changes and (by the right-hand rule) this reverses the direction of the resultant force and also the direction of movement of the cupula. b Schematic of slow-phase eye movements produced by stimulating individual semicircular canals. Equations below show a few combinations of stimulating/inhibiting more than one canal. c Example recording from subject S2. The main components of nystagmus are horizontal and torsional and both reverse after reversing the position of the subject
Each semicircular canal responds optimally to head rotations in a plane parallel to their orientation in the skull (Ewald’s first law, Fig. 1b). Excitation of a semicircular canal afferent (above its resting level of activity) provides the stimulus for the vestibular–ocular reflex (VOR) that rotates the eye in the same plane but in the opposite direction as the rotation of the head. The canals are arranged in coplanar pairs so for a given head rotation semicircular canal afferents in one ear are excited (increase their response) while the coplanar canals in the other ear are inhibited. If multiple pairs of canals are stimulated, the composite eye movement reflects the vector sum of the contribution of each canal (Fig. 1b). Conversely, by observing the three components of eye movements (horizontal, vertical and torsional), one can infer which canals are being stimulated.
Initial experiments in MVS in normal human subjects focused on the horizontal component and did not report torsional or vertical components [8, 12]. Torsional eye movements were not measured quantitatively and they can be difficult to appreciate when superimposed on a large horizontal movement. In healthy subjects, if only lateral and anterior canals are activated by MVS one would predict eye movements that have a large horizontal component, no vertical component and a large torsional component (Fig. 1b). The horizontal component of MVS is easily explained by excitation of the lateral semicircular canal in one labyrinth and inhibition of the lateral semicircular canal in the other. The pattern of vertical and torsional components is more complicated. Recall that excitation of either anterior canal produces an upward and torsional (top pole rolls towards the opposite ear) movement. Thus, the combined pattern of stimulation by MVS of both anterior canals causes the anterior canal of one labyrinth to be excited and the other anterior canal to be inhibited, thereby resulting in subtraction of the vertical and addition of the torsional components (Fig. 1b). One also predicts that a vertical component should emerge in patients with unilateral vestibular loss and the direction of the vertical component would depend on the side of the vestibular loss [11] as predicted by Ewald’s third law (for the vertical semicircular canals ampullafugal motion is excitatory [5]).
The objective of this study was to record three-dimensional eye rotation and test the hypothesis that both lateral and anterior canals would be affected during MVS in normal subjects. We also wanted to reconcile prior observations from healthy subjects and patients with unilateral vestibular loss, in whom the pattern of torsion appeared less obvious.
Methods
Five healthy normal volunteers (four males, one female; 33–71 years) participated in the study. Experiments were approved by the Johns Hopkins institutional review board and informed consent was obtained from all participants.
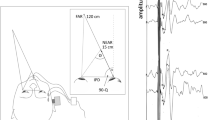
For all experiments, subjects were supine while outside and then inside a Philips Achieva 7T MRI magnetic field (Philips research, Hamburg, Germany). For this MRI machine, the magnetic field vector B was directed from the subject’s head toward the feet when entering the MRI supine and head first. We used the RealEyes xDVR system (Micromedical Technologies Inc.) and custom software to measure binocular eye position in three dimensions. This system uses two cameras (Firefly MV, PointGrey Research Inc., Richmond, BC, Canada) mounted on goggles to capture infrared images of each eye. Real-time binocular eye position was tracked at 100 Hz in the horizontal and vertical positions using the pupil and torsional position based on the iris pattern [7]. Eye movements were calibrated outside the magnetic field while supine with the head in a neutral position on the table and looking directly at a target screen above. Eye velocity for the slow-phase (SPV) component of nystagmus was calculated from the position versus time traces. During the experiments, vision was occluded with a double layer of black felt to ensure complete darkness.
Subjects were first placed supine on the MRI table with their head near the bore in a neutral position. Eye movements were recorded for 2 min, after which the subject was moved into the MRI bore using the fixed-speed table motor drive (10.8 cm/s over 2 m travel). Recordings continued in the MRI for 5 min. Subjects were then moved to just outside the MRI bore and were recorded for another 2 min. Each subject repeated the experiment twice, once entering the magnet with head first and once with feet first, always in supine position.
Results
We recorded eye position in three dimensions before, during and after exposure to the magnetic field of 7 T MRI machine. Subjects entered the bore of the magnet either with head or feet first and remained inside for 5 min. Figure 1c shows an example recording with one second of nystagmus, demonstrating that the main components are horizontal and torsional and that both components reverse direction when the subject enters the magnetic bore feet first instead of head first.
All subjects showed this pattern of activation although the absolute and relative amplitudes of each component varied. However, in all cases the torsional and horizontal component reversed with the reversal of the position of the subject (head first to feet first) and the vertical component was either absent or always the smallest of the three (Fig. 2). To confirm that the torsional component was not an artifact of our recording system, we also recorded the eye movements of subject S1 with the same system while continuously accelerating in the yaw plane in a rotary chair. Data show (Fig. 2, inset) that a horizontal slow phase of the same velocity was induced as in the MRI machine but with no torsional component. This finding confirms that the torsion recorded with the horizontal component due to MVS was not an artifact.
To determine which canals are being stimulated, we used the matrix approach as developed by Robinson and others [9, 10]. The three-dimensional rotational velocity of the head can be represented by a vector with three components. Then, the canal activation can be derived by multiplying that vector by a 3 × 3 matrix that indicates how much each canal is activated by rotation of the head in each plane. Two other matrices are similarly used to calculate the brainstem activation given the canal activation, and the velocity of the eyes given the brainstem activation.
Tweed and colleagues calculated an average gain matrix of the full system [10] by rotating subjects in the dark around axes in all three dimensions, measuring the resultant eye movements and calculating the VOR gain. Similar to the low-frequency characteristic of MVS, their rotational stimulus was also low frequency. The most relevant feature of this matrix for our study is the lower gain of the torsional component, which is about half of the gain of the vertical and the horizontal components. That is, if the head rotates horizontally (yaw) or vertically (pitch), the eyes will rotate twice as much than if the head rotates torsionally (roll).
There is an important consideration when using the matrix analysis for MVS vs. natural head rotation. During head rotation, the canals are paired given their anatomical orientation relative to the head, right anterior with left posterior and left anterior with right posterior (LARP and RALP). During MVS, however, the canals are paired given their symmetrical anatomical relationship to the utricle and their connection via endolymph, right anterior with left anterior and right posterior with left posterior (LPRP and LARA). One can easily convert from one representation to the other since the relationship is linear and both have only three degrees of freedom. Thus, \({\text{Act}}_{\text{LPRP}} {\text{ = Act}}_{\text{LARP}} {\text{ + Act}}_{\text{RALP}}\) and \({\text{Act}}_{\text{LARA}} {\text{ = Act}}_{\text{LARP}} - {\text{Act}}_{\text{RALP}}\).
By using the matrices reported by Robinson, Tweed and colleagues [9, 10], we could infer the relative canal activation given our recordings of the three components of slow-phase eye velocity. In all subjects, we saw a significant activation of the lateral and anterior canals with little activation of the posterior canals (Fig. 3). Although there was some variability across subjects in the relative activation of the lateral and anterior canals (Fig. 3c), on average their contribution was comparable (Fig. 3d).
a Peak slow-phase velocity (SPV) of each component of nystagmus (horizontal, vertical and torsional) for each subject. b Average across subjects for each component (error bars represent sem). c Inferred activation of each canal (pair) given the eye movement pattern. Activations are normalized by the maximum. d Average across subjects for each canal pair (error bars represent the standard error of the mean)
Discussion
Our recordings of three-dimensional eye movements in normal humans exposed to a strong magnetic field show a mixed horizontal–torsional nystagmus. The direction of nystagmus of both components reverses when the position of the body is reversed inside the magnetic field (head first vs. feet first). The pattern of nystagmus is consistent with combined activation of lateral and anterior semicircular canals. In the head-first configuration in our magnet, the induced force excites the right lateral canal and inhibits the left, simulating a rightward head rotation in the yaw plane. This stimulation produces a horizontal nystagmus with slow-phase velocity towards the left (negative). The force also affects both anterior canals exciting the left anterior canal and inhibiting the right, simulating a head rotation in the roll plane towards the left shoulder. This stimulation produces little vertical nystagmus because the vertical contributions from the two canals cancel, however, a torsional nystagmus does occur because the torsional components (the top pole of the slow-phase eye movement rotating towards the left shoulder) are summed. Our results are consistent with previous recordings in the MRI machine of patients with unilateral vestibular loss in whom the vertical component of nystagmus was not canceled and had a direction that depended on which side had lost function [11].
Our results are also consistent with the perception of rotation that subjects experience inside the 7 T MRI. Mian and colleagues [6] found that most subjects experience a perception of roll, the direction of which is consistent with the added torsional component that one would expect from excitation of one and inhibition of the other anterior canal. Why is the perception of motion in the roll plane more salient than the perception in the yaw plane even though the torsional component of the nystagmus is lower velocity? There are several possibilities. First, while the gain of the VOR for roll (torsion) is smaller than that for yaw [10], the perceptual gain need not be less than for horizontal. Prior work has demonstrated that retinal slip was not correlated with perception of oscillopsia [3]. Second, while laying supine in the MRI machine, the gravity vector is aligned with the roll axis and perpendicular to the yaw axis (both relative to the head). Thus, the perceived yaw rotation in the MRI machine from activation of the semicircular canals is not accompanied by a relative change of orientation between the body and gravity as it would be in natural yaw rotations around an earth-horizontal axis. In the latter case, the body is continuously reoriented relative to the gravity vector thereby stimulating the otoliths as well as the semicircular canals. We propose that MVS does not activate the otoliths, therefore the perception of yaw-axis rotation may be relatively suppressed because of the sensory conflict imposed by the absence of concurrent otolith stimulation. In contrast, there is no sensory conflict for roll axis rotation.
Another question in MVS is the cause of inter-subject variability. The velocity of the slow-phase eye movements, the relative horizontal/torsional contributions and as reported previously, the head pitch orientation that corresponds with a subject’s null position where nystagmus is absent [8], vary considerably among subjects. Possible explanations cover all aspects of MVS, from variations in the skull anatomy, the source of the force, how the force is communicated to the canals and how it affects the sensory epithelia. There could also be differences in central mechanisms such as velocity storage, adaptive mechanisms, and how the brain weighs the interaction between conflicting otolith and canal inputs.
While there are some limitations in our study, these do not change the main message. First, the VOG goggles moved inside the bore due to some magnetic pull during the movement of the subject towards or away from the magnet. This translation in goggle position could produce an offset of eye position; however, it would not affect velocity as the translation does not change the distance between the cameras and the eye so the gain of the system remains constant. Second, since subjects were in complete darkness, it was not possible to maintain a constant gaze position. We continuously monitored the eyes of subjects and instructed them to correct their gaze toward straight ahead if it deviated. Finally, we relied on several assumptions about the positions of the head, eye, and canals during experiments for the matrix calculations to extrapolate the canal activations. Small deviations from these assumptions would have minimal impact on the results.
In summary, using 3D eye movement recordings of healthy subjects inside a 7 T MRI, we have corroborated the hypothesis that the mechanism of MVS affects both lateral and anterior canals.
References
Antunes A, Glover PM, Li Y, Mian OS, Day BL (2012) Magnetic field effects on the vestibular system: calculation of the pressure on the cupula due to ionic current-induced Lorentz force. Phys Med Biol 57:4477–4487
Glover PM, Cavin I, Qian W, Bowtell R, Gowland PA (2007) Magnetic-field-induced vertigo: a theoretical and experimental investigation. Bioelectromagnetics 28:349–361
Grunfeld EA, Okada T, Bronstein AM et al (2000) The effect of habituation and plane of rotation on vestibular perceptual responses. J Vestib Res 10:193–200
Jareonsettasin P, Otero-Millan J, Ward BK, Roberts DC, Schubert MC, Zee DS (2016) Multiple time courses of vestibular set-point adaptation revealed by sustained magnetic field stimulation of the labyrinth. Curr Biol 26:1359–1366
Leigh R, Zee D (2015) The neurology of eye movements, 5th edn. Oxford University Press, New York
Mian OS, Li Y, Antunes A, Glover PM, Day BL (2013) On the vertigo due to static magnetic fields. Available at http://dx.plos.org/10.1371/journal.pone.0078748. Accessed 17 Feb 2017
Otero-Millan J, Roberts DC, Lasker A, Zee DS, Kheradmand A (2015) Knowing what the brain is seeing in three dimensions: a novel, noninvasive, sensitive, accurate, and low-noise technique for measuring ocular torsion. J Vis 15:11
Roberts DC, Marcelli V, Gillen JS, Carey JP, Della Santina CC, Zee DS (2011) MRI magnetic field stimulates rotational sensors of the brain. Curr Biol 21:1635–1640
Robinson DA (1982) The use of matrices in analyzing the three-dimensional behavior of the vestibulo-ocular reflex. Biol Cybern 46:53–66
Tweed D, Sievering D, Misslisch H, Fetter M, Zee D, Koenig E (1994) Rotational kinematics of the human vestibuloocular reflex. J Neurophysiol 72:2467–2479
Ward BK, Roberts DC, Della Santina CC, Carey JP, Zee DS (2014) Magnetic vestibular stimulation in subjects with unilateral labyrinthine disorders. Front Neurol 5 Available at: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3952138/. Accessed 17 Feb 2017
Ward BK, Roberts DC, Della Santina CC, Carey JP, Zee DS (2015) Vestibular stimulation by magnetic fields. Ann NY Acad Sci 1343(1):69–79
Acknowledgements
This study was funded by the Fight for Sight and Leon Levy Foundations, the Johns Hopkins School of Medicine Discovery Fund, and the Cinquegrana, Lott, and Schwerin families.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Ethical standards
This study has been approved by the institutional review board at the Johns Hopkins University School of Medicine and has been performed in accordance with the ethical standards established in the 1964 Declaration of Helsinki.
Additional information
This manuscript is part of a supplement sponsored by the German Federal Ministry of Education and Research within the funding initiative for integrated research and treatment centers.
Rights and permissions
About this article
Cite this article
Otero-Millan, J., Zee, D.S., Schubert, M.C. et al. Three-dimensional eye movement recordings during magnetic vestibular stimulation. J Neurol 264 (Suppl 1), 7–12 (2017). https://doi.org/10.1007/s00415-017-8420-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-017-8420-4