Abstract
We investigated whether vestibular dysfunction may cause or contribute to postural imbalance and falls in patients with Niemann-Pick type C disease (NP-C). Eight patients with NP-C disease and 20 healthy controls were examined using the video-based head impulse test (vHIT) and caloric irrigation to investigate horizontal canal function as well as ocular- and cervical vestibular evoked myogenic potentials (o- and cVEMP), and binocular subjective visual vertical estimation (SVV) for otolith function, and static posturography. There were no significant differences in vestibulo-ocular gain, caloric excitability, o-/cVEMP measures or SVV between the two groups. Posturographic total sway path (tSP) and root mean square (RMS) were significantly higher in NP-C than in controls in 3 out of 4 conditions. The Romberg quotient (RQ) to assess the amount of visual stabilization was significantly lower in the NP-C than in the HC group. In contrast to other inherited metabolic disorders, such as Morbus Gaucher type 3, we did not find any evidence for an impairment of canal or otolith function in patients with NP-C as their cause of postural imbalance. Since RQ was low in NP-C patients, indicating proper sensory input, the observed increased postural sway is most likely due to a cerebellar dysfunction in NP-C, which may therefore, explain postural imbalance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Niemann-Pick type C (NP-C) is a rare, multisystemic disease caused by pathological lipid storage and presenting with systemic, neurologic and psychiatric symptoms [1]. The cardinal symptom in all disease forms is vertical supranuclear saccade palsy, leading to complete gaze palsy in some patients, found in 70 % of patients from an international disease registry [2], but other ocular motor systems can also be impaired [3, 4]. One of the most prominent neurologic symptoms, especially in juvenile and adult forms is postural imbalance and gait disorder with recurrent falls. This may be due to impaired vestibular function, i.e., bilateral vestibulopathy (as in Morbus Gaucher type 3 [5] or chronic progressive external ophthalmoplegia [6]), cerebellar ataxia [2] or a combination of both as in cerebellar ataxia, neuropathy, vestibular areflexia syndrome (CANVAS) [7, 8].
A vestibular deficit is plausible, because hearing is also impaired in patients with NP-C. A prior study showed high-frequency sensorineural hearing loss with retrocochlear involvement, with hearing aids required at least in later stages of the disease [9], however, in the prior sibling studies, a normal vestibulo-ocular reflex (VOR) was observed [10, 11].
Intact function of the vestibular, proprioceptive and cerebellar systems is necessary for good balance and postural stability, which can be assessed by posturography [12]. With this tool, identification of the nature of the balance disturbance and topo-anatomical differentiation of the cerebellar impairment are possible [13].
The function of the vestibular system can be easily quantified nowadays. The angular VOR (aVOR) gain, defined as the ratio of eye velocity to head velocity, can be assessed by the video-based head impulse test (vHIT) in the high-frequency range [14] and by caloric irrigation in the low-frequency range [15]. Otolith function can be examined by ocular vestibular evoked myogenic potentials (oVEMP) for the utricle [16] and cervical VEMP for the saccule [17]. Furthermore, graviceptive pathways can be assessed by examination of the static subjective visual vertical (SVV) with the help of the bedside bucket test [18]. In this study, we systematically examined the function of the vestibular system and postural balance in a cohort of eight patients with NP-C using the abovementioned clinical tools.
Methods
Subjects
This study was conducted at a large tertiary outpatient clinic for vestibular and ocular motor disorders. Six patients with genetically confirmed and two patients with biochemically confirmed NP-C disease (2 females; age 27.3 ± 10.4 years (mean ± SD), range 17–51 years, mean age of onset 9.4 ± 4.4 years, mean age at diagnosis 18.4 ± 13.8 years, mean disease duration 18 ± 12.4 years) were included. Results were compared with those of twenty age-matched healthy controls (HC) (11 females, 28.0 ± 10.9, range 11–57 years), with no history of vestibular, neuro-ophthalmologic or neurologic disease (Table 1). Clinical data of 5 out of 8 patients have been reported elsewhere [19].
Neurological examination
All patients received a thorough neurologic, neuro-ophthalmologic and neuro-otologic examination. To evaluate the overall status of NP-C disease, the modified Disability Rating Scale (mDRS) [20, 21] was administered; cerebellar function was assessed by administration of the Scale for the Assessment and Rating of Ataxia (SARA) [22, 23]. The neurologic examination furthermore involved: examination of the cranial nerves, examination of the reflexes of the upper and lower extremities with pyramidal signs and extremity tonus. Proprioception was assessed clinically by the tuning fork examination, with a normal finding rated as 6-8/8 and pathological proprioception rated as <5/8, and electrophysiologically by measuring somatosensory evoked potentials (SSEP) of the tibial and median nerves. Neuropsychologic status was assessed by the Montreal Cognitive Assessment [24]. All patients underwent brain magnetic resonance imaging (MRI) (Table 1).
The neuro-ophthalmologic and neuro-otologic examination comprised examination with and without Frenzel’s glasses to detect nystagmus, gaze-evoked nystagmus, head-shaking nystagmus, smooth pursuit, saccades, and optokinetic nystagmus.
Ocular and cervical vestibular evoked myogenic potentials (o/cVEMP)
We used the same methodology as the one employed in previous studies [25, 26]. For oVEMP, subjects lay in a supine position and were instructed to foveate a red dot fastened at the minishaker margin during oVEMP stimulation. Tap stimuli were delivered with a Bruel and Kjaer Mini-Shaker Type 4810 (2-ms clicks of positive polarity, with a repetition rate of 2 per second) at the midline of the hairline, 30 % of the distance between the inion and nasion. The recording electrode was positioned over the inferior oblique muscle bilaterally, approximately 3 mm below the eye and centered beneath the pupil; a reference electrode was placed on the chin and a ground electrode under the chin. The responses to 50–100 stimuli were averaged. n1 and p1 were identified as the first negative and positive peaks that occurred between 10 and 20 ms after stimulus onset [27]. For cVEMP, participants were instructed to lift their heads with active straining of the sternocleidomastoid muscles on both sides to provide tonic background muscle activity during stimulation and recording. Air-conducted 500-Hz, 100-dB SPL tone bursts were delivered monaurally via intra-auricular headphones. Cervical VEMP were recorded from an electrode montage consisting of a recording electrode placed at the midpoint of the ipsilateral sternocleidomastoid muscle belly, a reference electrode placed on the manubrium sterni, and a ground electrode placed on the forehead. p1 and n2 peaks were identified as the first positive and negative peaks that occurred between 13 and 23 ms after stimulus onset. Peak-to-peak (PP) amplitudes, calculated as the sum of p1 and n2 peaks, were then divided by mean electromyographic activity recorded after the stimulus onset to check for background muscle activity. Corrected cVEMP PP amplitudes and p1 latencies were evaluated.
Video-based head impulse testing (vHIT)
The aVOR gain (eye velocity/head velocity) [28], assessed by the administration of the HIT with the EyeSeeCam system [29], was evaluated. The course of VOR was visually assessed for the presence of catch-up saccades.
Eye and head movements were recorded monocularly on the left eye with 2-dimensional video-oculography (VOG) [30]. Each participant was instructed to binocularly foveate a visual target comprising a visual angle of 0.3° presented in the center of the 22′′ large computer monitor (LG, FLATRON W2242PK–SS, LG Electronics, Germany) running at 60 Hz. Monitor luminance was 250 cd/m2. The monitor was positioned 60 cm from the participant’s nasion and subtended a visual angle of 43.2° horizontally by 27.7° vertically. Eye dominance was not determined. The VOG system was calibrated for each participant by recording eye fixations at the central and eccentric positions aligned in 8.5° array across a range of ±15°. Calibration recordings were visually inspected to exclude artifacts. 10 ± 2 head impulses were performed to each side.
Caloric testing
Caloric testing was performed with bithermal caloric irrigation (water temperature 30° and 44°, duration of irrigation 30 s). Patients lay in a supine position with their heads turned to the opposite ear during the measurement. Caloric-induced nystagmus was recorded for 2 min by means of the VOG system (EyeSeeCam® [29]). To calculate the slow-phase velocity (SPV) of the caloric-induced nystagmus, eye velocity was calculated using numerical three-point differentiation of eye position and subsequent Gaussian low-pass filtering with a corner frequency of 30 Hz. The high-frequency velocity peaks of nystagmus quick phases, saccades and blink artifacts were removed from eye velocity using an absolute acceleration threshold and subsequent floating median filter with a time window of 0.25 s. For robust extreme value (maximum or minimum) determination, the squared fit was calculated from the SPV data window (±15 s about the extrema), which was previously filtered by a zero-phase digital filter. The peak slow-phase velocity (PSPV) of the caloric-induced nystagmus was calculated as an extreme value of the fitted curve.
All patients underwent caloric irrigation; the results of the caloric testing of 3 out of 8 patients (patients no. 3, 6 and 8) were not quantitatively analyzed due to non-compliance or artifacts.
Directional preponderance was calculated using the standard Jongkees formula [27] and PSPV values <5°/s were considered pathological. The asymmetry ratio was considered abnormal when ≥25 %.
Subjective visual vertical (SVV)
SVV was determined by binocular estimation of the dark straight line at the bottom of the bucket which was rotated clockwise or counterclockwise. A mean of 10 measurements, exceeding the range of values 0° ± 2.3°, was considered a criterion for a pathological SVV tilt [18].
Posturography
The posturographic examination was performed in the upright position with eyes open and closed, on firm ground (conditions 1 and 2) and on a slab of foam rubber (conditions 3 and 4) [12]. The total body sway (tSP) in 30 s of the posturographic measurement, expressed as the sway path values [m/min], root mean square (RMS) [mm] and frequency spectrum between 2.4 and 3.5 Hz (Fast Fourier Transform, FFT) of the z axis (head-vertical) (kgf/Hz) of the measurements were analyzed. The Romberg quotient (RQ), a ratio of the tSP with eyes closed and open, to assess the amount of visual stabilization, was calculated [31].
Statistical methods
Statistical analysis and figure design were performed using SPSS version 22.0.0 (IBM, New York, NY, USA) and MATLAB (The Mathworks Inc). Differences were considered significant if p < 0.05. Normality of data distribution was tested using the mean, median, standard deviation and visual inspection of normal Q–Q plots and box plots. As data were not normally distributed, Wilcoxon related-samples rank test and non-related samples Mann–Whitney U test were conducted. To assess the relationships between tested variables, Spearman rank correlation coefficient was used. Patients who were not physically capable of performing the particular score tasks or who did not perform the test for other reasons were excluded from the analysis.
Results
Angular VOR
Representative raw data of a patient with NP-C and an HC subject are presented in Fig. 1a. The mean aVOR gain in patients with NP-C was 1.07 ± 0.12 and in controls 1.10 ± 0.12 (p = 0.469) (Fig. 2a). There were no statistically significant relationships with other vestibular and neurologic tests (SVV: ρ = 0.250, p = 0.589; oVEMP n1 amplitude: ρ = 0.069, p = 0.727; cVEMP PP amplitude: ρ = 0.163, p = 0.408; SARA: ρ = −0.198, p = 0.670; mDRS: ρ = 0.146, p = 0.729). There was also no correlation with age (ρ = 0.157, p = 0.426), disease duration (ρ = 0.405, p = 0.320) or age of onset (ρ = 0.217, p = 0.606).
Angular vestibulo-ocular reflex, ocular and cervical vestibular evoked myogenic potentials in a patient with Niemann-Pick type C and a control. Representative raw traces of the angular vestibulo-ocular reflex (aVOR) (a), ocular and cervical vestibular evoked myogenic potentials (o- and cVEMP) (b) of a patient with Niemann-Pick type C (patient no. 5) and a healthy control. The blue line (a) represents the mean of performed video-based head impulse tests (vHIT). The red line (b) indicates the function of the otolith organs on the left side, the blue line of those on the right side
Angular vestibulo-ocular reflex, ocular and cervical vestibular evoked myogenic potentials amplitudes and latencies in patients with Niemann-Pick type C and controls. Box plot representation of the angular vestibulo-ocular reflex (aVOR) gain (a), ocular vestibular evoked myogenic potentials (oVEMP) n1 amplitudes and n1 latencies, respectively, (b) and cervical VEMP peak-to-peak (PP) amplitudes and p1 latencies (cVEMP) (c) in patients with Niemann-Pick type C disease (NP-C) and healthy controls (HC). The light green depicts the VEMP amplitudes in HC, the dark green depicts the latencies in HC. The light blue depicts the VEMP amplitudes in NP-C and the dark blue depicts the VEMP latencies in NP-C. No statistically significant differences between the NP-C and HC groups were found for the vestibular measurements. The length of the boxes indicates the interquartile space (P25–P75); the horizontal line into the box represents the median (P50) and the whiskers indicate the adjacent values. The circles indicate the outliers
Caloric irrigation testing
Mean PSPV of caloric-induced nystagmus was 18.5 ± 6.8°/s in response to warm water and 12.6 ± 5.3°/s in response to cold water. After excluding the patient who suffered a left labyrinth contusion with bleeding in 2008 after a bike accident with a left-side canal paresis with PSPV of 2°/s (patient no. 6), the asymmetry ratio was 10 ± 1.6 %.
Ocular and cervical VEMP
Representative raw data of the NP-C and HC subjects are presented in Fig. 1b. Mean oVEMP n1 amplitude was 11.5 ± 5 µV in NP-C and 12.0 ± 4.8 µV (p = 0.784) in HC groups. Mean oVEMP n1 latency was 12.1 ± 1.5 ms in NP-C patients and 10.8 ± 3.1 ms (p = 0.199) in HC subjects. Mean corrected cVEMP PP amplitude was 1.07 ± 0.5 µV in NP-C and 1.1 ± 0.5 µV in HCs (p = 0.862). Mean cVEMP p1 latency was 16.3 ± 1.4 ms in NP-C and 16.6 ± 1.4 ms in HC groups (p = 0.980) (Fig. 2b, c).
Correlation analysis also showed no significant relationships between age of onset (oVEMP n1 amplitude: ρ = −0.639, p = 0.088; n1 latencies: ρ = −0.012, p = 0.977; cVEMP PP amplitudes: ρ = −0.566, p = 0.143; p1 latencies: ρ = 0.446, p = 0.268) and o- and cVEMP amplitudes and latencies, as well as duration of disease (oVEMP n1 amplitudes: ρ = 0.190, p = 0.651; n1 latencies: ρ = 0.262, p = 0.531; cVEMP PP amplitudes: ρ = 0.190, p = 0.651; p1 latencies: ρ = −0.238, p = 0.570) and o- and cVEMP amplitudes and latencies.
There was no relation between neurological status, as assessed by mDRS and SARA and o-/cVEMP parameters in NP-C patients (mDRS: oVEMP n1 amplitude: ρ = −0.586, p = 0.127, n1 latency: ρ = −0.073, p = 0.863; cVEMP PP amplitudes: ρ = −0.488, p = 0.220, p1 latency: ρ = 0.610, p = 0.108; SARA: oVEMP n1 amplitude: ρ = −0.419, p = 0.301, n1 latency: ρ = −0.299, p = 0.471; cVEMP PP amplitudes: ρ = −0.275, p = 0.509, p1 latency: ρ = 0.575, p = 0.136).
Subjective visual vertical
SVV was tilted in three out of eight patients with NP-C. The mean SVV was −0.18 ± 2.9 [(95 % CI for the mean) −2.87 to +2.51] and was thus slightly higher than the range described previously [18]. There was no significant relationship with any of the tests.
Posturography
An overview of the posturographic results of all subjects can be found in Table 2.
Condition 1 (standing on firm ground with eyes open) The tSP was 1.8 ± 0.7 (1.2–2.4) m/min in NP-C patients [mean ± SD, (95 % CI for the mean)] and 0.7 ± 0.1 (0.7–0.8) m/min in controls (Z = −3.818, p = 4.5 × 10−6) (difference: 1.1 m/min), the RMS was 12.5 ± 5.2 (7.7–17.3) mm in NP-C patients and 4.9 ± 1.9 (4.0–5.8) mm in controls (Z = −3.375, p = 2.117 × 10−4) (difference: 7.6 mm). The integral of the frequency spectrum (FFT Z) was 36.9 ± 41.6 (−1.6-75.4) kgf/Hz in NP-C patients and 7.3 ± 3.5 (5.6–8.9) kgf/Hz in controls (Z = −1.881, p = 0.06).
Condition 2 (standing on firm ground with eyes closed) The tSP was 1.8 ± 0.7 (1.1–2.4) m/min in NP-C patients and 0.9 ± 0.1 (0.8–1) m/min in controls (Z = −3.818, p = 4.504 × 10−6) (difference: 0.9 m/min), the RMS was 9.4 ± 5.1 (4.7–14.1) mm in NP-C patients and 5.2 ± 1.9 (4.0–6.1) mm in controls (Z = −2.434, p = 0.013) (difference: 4.2 mm). The FFT Z was 19.4 ± 18.9 (1.9–36.9) kgf/Hz in NP-C patients and 6.1 ± 1.8 (5.3–7) kgf/Hz in controls (Z = −3.150, p = 0.002).
Condition 3 (standing on foam with eyes open) The tSP of NP-C patients yielded 3.8 ± 2.8 (1.3–6.4) m/min and the tSP of controls 1.2 ± 0.3 (1.1–1.3) m/min (difference: 2.6 m/min) (Z = −2.43, p = 1.486 × 10−4). The RMS was 20 ± 6.6 (13.9–26.1) mm in NP-C patients and 8.3 ± 2.7 (7 ± 9.6) mm in controls (difference: 11.7 mm) (Z = −3.486, p = 1.013 × 10−4). The FFT Z was 123.1 ± 131.5 (1.4–244.7) kgf/Hz in NP-C patients and 33 ± 12.8 (27–39) kgf/Hz in controls (Z = −1.771, p = 0.077).
Condition 4 (standing on foam with eyes closed) The tSP of NP-C patients yielded 4.8 ± 2.5 (2.5–7.2) m/min and in controls 3.3 ± 1 (2.8–3.7) m/min (difference: 1.5 m/min) (Z = −1.439, p = 0.162). The RMS was 24.9 ± 7.6 (17.9–32) mm in NP-C and 19.6 ± 27 (16.2 ± 22.9) mm in controls (difference: 5.5 mm) (Z = −1.605, p = 0.109). The FFT Z was 132.8 ± 93.8 (46.1–219.5) kgf/Hz in NP-C patients and 98.2 ± 42.6 (77.6–118.7) kgf/Hz in controls (Z = −0.665, p = 0.506).
The amount of visual stabilization, as assessed by the RQ on both firm ground and foam was significantly different across the groups, being higher in controls (firm ground: NP-C 1.01 ± 0.24 (0.8–1.2), HC 1.27 ± 0.25 (1.2–1.4) p = 0.022; foam: NP-C 1.4 ± 0.6 (0.9–1.9), HC 2.7 ± 0.8 (2.4–3.1), p = 2.117 × 10−4).
In one patient, discrete cerebellar 3 Hz postural sway was seen (patient no 4. in Table 1). This finding has a morphological correlate in a cerebellar atrophy, as seen in MRI.
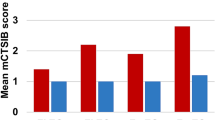
The frequency plot of an NP-C patient, a patient with a cerebellar 3-Hz sway and a healthy subject are shown in Fig. 3a, and a graphical representation of the tSP and RMS values are presented in Fig. 3b.
Posturographic results in patients with Niemann-Pick type C and healthy controls. Frequency plot of the z axis (kgf/Hz) in a patient with Niemann-Pick type C (NP-C) (blue line), a patient with cerebellar sway (red line) and a normal subject (green line) standing on foam with eyes closed (a). Note that the frequency pattern of the NP-C patient is not significantly different from that of a healthy subject with a normal frequency distribution. Bar representation of the differences in total sway path (tSP) and root mean square (RMS) values in patients with Niemann-Pick type C and healthy controls (b). Asterisk indicates significant difference at the 0.05 level. Double asterisk indicates significant difference at the 0.001 level
Correlation analyses
There was no significant association between neurologic status, as assessed by mDRS and SARA, and posturographic parameters in NP-C patients (mDRS: tSP in condition 1: ρ = 0.296, p = 0.518, RMS: ρ = 0.074, p = 0.875; tSP in condition 2: ρ = 0.222, p = 0.632; RMS: ρ = 0.667, p = 0.102, tSP in condition 3: ρ = −0.037, p = 0.937, RMS: ρ = 0.259, p = 0.574; tSP in condition 4: ρ = −0.074, p = 0.875, RMS: ρ = 0.185, p = 0.691; SARA: tSP in condition 1 ρ = 0.559, p = 0.192, RMS: ρ = 0.721, p = 0.068; tSP in condition 2: ρ = 0.126, p = 0.788; RMS: ρ = 0.631, p = 0.129, tSP in condition 3 ρ = −0.180, p = 0.699, RMS: ρ = 0.487, p = 0.268; tSP in condition 4: ρ = −0.595, p = 0.159, RMS: ρ = 0.523, p = 0.229).
No significant relationships between the posturographic and VEMP parameters, but one (tSP value in condition 4 and cVEMP PP amplitudes) were seen (oVEMP n1 amplitude: tSP in condition 1: ρ = 0.170, p = 0.397, RMS: ρ = 0.192, p = 0.338; tSP in condition 2: ρ = −0.036, p = 0.858; RMS: ρ = −0.125, p = 0.536, tSP in condition 3: ρ = 0.148, p = 0.462, RMS: ρ = 0.191, p = 0.340; tSP in condition 4: ρ = 0.001, p = 0.998, RMS: ρ = 0.155, p = 0.440; cVEMP PP amplitudes: tSP in condition 1: ρ = −0.001, p = 0.998, RMS: ρ = 0.018, p = 0.930; tSP in condition 2: ρ = −0.150, p = 0.455; RMS: ρ = −0.270, p = 0.172, tSP in condition 3: ρ = 0.015, p = 0.940, RMS: ρ = 0.051, p = 0.799; tSP in condition 4: ρ = −0.384, p = 0.048, RMS: ρ = −0.281, p = 0.155).
Discussion
The major findings of this study are as follows: first, we did not find any evidence for an impairment of angular VOR function (in either the high-frequency or in the low-frequency range) or otolith function (in either the utricle or the saccule); second, patients showed remarkable postural instability compared with normal subjects; third, analysis of the posturographic findings indicated diffuse cerebellar disturbance with potential involvement of the vestibulo-cerebellum. Based on our results, vestibular horizontal canal and otolith function is intact.
These findings were unexpected in light of the fact that the vestibular system is commonly affected in neurodegenerative disorders [32, 33]. In fact, in another lysosomal storage disease, Gaucher disease type 3 (neuronopathic type, GD3), impaired otolith pathways and aVOR deficits with absent horizontal refixation saccades were described [5]. This might be explained by the neuronal loss and functional disturbance of the vestibular nuclei in the medulla and paramedian pontine reticular formation (PPRF), respectively, leading to horizontal saccade palsy in GD3 disease. Vestibular nuclei and their afferent and efferent projections seem to be functionally intact in NP-C disease. NP-C is a chronic progressive disease with a relatively slow progressive rate, which might explain the lack of vestibular involvement, also seen in acute cerebellar disturbance caused by a stroke or cerebellitis [34, 35]. Vestibular regions analogous to the ocular motor nuclei and supranuclear structures for gaze-holding, such as the interstitial nucleus of Cajal (INC), or the nucleus prepositus hypoglossi (NPH), and centers for horizontal saccades are relatively spared by the disease process, nevertheless, as the disease progresses, these structures might also degenerate at more severe stages of the disease. Thus, the specific pattern of impairment in NP-C and GD3 diseases suggests a different neuronal susceptibility to the toxic effects of the storage material, probably based on their biochemical properties. For instance, downward saccades are often more impaired than upward saccades in NPC disease [36]. This might be explained by a calretinin-positive excitatory input to motor centers mediating upgaze, arising from premotor centers, such as the INC, the rostral interstitial nucleus of the medial longitudinal fascicle (riMLF) and y-group [37–39]. Calretinin is a calcium-binding protein, but its functional significance is yet unclear. We can speculate whether it contributes to an improvement of the lipid storage in upward neurons, since there is a well-known calcium dyshomeostasis in patients with NP-C [40]. The lack of this input in downgaze pathways might explain the accentuated downgaze deficits in NP-C disease [41]. In addition, a lack of correlation between any of the vestibular tests and clinical rating scales also supports the intactness of the vestibular organs.
In terms of posturography, we found that patients with NP-C had increased body sway compared with controls in three out of four conditions. Disturbance of the somatosensory input in condition four led to an equal increase of the total sway path in both groups. The effect of vision was significantly more pronounced in controls, since the Romberg quotient was significantly higher in controls than in patients with NP-C. This finding also indicates the functionally intact vestibular organs, as visual cue is known to be of high importance in peripheral vestibulopathy to compensate for the vestibular loss [12, 42]. Moreover, due to the saccadic deficits, motor performance and orientation in space that require visual-vestibular interaction in patients with NP-C are impaired. It is likely that the balance network compensates for these ocular deficits by enhancing the other sensory input, especially vestibular and somatosensory input. Balance is not based on a fixed set of equilibrium reflexes but on a flexible, functional motor skill that can adapt with training and experience, and presumably reflects noise and regulatory activity within afferent-efferent control loops, which are plastic enough to compensate for the existing deficits.
Visual stabilization had no remarkable influence on the postural stability, as indicated by the low Romberg quotient of the total sway path in patients with NP-C, even though the proprioception was diminished in three patients. A low proportion of visual stabilization suggests that the spino-cerebellum and its spinal afferents are not primarily affected by the disease process. Nevertheless, atrophy of the cerebellar vermis in severe cases of NP-C disease has been previously described [43, 44].
In the frequency analysis, there was no consistent 3 Hz anteroposterior cerebellar sway in patients with NP-C. One patient with cerebellar atrophy in MRI had increased postural sway at 2–3 Hz frequency, but he did not reach values seen in patients with anterior lobe lesions [13].
On the whole, the constellation of the posturographic findings (pathological sway parameters with poor visual stabilization without 3 Hz sway) suggests a rather diffuse cerebellar disturbance with a possible involvement of the vestibulo-cerebellum, rather than isolated impairment of the spino-cerebellum, anterior lobe or cerebellar hemispheres [13, 45].
In contrast, a patterned degeneration of the Purkinje cells from anterior to posterior, with surviving Purkinje cells in lobules IX and X at the terminal stages of the disease has been described in an NP-C mouse model [46]. Our NP-C cohort was relatively young (mean age ± SD 27.3 ± 10.4 years) with a rather mild cerebellar disturbance (mean SARA score 9.3/40) and lacking severe involvement of any circumscribed region of the cerebellum. Thus, the pattern of neurodegeneration described in the mouse model could not be observed.
The isolated cerebellar impairment without vestibular involvement is also in line with previously shown increased metabolism in the vestibulo-cerebellum in a rat model of peripheral vestibulopathy under therapy with acetyl-DL-leucine [47]. The beneficial effect of this therapy was seen recently in a cohort of 12 NP-C patients [19], improving stance and gait, diadochokinesis and diminishing the intensity of the square wave jerks, indicating a stabilizing effect on impaired cerebellar Purkinje cells, similar to the effect on neurons of medial vestibular nucleus in an unilateral-vestibular-loss guinea-pig model [48]. This study has some limitations. First, the sample size is small, given that the NP-C disease is an orphan disease, meaning that this study might be underpowered. Second, as patients become fatigued very quickly, some tests were not performed due to the lack of compliance or due to physical disability or cognitive impairment. Third, there is an ongoing discussion about the sensitivity and specificity of VEMP investigation, principally because of its high interindividual, but also interrater variability [49].
References
Vanier MT (2013) Niemann-Pick diseases. Handb Clin Neurol 113:1717–1721. doi:10.1016/B978-0-444-59565-2.00041-1
Patterson MC, Mengel E, Wijburg FA et al (2013) Disease and patient characteristics in NP-C patients: findings from an international disease registry. Orphanet J Rare Dis 8:12. doi:10.1186/1750-1172-8-12
Abel LA, Walterfang M, Fietz M et al (2009) Saccades in adult Niemann-Pick disease type C reflect frontal, brainstem, and biochemical deficits. Neurology 72:1083–1086. doi:10.1212/01.wnl.0000345040.01917.9d
Rottach KG, von Maydell RD, Das VE et al (1997) Evidence for independent feedback control of horizontal and vertical saccades from Niemann-Pick type C disease. Vision Res 37:3627–3638. doi:10.1016/S0042-6989(96)00066-1
Chen L, Halmagyi GM, Todd MJ, Aw ST (2014) Vestibular and saccadic abnormalities in Gaucher’s disease. JIMD Rep 13:111–118. doi:10.1007/8904_2013_264
Ritchie AE, Griffiths PG, Chinnery PF, Davidson AW (2010) Eye movement recordings to investigate a supranuclear component in chronic progressive external ophthalmoplegia: a cross-sectional study. Br J Ophthalmol 94:1165–1168. doi:10.1136/bjo.2009.165639
Kirchner H, Kremmyda O, Hufner K et al (2011) Clinical, electrophysiological, and MRI findings in patients with cerebellar ataxia and a bilaterally pathological head-impulse test. Ann N Y Acad Sci 1233:127–138. doi:10.1111/j.1749-6632.2011.06175.x
Szmulewicz DJ, Waterston JA, Halmagyi GM et al (2011) Sensory neuropathy as part of the cerebellar ataxia neuropathy vestibular areflexia syndrome. Neurology 76:1903–1910. doi:10.1212/WNL.0b013e31821d746e/WNL.0b013e31821d746e
King KA, Gordon-Salant S, Yanjanin N et al (2014) Auditory phenotype of Niemann-Pick disease, type C1. Ear Hear 35:110–117. doi:10.1097/AUD.0b013e3182a362b8
Lengyel D, Weissert M, Schmid L, Gottlob I (1999) Eye movement abnormalities as a sign for the diagnosis in Niemann-Pick disease type C. Klin Monbl Augenheilkd 214:50–52. doi:10.1055/s-2008-1034748
Solomon D, Winkelman AC, Zee DS et al (2005) Niemann-Pick type C disease in two affected sisters: ocular motor recordings and brain-stem neuropathology. Ann N Y Acad Sci 1039:436–445. doi:10.1196/annals.1325.041
Krafczyk S, Tietze S, Swoboda W et al (2006) Artificial neural network: a new diagnostic posturographic tool for disorders of stance. Clin Neurophysiol 117:1692–1698
Diener HC, Dichgans J, Bacher M, Gompf B (1984) Quantification of postural sway in normals and patients with cerebellar diseases. Electroencephalogr Clin Neurophysiol 57:134–142
Agrawal Y, Schubert MC, Migliaccio AA et al (2014) Evaluation of quantitative head impulse testing using search coils versus video-oculography in older individuals. Otol Neurotol 35:283–288. doi:10.1097/MAO.0b013e3182995227
Halmagyi GM, Cremer PD, Anderson J et al (2000) Isolated directional preponderance of caloric nystagmus: I. Clinical significance. Am J Otol 21:559–567
Curthoys IS, Vulovic V, Manzari L (2012) Ocular vestibular-evoked myogenic potential (oVEMP) to test utricular function: neural and oculomotor evidence. Acta Otorhinolaryngol Ital 32:41–45
Rosengren SM, Kingma H (2013) New perspectives on vestibular evoked myogenic potentials. Curr Opin Neurol 26:74–80. doi:10.1097/WCO.0b013e32835c5ef3
Zwergal A, Rettinger N, Frenzel C et al (2009) A bucket of static vestibular function. Neurology 72:1689–1692
Bremova T, Malinová V, Amraoui Y et al (2015) Acetyl-dl-leucine in Niemann-Pick type C: a case series. Neurology 85:1368–1375. doi:10.1212/WNL.0000000000002041
Iturriaga C, Pineda M, Fernández-Valero EM et al (2006) Niemann-Pick C disease in Spain: clinical spectrum and development of a disability scale. J Neurol Sci 249:1–6. doi:10.1016/j.jns.2006.05.054
Pineda M, Wraith JE, Mengel E et al (2009) Miglustat in patients with Niemann-Pick disease Type C (NP-C): a multicenter observational retrospective cohort study. Mol Genet Metab 98:243–249. doi:10.1016/j.ymgme.2009.07.003
Subramony SH (2007) SARA–a new clinical scale for the assessment and rating of ataxia. Nat Clin Pract Neurol 3:136–137. doi:10.1038/ncpneuro0426
Weyer A, Abele M, Schmitz-Hübsch T et al (2007) Reliability and validity of the scale for the assessment and rating of ataxia: a study in 64 ataxia patients. Mov Disord 22:1633–1637. doi:10.1002/mds.21544
Nasreddine ZS, Phillips NA, Bédirian V et al (2005) The montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 53:695–699. doi:10.1111/j.1532-5415.2005.53221.x
Agrawal Y, Bremova T, Kremmyda O, Strupp M (2013) Semicircular canal, saccular and utricular function in patients with bilateral vestibulopathy: analysis based on etiology. J Neurol 260:876–883. doi:10.1007/s00415-012-6724-y
Bremova T, Bayer O, Agrawal Y et al (2013) Ocular VEMPs indicate repositioning of otoconia to the utricle after successful liberatory maneuvers in benign paroxysmal positioning vertigo. Acta Otolaryngol 133:1297–1303. doi:10.3109/00016489.2013.829922
Jongkees LB, Maas JP, Philipszoon AJ (1962) Clinical electronystagmography: a detailed study of electronystagmography in 341 patients with vertigo. Pract Otorhinolaryngol Basel 24:65–93
Aw ST, Haslwanter T, Halmagyi GM et al (1996) Three-dimensional vector analysis of the human vestibuloocular reflex in response to high-acceleration head rotations. I. Responses in normal subjects. J Neurophysiol 76:4009–4020
Schneider E, Villgrattner T, Vockeroth J et al (2009) EyeSeeCam: an eye movement-driven head camera for the examination of natural visual exploration. Ann N Y Acad Sci 1164:461–467. doi:10.1111/j.1749-6632.2009.03858.x
Bartl K, Lehnen N, Kohlbecher S, Schneider E (2009) Head impulse testing using video-oculography. Ann N Y Acad Sci 1164:331–333. doi:10.1111/j.1749-6632.2009.03850.x
Njiokiktjien CJ, Van Parys JA (1976) Romberg’s sign expressed in a quotient. II. Pathology. Agressologie 17:19–23
Shaikh AG, Marti S, Tarnutzer AA et al (2011) Ataxia telangiectasia: a “disease model” to understand the cerebellar control of vestibular reflexes. J Neurophysiol 105:3034–3041. doi:10.1152/jn.00721.2010
Strupp M, Kremmyda O, Adamczyk C et al (2014) Central ocular motor disorders, including gaze palsy and nystagmus. J Neurol 261(Suppl 2):542–558. doi:10.1007/s00415-014-7385-9
Choi J-Y, Kim J-S, Jung J-M et al (2013) Reversed corrective saccades during head impulse test in acute cerebellar dysfunction. Cerebellum 13:243–247. doi:10.1007/s12311-013-0535-2
Choi SY, Lee S-H, Kim HJ, Kim J-S (2014) Impaired modulation of the otolithic function in acute unilateral cerebellar infarction. Cerebellum 13:362–371. doi:10.1007/s12311-013-0544-1
Solomon D, Winkelman AC, Zee DS et al (2005) Niemann-pick type C disease in two affected sisters: ocular motor recordings and brain-stem neuropathology. Ann N Y Acad Sci 1039:436–445. doi:10.1196/annals.1325.041
Che Ngwa E, Zeeh C, Messoudi A et al (2014) Delineation of motoneuron subgroups supplying individual eye muscles in the human oculomotor nucleus. Front Neuroanat 8:2. doi:10.3389/fnana.2014.00002
Zeeh C, Hess BJ, Horn AKE (2013) Calretinin inputs are confined to motoneurons for upward eye movements in monkey. J Comp Neurol 521:3154–3166. doi:10.1002/cne.23337
Zeeh C, Mustari MJ, Hess BJM, Horn AKE (2015) Transmitter inputs to different motoneuron subgroups in the oculomotor and trochlear nucleus in monkey. Front Neuroanat 9:95. doi:10.3389/fnana.2015.00095
Lloyd-Evans E, Morgan AJ, He X et al (2008) Niemann-Pick disease type C1 is a sphingosine storage disease that causes deregulation of lysosomal calcium. Nat Med 14:1247–1255. doi:10.1038/nm.1876
Adamczyk C, Strupp M, Jahn K, Horn AKE (2015) Calretinin as a marker for premotor neurons involved in Upgaze in human brainstem. Front Neuroanat 14(9):153. doi:10.3389/fnana.2015.00153
Nashner LM, Black FO, Wall CI (1982) Adaptation to altered support and visual conditions during stance: patients with vestibular deficits. J Neurosci 2:536–544
Huang J-Y, Peng S-F, Yang C-C et al (2011) Neuroimaging findings in a brain with Niemann-Pick type C disease. J Formos Med Assoc 110:537–542. doi:10.1016/S0929-6646(11)60080-6
Fusco C, Russo A, Galla D et al (2013) New Niemann-Pick Type C1 gene mutation associated with very severe disease course and marked early cerebellar vermis atrophy. J Child Neurol 28:1694–1697. doi:10.1177/0883073812462765
Schwesig R, Becker S, Lauenroth A, Kluttig A, Leuchte S, Esperer HD (2009) A novel posturographic method to differentiate sway patterns of patients with Parkinson’s disease from patients with cerebellar ataxia. Biomed Tech 54:347–356. doi:10.1515/BMT.2009.041
Sarna JR, Larouche M, Marzban H et al (2003) Patterned Purkinje cell degeneration in mouse models of Niemann-Pick type C disease. J Comp Neurol 456:279–291. doi:10.1002/cne.10522
Günther L, Beck R, Xiong G et al (2015) N-acetyl-L-leucine accelerates vestibular compensation after unilateral labyrinthectomy by action in the cerebellum and thalamus 10(3):e0120891. doi:10.1371/journal.pone.0120891
Vibert N, Vidal PP (2001) In vitro effects of acetyl-DL-leucine (tanganil) on central vestibular neurons and vestibulo-ocular networks of the guinea-pig. Eur J Neurosci 13:735–748
Ertl M, Boegle R, Kirsch V, Dieterich M (2016) On the impact of examiners on latencies and amplitudes in cervical and ocular vestibular-evoked myogenic potentials evaluated over a large sample (N = 1,038). Eur Arch Oto Rhino Laryngol 273:317–323
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical standards
The study was performed in accordance with the Helsinki II Declaration and was approved by the ethics committee of the Ludwig-Maximilians University Medical Faculty (No. 379-12). All participants gave their informed consent prior to their inclusion in the study.
Conflicts of interest
T.B. received honoraria for lecturing from Actelion. S.K. and S.B. have nothing to report. J.R. received speaker’s honoraria from BioMarin, Shire, Genzyme and Actelion. M.S. is Joint Editor-in-Chief of the Journal of Neurology, Editor-in-Chief of Frontiers of Neuro-otology and Section Editor of F1000. He received speaker’s honoraria from Abbott, Actelion, UCB, GSK, TEVA, Heel, Biogen, Pierre-Fabre, Eisai and Hennig Pharma. He also works as a consultant for Abbott, Heel, Synthon and Actelion.
Rights and permissions
About this article
Cite this article
Bremova, T., Krafczyk, S., Bardins, S. et al. Vestibular function in patients with Niemann-Pick type C disease. J Neurol 263, 2260–2270 (2016). https://doi.org/10.1007/s00415-016-8247-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-016-8247-4