Abstract
The measurement of autoregulatory delay by near-infrared spectroscopy (NIRS) has been proposed as an alternative technique to assess cerebral autoregulation, which is routinely assessed via transcranial Doppler sonography (TCD) in most centers. Comparitive studies of NIRS and TCD, however, are largely missing. We investigated whether cerebrovascular reserve (CVR), as assessed via TCD, correlates with the delay of the autoregulatory response to changes in arterial blood pressure (ABP) as assessed by NIRS, i.e., if impaired upstream vasomotor reactivity is reflected by downstream cortical autoregulation. Twenty patients with unilateral high-grade steno-occlusion of the middle cerebral artery (MCA) underwent bilateral multichannel NIRS of the cortical MCA distributions over a period of 6 min while breathing at a constant rate of 6 cycles/min to induce stable oscillations in ABP. The phase shift φ between ABP and cortical blood oxygenation was calculated as a measure of autoregulatory latency. In a subgroup of 13 patients, CO2 reactivity of the MCAs was determined by TCD to assess CVR in terms of normalized autoregulatory response (NAR). Mean phase shift between ABP and blood oxygenation was significantly increased over the hemisphere ipsilateral to the steno-occlusion (n = 20, p = 0.042). The interhemispheric difference Δφ in phase shift was significantly larger in patients with markedly diminished or exhausted CVR (NAR < 10) than in patients with normal NAR values (NAR ≥ 10) (p = 0.007). Within the MCA core distribution territory, a strong correlation existed between Δφ and CO2 reactivity of the affected MCA (n = 13, r = −0.78, p = 0.011). NIRS may provide an alternative or supplementary approach to evaluate cerebral autoregulation in risk assessment of ischemic events in steno-occlusive disease of cerebral arteries, especially in patients with insufficient bone windows for TCD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Impaired cerebral autoregulation (CA) is an important consideration in risk assessment of ischemic events in steno-occlusion of brain supplying arteries [1]. In particular, reduced cerebrovascular reserve (CVR), i.e., a diminished capacity of downstream resistance vessels to dilate in response to decreased cerebral perfusion pressure (CPP), has been associated with an increased risk of ischemic stroke [2, 3]. A clinically established surrogate measure for CVR is the increase of arterial blood flow velocity in response to standardized vasodilatory stimuli as assessed by transcranial Doppler (TCD) sonography of the affected artery [4, 5]. This measure reflects the autoregulatory capacity of the entire downstream resistance vasculature, and thus may not be sufficiently sensitive to identify isolated hemodynamic risk zones within the distribution territory of the insonated artery. Identification of such risk zones may improve the understanding of autoregulatory dysfunction in the cerebrovascular network and, ultimately, may optimize patient stratification [6].
Near-infrared spectroscopy (NIRS) enables the measurement of cortical blood oxygenation, and thereby allows the assessment of CA at a microvascular level [7]. The delay between changes in arterial blood pressure (ABP) and cortical blood oxygenation has already been used in different pathologies to characterize CA [6, 8–14]. Here, we compared this approach with CVR assessment using TCD. We investigated, in particular, whether in steno-occlusive disease of the middle cerebral artery (MCA), phase shift between induced oscillations in ABP and blood oxygenation, as assessed by multichannel NIRS in the cortical MCA distribution, is increased and whether it correlates with CVR as assessed by TCD, i.e., if impaired upstream vasomotor reactivity is reflected by downstream cortical autoregulation.
Methods
Study population
Twenty patients (mean age 55.3 ± 12.5 years) in the chronic stage of unilateral high-grade stenosis or occlusion of the MCA were enrolled in this study. Patients were recruited from our hospital-based neurovascular outpatient clinic. All patients underwent standardized color-coded duplex sonography of the extra- and intracranial arteries to confirm cerebral macroangiopathy and to exclude relevant concomitant vascular pathology of the supraaortic vessels; in all patients, steno-occlusion was established by two independent, experienced investigators. Assessment of bilateral cerebrovascular reserve (CVR) by standard CO2 test was possible in 13 patients, whereas continuous measurement of MCA blood flow velocities could not be performed in seven patients, possibly due to insufficient temporal bone windows. None of the patients had a history of major stroke or presented with significant neurological signs at the time of investigation, i.e., no ischemic events for at least 6 months. Detailed patient characteristics are given in Table 1. The institutional ethics committee (Ethics Committee of the Otto-von-Guericke University Magdeburg) approved the study protocol. All patients gave written informed consent prior to enrollment.
Ultrasound
Patients underwent standardized ultrasound examination of the extra- and intracranial arteries using an Aplio XG US system (Toshiba, Tokyo, and Japan). Transcranial duplex sonography was applied to confirm MCA stenosis based on intra-stenotic blood flow velocity. High-grade stenosis was assumed at a peak systolic velocity greater than 220 cm/s [15]. Segmental occlusion was diagnosed in the case of absent or minimal flow signals along the M1 segment of the MCA. Post-occlusion blood flow velocities obtained in these patients likely result from collateral blood flow.
Doppler CO2 test
CVR capacity was assessed by measuring CO2 reactivity using bitemporal fixed 2-MHz probes (Multi-Dop X4; DWL, Sipplingen, Germany), which allowed for continuous measurement of MCA blood flow velocities in both hemispheres. For the affected hemisphere, measurements were performed distal to the steno-occlusion. End-tidal CO2 concentration was simultaneously monitored using a capnometer (Normocap 200; Datex-Ohmeda, Helsinki, Finland). For both hemispheres, normalized autoregulatory response (NAR; according to the formula:
CBFV: post-stenotic cerebral blood flow velocity of the middle cerebral artery under normal (normal) and hypercapnic (hyper) conditions; ECTO2: percentage of end-tidal CO2 under normal (normal) and hypercapnic (hyper) conditions [16]) was determined as the increase in steady-state blood flow velocity per 1 % increase in end-tidal CO2 concentration, as induced by inspiration of carbogen gas for 2–3 min (95 % O2, 5 % CO2) as previously described [17]. CVR was considered sufficient for NAR ≥ 10, markedly diminished if NAR < 10, and nearly or completely absent if NAR < 5 [16]. NAR could not be assessed in seven patients due to insufficient temporal bone windows.
Near-infrared spectroscopy
Cortical concentrations of oxygenated hemoglobin (oxyHb) were measured using multichannel continuous wave NIRS (ETG 4000; Hitachi, Tokyo, Japan) employing 4 × 4 probe holders for simultaneous measurement of up to 24 channels per hemisphere. Inter-optode distance was 3 cm. Data were acquired over a period of 6 min at a sampling rate of 10 Hz. For both hemispheres, the lowermost seven channels were ignored to avoid signal contamination by the temporalis muscle. According to the international 10–20 system, reference optodes were aligned to F3/4 and C3/4 to cover the cortical distribution territory of the MCA and its transition to the parasagittal territory of the anterior cerebral artery. For visualization, optode positions were determined relative to three anatomical landmarks (nasion, right and left preauricular point) using an electromagnetic digitizer system (Polhemus ISOTRAK II; Inition, London, UK).
Examinations took place in a dark and quiet environment with patients in the supine position. Patients were asked to breathe at a rate of six breaths per minute to reinforce Mayer oscillations in ABP at 0.1 Hz [18]. ABP was simultaneously recorded via servo-controlled finger plethysmograph (Ohmeda 2300; Englewood, USA).
Data analysis
Data analysis was performed using the in-house software based on MATLAB (R2009b; The Mathworks Inc, Natick, MA, USA). ABP and oxyHb time series were bandpass filtered at 0.08–0.12 Hz using a Hamming window-based finite-response filter successively applied in forward and backward directions to avoid phase distortion. The phase shift between the resulting time series was then determined as the temporal mean of the phase difference between the respective analytical signals obtained by Hilbert transformation [19].
Statistical analysis
The interhemispheric difference Δφ in mean phase shift between ABP and oxyHb was tested for statistical significance at the group level using Student’s paired t test. Subgroups were tested for significant differences in Δφ using the unpaired t test. A stepwise permutation method employing Student’s t as the test statistic, applying the maximum statistic approach to correct p values for multiple testing, was used to compare hemispheres on a per region basis [20]. Similarly, statistical inference of the correlation between phase shift and CO2 reactivity (NAR) was conducted by permutation testing employing Pearson’s r as the test statistic and the maximum statistic approach to correct p values [21].
Statistical analysis was performed using MATLAB. Mean values are given as mean ± SD. Throughout the text, φ denotes the phase shift between ABP and oxyHb, and Δφ denotes the interhemispheric difference in phase shift or, equivalently, the phase shift between the affected and contralateral hemisphere.
Results
Steno-occlusion of the left MCA was diagnosed in 11 patients, whereas the right MCA was affected in nine patients. Mean phase shift between ABP and oxyHb averaged over all channels was significantly increased by Δφ = 10.49 ± 17.26° over the affected hemisphere when compared to the contralateral hemisphere (p = 0.014). The smallest increase was found for parasagittal regions B (Δφ = 5.87 ± 15.85) and C (Δφ = 4.26 ± 26.15°) and the largest increase for regions G (Δφ = 16.42 ± 31.35°) and F (Δφ = 14.34 ± 21.38°) in the core distribution of the MCA (Fig. 1a). Statistical significance of the regional differences, however, withstood correction for multiple testing only for region F (p = 0.042).
NIRS data acquisition and analysis. Optodes were arranged on the scalp according to the 10–20 EEG system (a). Red and blue spheres mark light sources and detectors, respectively. Numbers indicate the location of the corresponding channels, each between a source-detector pair. Yellow circles denote the optodes aligned to F3 and C3 over the left hemisphere. Corresponding optodes over the right hemisphere were aligned to F4 and C4. Letters denote areas defined for the evaluation of regional differences. Each area encompassed four adjacent channels. For each channel, the phase shift φ between oscillations in systemic arterial blood pressure (ABP) and oxygenated hemoglobin (oxyHb) was determined as a measure of autoregulatory latency (b)
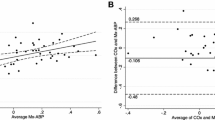
Among the 13 patients suitable for the CO2 inhalation test, three patients displayed a nearly or completely exhausted reactivity (NAR = −19, −0.9, and 4.6) of the affected MCA. In two patients, CO2 reactivity was markedly diminished (NAR = 6.6 and 9.7), whereas in the remaining eight patients, reactivity was normal (NAR = 49.4 ± 12.9). Mean NAR of the non-affected MCA was 59.3 ± 38.6 %. The difference in phase shift between affected and contralateral hemisphere was significantly increased by 22.7 ± 6.4° in patients with markedly diminished or exhausted CVR (NAR < 10) when compared to patients with sufficient CVR (Fig. 2, p = 0.007). A strong negative correlation between NAR of the affected MCA and the interhemispheric difference in phase shift existed for regions E (Fig. 3b, r = −0.78, p = 0.011) and H (r = −0.71, p = 0.04).
Interhemsipheric difference in autoregulatory latency between affected and contralateral hemispheres and cerebrovascular reserve (CVR) capacity in unilateral steno-occlusion of the middle cerebral artery (MCA). Color-coded representation of phase shift φ between systemic arterial blood pressure (ABP) and cortical blood oxygenation in the affected (right) and contralateral (left) hemispheres as determined by near-infrared spectroscopy (a). For region E in the MCA core distribution, Δφ versus normalized autoregulatory response (NAR) is given as a scatter plot with a regression line (red line and 95 % confidence interval) (b)
Discussion
The results of the present study suggest that hemodynamically relevant steno-occlusion of the MCA is associated with a significant delay in autoregulatory response to changes in ABP as assessed in the respective cortical distribution territory by NIRS. Patients with markedly diminished or absent CVR displayed a significantly larger delay than patients with sufficient CVR. Within the MCA core distribution, a strong inter-individual correlation existed between autoregulatory delay and CVR. The delay decreased towards the parasagittal distribution of the anterior cerebral artery. This spatial pattern suggests that multichannel NIRS may allow for pathophysiologically relevant cortical mapping of autoregulatory dysfunction [6]. This may be important in steno-occlusion of MCA branches that cannot be assessed by TCD sonography.
NIRS has previously been suggested as a promising modality for the assessment of cerebral autoregulation in different pathologies and clinical settings [6, 8–14]. Here, we compared an NIRS-based approach for the evaluation of dynamic autoregulation with a standard clinical routine based on TCD. In accordance with a previous study conducted on a macrovascular level [22], static and dynamic testings of cerebral autoregulation in the present study yielded similar results as reflected by a strong correlation between CVR and autoregulatory delay. This correlation may, in part, result from both CVR and autoregulatory delay being dependent on vascular tone. In hemodynamically relevant steno-occlusion, reduced CVR results from persistent arteriolar dilatation, and thus from a decrease in vascular tone. Hypercapnia has been shown to lead to the deceleration of the autoregulatory response to changes in ABP, suggesting that vascular tone may also affect dynamic autoregulation [23]. Whether CVR is more sensitive to clinically relevant hemodynamic compromise in macroangiopathy [24, 25] will have to be investigated in future studies with the long-term follow-up. Future studies may also address the sensitivity of this approach to pathophysiological parameters, such as the individual pattern of collateral flow [26, 27].
The following study limitations should be taken into account: the number of subjects is small, but leads to highly significant results, possibly reflecting adequate sensitivity of this NIRS-based approach. The present NIRS results need to be verified in patients without temporal bone window for TCD.
In conclusion, our results render NIRS a potential alternative to the standard Doppler CO2 test for the identification of hemodynamic risk states in patients with cerebrovascular disease. Although the application of NIRS in the present study is restricted to unilateral steno-occlusion, it supplements clinical practice, as TCD cannot be performed in up to 18–35 % of patients due to insufficient temporal bone windows [28–30]. Further studies evaluating the clinical outcome in patients with macroangiopathy are warranted to assess the sensitivity and reliability of NIRS in clinical routine.
References
Markus HS (2004) Cerebral perfusion and stroke. J Neurol Neurosurg Psychiatry 75(3):353–361
Reinhard M, Schwarzer G, Briel M, Altamura C, Palazzo P, King A, Bornstein NM, Petersen N, Motschall E, Hetzel A, Marshall RS, Klijn CJ, Silvestrini M, Markus HS, Vernieri F (2014) Cerebrovascular reactivity predicts stroke in high-grade carotid artery disease. Neurology 83(16):1424–1431. doi:10.1212/WNL.0000000000000888
Gupta A, Chazen JL, Hartman M, Delgado D, Anumula N, Shao H, Mazumdar M, Segal AZ, Kamel H, Leifer D, Sanelli PC (2012) Cerebrovascular reserve and stroke risk in patients with carotid stenosis or occlusion: a systematic review and meta-analysis. Stroke 43(11):2884–2891. doi:10.1161/STROKEAHA.112.663716
Willie CK, Colino FL, Bailey DM, Tzeng YC, Binsted G, Jones LW, Haykowsky MJ, Bellapart J, Ogoh S, Smith KJ, Smirl JD, Day TA, Lucas SJ, Eller LK, Ainslie PN (2011) Utility of transcranial Doppler ultrasound for the integrative assessment of cerebrovascular function. J Neurosci Methods 196(2):221–237. doi:10.1016/j.jneumeth.2011.01.011
Wolf ME (2015) Functional TCD: regulation of cerebral hemodynamics–cerebral autoregulation, vasomotor reactivity, and neurovascular coupling. Front Neurol Neurosci 36:40–56. doi:10.1159/000366236
Reinhard M, Schumacher FK, Rutsch S, Oeinck M, Timmer J, Mader I, Schelter B, Weiller C, Kaller CP (2014) Spatial mapping of dynamic cerebral autoregulation by multichannel near-infrared spectroscopy in high-grade carotid artery disease. J Biomed Opt 19(9):97005. doi:10.1117/1.JBO.19.9.097005
Reinhard M, Wehrle-Wieland E, Grabiak D, Roth M, Guschlbauer B, Timmer J, Weiller C, Hetzel A (2006) Oscillatory cerebral hemodynamics—the macro- vs. microvascular level. J Neurol Sci 250(1–2):103–109. doi:10.1016/j.jns.2006.07.011
Kainerstorfer JM, Sassaroli A, Tgavalekos KT, Fantini S (2015) Cerebral autoregulation in the microvasculature measured with near-infrared spectroscopy. J Cereb Blood Flow Metab. doi:10.1038/jcbfm.2015.5
Muller MW, Osterreich M, Muller A, Lygeros J (2016) Assessment of the brain’s macro- and micro-circulatory blood flow responses to CO2 via transfer function analysis. Front Physiol 7:162. doi:10.3389/fphys.2016.00162
Obrig H, Neufang M, Wenzel R, Kohl M, Steinbrink J, Einhaupl K, Villringer A (2000) Spontaneous low frequency oscillations of cerebral hemodynamics and metabolism in human adults. Neuroimage 12(6):623–639. doi:10.1006/nimg.2000.0657
Papademetriou MD, Tachtsidis I, Elliot MJ, Hoskote A, Elwell CE (2012) Multichannel near infrared spectroscopy indicates regional variations in cerebral autoregulation in infants supported on extracorporeal membrane oxygenation. J Biomed Opt 17(6):067008. doi:10.1117/1.JBO.17.6.067008
Phillip D, Iversen HK, Schytz HW, Selb J, Boas DA, Ashina M (2013) Altered low frequency oscillations of cortical vessels in patients with cerebrovascular occlusive disease—a NIRS study. Front Neurol 4:204. doi:10.3389/fneur.2013.00204
Pierro ML, Sassaroli A, Bergethon PR, Ehrenberg BL, Fantini S (2012) Phase-amplitude investigation of spontaneous low-frequency oscillations of cerebral hemodynamics with near-infrared spectroscopy: a sleep study in human subjects. Neuroimage 63(3):1571–1584. doi:10.1016/j.neuroimage.2012.07.015
Tian F, Tarumi T, Liu H, Zhang R, Chalak L (2016) Wavelet coherence analysis of dynamic cerebral autoregulation in neonatal hypoxic-ischemic encephalopathy. Neuroimage Clin 11:124–132. doi:10.1016/j.nicl.2016.01.020
Baumgartner RW, Mattle HP, Schroth G (1999) Assessment of ≥50 % and <50 % intracranial stenoses by transcranial color-coded duplex sonography. Stroke 30(1):87–92
Widder B (1989) The Doppler CO2 test to exclude patients not in need of extracranial/intracranial bypass surgery. J Neurol Neurosurg Psychiatry 52(1):38–42
Donahue MJ, Dethrage LM, Faraco CC, Jordan LC, Clemmons P, Singer R, Mocco J, Shyr Y, Desai A, O’Duffy A, Riebau D, Hermann L, Connors J, Kirshner H, Strother MK (2014) Routine clinical evaluation of cerebrovascular reserve capacity using carbogen in patients with intracranial stenosis. Stroke 45(8):2335–2341. doi:10.1161/STROKEAHA.114.005975
Schytz HW, Hansson A, Phillip D, Selb J, Boas DA, Iversen HK, Ashina M (2010) Spontaneous low-frequency oscillations in cerebral vessels: applications in carotid artery disease and ischemic stroke. J Stroke Cerebrovasc Dis 19(6):465–474. doi:10.1016/j.jstrokecerebrovasdis.2010.06.001
Gabor D (1946) Theory of communication. J IEEE 93(26):429–441
Troendle JW (1995) A stepwise resampling method of multiple hypothesis testing. J Am Stat Assoc 90(429). doi:10.1080/01621459.1995.10476522
Groppe DM, Urbach TP, Kutas M (2011) Mass univariate analysis of event-related brain potentials/fields I: a critical tutorial review. Psychophysiology 48(12):1711–1725. doi:10.1111/j.1469-8986.2011.01273.x
Tiecks FP, Lam AM, Aaslid R, Newell DW (1995) Comparison of static and dynamic cerebral autoregulation measurements. Stroke 26(6):1014–1019
Aaslid R, Lindegaard KF, Sorteberg W, Nornes H (1989) Cerebral autoregulation dynamics in humans. Stroke 20(1):45–52
Haubrich C, Klemm A, Diehl RR, Moller-Hartmann W, Klotzsch C (2004) M-wave analysis and passive tilt in patients with different degrees of carotid artery disease. Acta Neurol Scand 109(3):210–216
Hu HH, Kuo TB, Wong WJ, Luk YO, Chern CM, Hsu LC, Sheng WY (1999) Transfer function analysis of cerebral hemodynamics in patients with carotid stenosis. J Cereb Blood Flow Metab 19(4):460–465. doi:10.1097/00004647-199904000-00012
Choi JW, Kim JK, Choi BS, Lim HK, Kim SJ, Kim JS, Suh DC (2010) Angiographic pattern of symptomatic severe M1 stenosis: comparison with presenting symptoms, infarct patterns, perfusion status, and outcome after recanalization. Cerebrovasc Dis 29(3):297–303. doi:10.1159/000275508
Oldag A, Goertler M, Bertz AK, Schreiber S, Stoppel C, Heinze HJ, Kopitzki K (2012) Assessment of cortical hemodynamics by multichannel near-infrared spectroscopy in steno-occlusive disease of the middle cerebral artery. Stroke 43(11):2980–2985. doi:10.1161/STROKEAHA.112.656710
Kaps M, Damian MS, Teschendorf U, Dorndorf W (1990) Transcranial Doppler ultrasound findings in middle cerebral artery occlusion. Stroke 21(4):532–537
Kwon JH, Kim JS, Kang DW, Bae KS, Kwon SU (2006) The thickness and texture of temporal bone in brain CT predict acoustic window failure of transcranial Doppler. J Neuroimaging 16(4):347–352. doi:10.1111/j.1552-6569.2006.00064.x
Wijnhoud AD, Franckena M, van der Lugt A, Koudstaal PJ, Dippel ED (2008) Inadequate acoustical temporal bone window in patients with a transient ischemic attack or minor stroke: role of skull thickness and bone density. Ultrasound Med Biol 34(6):923–929. doi:10.1016/j.ultrasmedbio.2007.11.022
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Ethical standards
The Ethics Committee of the Otto-von-Guericke University Magdeburg approved the protocol for this study and all procedures were in accordance with the ethical standards laid down in the Declaration of Helsinki. All patients provided written informed consent prior to enrollment.
Rights and permissions
About this article
Cite this article
Oldag, A., Neumann, J., Goertler, M. et al. Near-infrared spectroscopy and transcranial sonography to evaluate cerebral autoregulation in middle cerebral artery steno-occlusive disease. J Neurol 263, 2296–2301 (2016). https://doi.org/10.1007/s00415-016-8262-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-016-8262-5