Abstract
Multiple sclerosis (MS) is an immune-mediated disease of the central nervous system with a complex and heterogeneous pathology that may ultimately lead to neurodegeneration and brain atrophy. Brain volume loss in MS is known to occur early in the disease course and to be clinically relevant, as it has been related to disability progression. Nowadays, brain volume loss is relatively easy to measure with different automated, reproducible and accurate software tools. Therefore, most of (if not all) the newest clinical trials have incorporated brain volume outcomes as a measure of treatment effect. With this review, we aimed to update and summarize all existing data regarding brain volume and RRMS treatment in clinical trials as well as in open-label observational studies of drugs with positive results in its primary outcome in at least one phase III trial as of March 2014.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
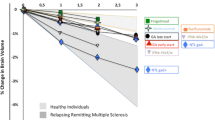
Multiple sclerosis (MS) is an immune-mediated disease of the central nervous system with a complex and heterogeneous pathology consisting in a combination of inflammation, demyelination, gliosis and axonal loss. These processes may ultimately lead to neurodegeneration, tissue damage and brain atrophy [1]. Brain atrophy is a common and early feature in MS patients; it occurs at an accelerated rate when compared with healthy controls, and it is clinically relevant as it has been related to disability [2, 3]. Current methods for measuring brain volume (BV) are usually automated softwares that either use a segmentation-based approach [such as the statistical parametric mapping (SPM) or SIENAX] for cross-sectional data or a registration-based approach (such as the structural imaging evaluation using normalization of atrophy—SIENA) for longitudinal analysis. BV measures yielded by the above-mentioned programs will be: percentage of BV change (PBVC) for the SIENA software, normalized BV (NBV) for SIENAX, and brain parenchymal fraction (BPF), grey matter fraction (GMF) and white matter fraction (WMF) for SPM software. These methodologies have gone through extensive testing and provide us now with an accurate, reproducible and efficient way of measuring in vivo this clinically relevant neurodegenerative process [4].
Thus, it is not surprising that recent clinical trials have incorporated BV outcomes as a measure of treatment effect [5]. A number of the disease modifying drugs available for MS patients, specially the newest ones, have shown to improve brain atrophy accrual when compared to placebo. In recent years, the amount of drugs released to the market for treating relapsing–remitting MS (RRMS) patients has grown considerably. In this review, we aimed to summarize all existing data regarding BV and RRMS treatment in clinical trials as well as in open-label observational studies of drugs with positive results in its primary outcome in at least one phase III trial as of March 2014. Most of the studies reported in this review have used the software packages mentioned above, with their respective measures, but in some, in-house software packages have been used.
Effect of therapies on BV: clinical trials data
Interferon beta
Brain volume analysis in subcutaneous (sc) interferon beta (IFN-β) clinical trials on clinically isolated syndromes (CIS) patients, yielded contradictory results. Compared to placebo, patients treated with a weekly dose of 22 μg sc IFN-β-1a in the ETOMS clinical trial, had a lower BV loss during the 2-year period analysed (PBVC: −1.18 versus –1.68 %; p = 0.0031) [6]. Surprisingly, the REFLEX clinical trial, evaluating the same IFN-β formulation, found no differences in BV loss over 2 years in patients receiving either weekly or three times a week high dose (44 μg) of sc IFN-β-1a as compared to the placebo arm [7]. In fact, patients receiving the highest frequency of interferon treatment seemed to have the largest loss of BV during the 2-year period of observation [7]. As for the sc IFN-β-1b clinical trial, BV results were only reported at year 3 and 5 after inclusion to the study [8, 9]. The study showed no differences in BV loss when comparing early versus delayed treatment, but no strict comparison to placebo was carried out [8, 9] (Table 1a). BV data for intramuscular (im) IFN-β-1a in CIS patients is not available.
Regarding RRMS treatment, the pivotal trial of im IFN-β-1a was the first to report atrophy outcomes [10]: although no significant differences were noted during the first year of treatment, IFN-β-treated patients presented lower BV loss during the second year of treatment as compared to placebo (−0.23 % in IFN-β-treated patients versus −0.51 % in the placebo arm, p = 0.03). When comparing first and second year of treatment, BV loss occurred at a different rate in IFN-β-treated patients with a greater volume loss occurring in the first year of treatment while there was no difference in BV loss rate between the two 1-year periods in the placebo arm. Along these lines, the European dose comparison study [11] evaluated BV changes during the first 3 years after starting im IFN-β-1a yielding similar results: a higher decrease of BPF occurred during the first year of treatment with the largest BV loss taking place in the first 4 months of therapy (Tables 1b, 2). The authors of these two studies evaluated the presence of inflammation as a possible confounder of subsequent BV loss and results were only partially in agreement. In one of the studies a correlation between the number of gadolinium-enhancing lesions at baseline and in-trial BV loss was observed but no significant correlation was found between the in-trial change in gadolinium-enhancing lesion volume and in-trial BV loss. Whereas in the other study, the rate of BV loss during the first months of treatment was paralleled by a drop in the number of gadolinium-enhancing lesions [10, 11]. The PRISMS clinical trial, evaluating two doses of sc IFN-β-1a versus placebo for treating RRMS patients, analysed BV measures in a long-term follow-up of up to 8 years [12]. Bearing in mind all the confounders inherent to these long-term designs, there were no differences in BV from baseline to last follow-up visit between all three arms. However, it is worth noting that, as previously described in the REFLEX CIS trial, during the double-blind phase (first 2 years) as well as when changing from placebo to active treatment in the open-label phase (from 2 to 4 years) BV loss was greater in the 44-μg group. The two pivotal clinical trials of IFN-β-1a [10, 12] found that, independently of treatment allocation, patients presenting higher disability progression were also those having greater BV loss than patients who did not progress. Data regarding treatment effect on BV loss for sc IFN-β-1b clinical trials in RRMS has not been published.
Glatiramer acetate
Glatiramer acetate (GA) demonstrated its efficacy for treating CIS patients in the PRECISE clinical trial [13]. The primary analysis of brain MRI outcomes allowed a strict comparison with placebo as it was performed using only the scans obtained before presenting a second relapse; with this measure the authors tried to correct for the confounding factor of starting open-label therapy in patients with RRMS regardless of the original group in which they were allocated. Compared to placebo, GA failed to prove reduction in BV loss measured as PBVC (−0.38 versus −0.33 %) [13]. However, a pre-planned open-label analysis showed that early treatment with GA significantly reduced brain atrophy when compared to patients with delayed treatment onset adjusting for study exposure (−0.99 versus −1.28 %, p = 0.021) [14] (Table 1a).
The first report of GA effects on BV in RRMS was in a subcohort of patients participating in the GA US Trial; in this small cohort, GA significantly reduced the rate of BV loss in the 2-year treatment period [15]. The initial analysis of the European/Canadian GA trial, measuring BV on a central portion of the brain with a semi-automated segmentation technique, showed no differences between placebo and GA-treated patients [16]. A posterior assessment of the same trial but using the SIENA software, showed a protective effect of GA in BV decrease at the end of the observation period (18 months); this beneficial effect was mainly due to a reduction of BV loss during the open-label phase in early treated patients [17]. These differences were no longer held when evaluating BV loss at 5 years after study entry [18] (Tables 1b, 2). The FORTE trial that evaluated two doses of GA (20 versus 40 mg administered daily) found no differences in BV measures between low- and high-dose treatment arms [19].
Three large studies have compared IFN-β formulations and GA, showing similar performance for both drugs in clinical and MRI outcomes [20–22]. Brain atrophy was assessed in all three trials, one study could demonstrate a significant reduction in BV loss for GA-treated patients as compared to sc IFN-β-1a [20], but no differences were observed in the other two [21, 22] (Tables 1b, 2). Noteworthy, in all these three trials most of the BV loss occurred during the first 6–12 months of therapy.
Natalizumab
Natalizumab was the first monoclonal antibody approved for the treatment of MS after proving its efficacy in two phase III clinical trials. Both trials reported similar results regarding atrophy data and demonstrated again an interesting pattern of BV loss in the active arm: compared to placebo, natalizumab-treated patients presented greater BV loss during the first year of the trial, whereas significantly lower rates of BV decrease during the second year of treatment were observed [23, 24]. This was interpreted by the authors as an initial pseudoatrophy effect and a later protective effect of natalizumab in preventing brain atrophy [23, 24], and is also consistent with the similar pattern observed in some IFN-β trials (Tables 1b, 2).
Fingolimod
Fingolimod was the first oral drug approved to treat MS patients; three phase III clinical trials (FREEDOMS, FREEDOMS II and TRANSFORMS) demonstrated its efficacy not only regarding inflammation parameters but also in reducing BV loss [25–27]. Compared to placebo, fingolimod significantly reduced BV loss down to 30–45 % after 2 years of treatment and this reduction was observed as early as 6 months after treatment onset [25, 27], specially in patients without baseline gadolinium-enhancing lesions [28] (Tables 1b, 2). Patients with baseline inflammation showed higher rates of BV loss during the first year of therapy, but this BV loss was never greater than the placebo arm [28]. Compared to im IFN-β-1a, patients receiving fingolimod also presented less BV loss during the first year of treatment [26] (Tables 1b, 2). These differences were held when subgroup analyses were performed [29]. In the extension study of the TRANSFORMS trial, patients switching from im IFN-β-1a to fingolimod treatment reduced their BV loss rate and no differences in BV loss between the core and the extension phase for patients continuing on fingolimod were observed [30].
Newest oral drugs
Results of brain atrophy for both phase III clinical trials with dimethyl-fumarate have been recently published [31, 32]. In the DEFINE clinical trial, comparing dimethyl-fumarate versus placebo in RRMS, and using the 6-month MRI as baseline for BV estimation, dimethyl-fumarate administered twice a day (BID) significantly reduced BV loss as compared to placebo; surprisingly, results for the three times a day (TID) posology on brain atrophy resulted negative [31]. In the CONFIRM trial, BV loss was analysed at different time-points: from baseline to the end of the trial (2 years), from baseline to year 1 and from year 1 to year 2. Compared to placebo, the BID dose seemed to reduce BV loss from baseline to year 2 (−0.660 vs. −0.945; p = 0.0645) and significantly reduced BV loss during the last year of follow-up (year 1 to year 2 of the clinical trial). Neither the TID dose nor glatiramer acetate significantly reduced BV loss at any point compared to placebo, although a trend towards statistical significance was observed from year 1 to year 2 for both drugs [32] (Tables 1b, 2).
Regarding teriflunomide, BV measures were only reported for the phase III TEMSO clinical trial: both doses of teriflunomide (7 and 14 mg.) failed to demonstrate a reduction in BV loss as compared to placebo [33] (Tables 1b, 2). However, when analysing not only global BV loss, but also tissue-specific BV changes, a significant reduction of white matter (WM) loss for both doses of teriflunomide as compared to placebo was observed [34].
As for laquinimod, its effect on BV loss was assessed in two phase III clinical trials [35, 36]. In the ALLEGRO clinical trial, adjusting for the baseline number of gadolinium-enhancing lesions, laquinimod significantly reduced BV loss as compared to placebo [35]. In the BRAVO study an active control arm with im IFN-β-1a for descriptive analysis was included: compared to placebo, laquinimod demonstrated a protective effect on BV loss reduction; conversely, IFN-β-1a failed to protect against BV loss, even showing non-significant greater reductions in BV compared to placebo [36] (Tables 1b, 2).
Newest monoclonal antibodies
Brain volume effects of alemtuzumab were first analysed in the phase II clinical trial CAMSS223: compared to 44 μg sc IFN-β-1a, alemtuzumab-treated patients showed a reduction in BV loss during the 3 years of the trial. BV changes occurring in the last 2 years of follow-up were also analysed (12–36 months) to find an even larger protective effect of alemtuzumab on BV loss [37]. Similar results, favouring alemtuzumab-treated patients compared to 44 μg sc IFN-β-1a, were obtained in both phase III CARE-MS-I and CARE-MS-II clinical trials [38, 39] (Tables 1b, 2). It is worth mentioning that brain volume loss reduction relative to 44 μg sc IFN-β-1a was more marked for treatment-naïve patients (about 40 % reduction in CARE-MS-I vs. 25 % reduction in CARE-MS-II) [38] and for patients originally randomized to the 24 mg arm (CARE-MS-II) [39].
Effect of therapies on BV: open-label observational studies
Interferon beta and glatiramer acetate
First open-label reports on BV changes under treatment were performed with the two formulations of sc IFN-β and with no control group [40, 41]. Both studies found that a greater BV loss occurred during the first months of therapy with a posterior slow down, specially after the second year of treatment [40, 41]; these findings were not modified by the presence of IFN-β neutralizing antibodies [41]. In one study, BV loss occurring during the first 6 years of therapy moderately correlated with EDSS worsening during the same time period; however, the authors did not find any early MRI variable that could predict disability progression over time [40]. More recent open-label studies did include a control group consisting of RRMS patients who decided not to start any treatment [42–44]. Despite the limitations of open-label studies, all injectable DMDs were shown to reduce global BV loss [42–44] and grey matter (GM) atrophy [42, 43] as compared to patients who did not receive any treatment. Whereas one of the studies seemed to favour IFN-β treatment on preventing GM pathology (specially development of new cortical lesions) [43], another study showed a larger effect of GA on reducing global BV [44]. Only one study assessed the effect of BV loss in predicting treatment response; the authors of this study found that BV loss occurring during the first year of IFN-β therapy significantly increased the risk of presenting treatment failure at year 3 [45].
Natalizumab
Similar to what has been described in natalizumab clinical trials [23, 24], three observational studies with no control group confirmed that most of the BV loss occurring while on natalizumab treatment takes place during the first months of therapy, and found that it was related to baseline clinical [46] and radiological [47], [48] disease activity. Specifically, the number of baseline gadolinium-enhancing lesions predicted global and WM but not GM volume loss during the first [47] and second [48] year of therapy. In a study comparing natalizumab-treated patients with patients treated with injectable therapies (IFN-β and GA) and to untreated patients, natalizumab significantly reduced the number of new cortical lesions as well as cortical thinning over a 2-year treatment period [49]. In one study, the reduction in global and cortical volume loss was associated with a lower cognitive deterioration during the same period [50].
Discussion
Using automated techniques to measure BV changes, differences between placebo and treated arms have been shown in randomized clinical trials for some of the presently available disease modifying MS therapies; head-to-head trials have also shown superiority of some drugs over active comparators. Even though BV measures have been shown to be accurate and reproducible, a number of issues should be taken into account when interpreting therapy effects on BV changes.
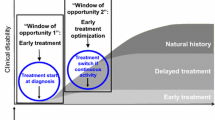
Among methodological aspects, it is worth mentioning the evolution of analysis techniques as well as improvements in the acquisition of images; as it has been shown for the GA trial in RRMS, improvements in the analysis techniques may increase the power of the studies so as to observe previously undetectable treatment effects [15, 16]. Even more, it should be taken into consideration that some of the earliest trials do not feature BV data because of the insurmountable difficulties of multicentre analyses of such kind at that time. Another important aspect refers to the methodology used to obtain BV change estimates, as a number of automated techniques have been used and both BPF (obtained with a number of different software tools) and PBVC measures have been obtained. Although most techniques have demonstrated to be accurate and reproducible [51], segmentation-based techniques (used in a number of studies reported here) are not as robust as registration-based techniques for longitudinal studies [52] and, in any case, the global magnitude of treatment effect cannot be compared across trials. Other physiological and disease-related factors that can influence BV changes should also be taken into account; in this regard, a well-known source of variation is the hydration status or the presence of on-going inflammation at treatment onset. Patients participating in clinical trials or starting treatment in clinical practice are usually active patients with clinical relapses and presence of MRI activity as demonstrated by gadolinium-enhancing lesions. Resolution of this inflammation will lead to an initial accelerated BV loss that has been described as a ‘pseudoatrophy’ effect [5]. Along these lines, drugs with a high impact on inflammation, such as natalizumab [23, 24, 46–48], fingolimod [28] or high-dose IFN-β [7, 12], will tend to produce larger than placebo BV decreases during the first months of therapy (specially in patients presenting with gadolinium-enhancing lesions [28, 47, 48]) that, at least in part, may be not related to true tissue damage.
It is also worth mentioning that therapy effects on BV loss in CIS patients may be even more difficult to interpret, not only because of the design of the clinical trials, but also because of specific factors related with disease pathophysiology at the early stages. This may end up resulting in contradictory findings for the same drug [6, 7]. CIS patients who will present a second attack and thus, convert to clinical definite MS (CDMS) will be also having greater BV loss [53, 54]; however, placebo patients who develop CDMS while on the clinical trial will be switched to the treatment arm before trial termination; if this is not taken into account, the placebo arm may end up contaminated with active therapy effects on BV. Lastly, when CIS occurs it is usually accompanied of brain inflammation that, as stated before, may affect BV loss during the subsequent follow-up.
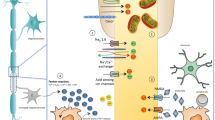
Obviously, it is also very important to recognize that treatment effects on BV may be different not only because of the methodological issues stated before but also because of specific aspects of the different drugs mechanism of action. Whereas all MS treatments have been shown to have an effect on the immune system that ultimately leads to decreased inflammation and which in consequence should decrease central nervous system damage, a neuroprotective effect, as measured by BV changes, has only been confirmed for a number of them. Neuroprotection, or the preservation of neuronal structures and its function, can be achieved by an indirect mechanism (due to the reduction of central nervous system damage) or to a direct mechanism (by increasing tissue resistance to critical damage or by promoting tissue repair). We could speculate that some of the drugs that have demonstrated a greater impact on BV loss, such as fingolimod, laquinimod or alemtuzumab have also been shown to have a direct neuroprotective effect, either by promoting secretion of neurotrophic factors [55–57], blocking the production of nitric oxide [58] or promoting myelin repair [59]. Some of these drugs, such as fingolimod and laquinimod, may have the capacity of crossing the blood–brain barrier and may exert part of their potential neuroprotective effect directly into the central nervous system [60–62]; in any case, penetration in the CNS does not ensure the existence of a neuroprotective effect and, on the contrary, neuroprotection could also be exerted through mechanisms initiated in the periphery [63]. However, the effect of these potential neuroprotective drugs on progressive forms of the disease still has to be demonstrated and, in fact, preliminary data coming from the INFORMS trial of fingolimod in primary progressive multiple sclerosis have been reported negative; interestingly, coupling of such negative brain volume results with negative results on the primary outcome (disability progression) in spite of positive effects on lesion-related parameters seems to emphasize the importance of BV outcomes. On the other hand, IFN-β preparations and natalizumab which have no or little capacity to cross the blood–brain barrier, have shown to have either no or little immediate effect on BV loss, and may probably exert their neuroprotective effect only by reducing brain inflammation and preventing lymphocytes to cross the blood–brain barrier and cause tissue damage [64, 65]. Therefore, less clear or compelling results with other drugs in terms of their final net impact on BV loss will be the result of a varying combination of both methodological issues, anti-inflammatory properties and neuroprotective effects; this might be the case of GA [55], dimethyl-fumarate (another drug with a possible neuroprotective effect [66]) which has shown positive results on BV loss only with the BID dose and teriflunomide (a drug without a known neuroprotective effect [67]) which has been demonstrated to reduce white matter volume loss only. Finally, we should keep in mind that global BV loss measures are not reflecting all real tissue damage occurring in MS patients, as they simply give us an estimate of a non-specific global effect that is the final net result of a number of pathogenic processes occurring in parallel in the brain (such as axonal degeneration, inflammation, new lesion formation, glial scarring, and others). Other, more pathologically specific, MRI techniques might be useful for that purpose once technical limitations have been overcome [68].
In summary, a number, but not all, of the available DMDs for treating RRMS patients have been demonstrated to reduce the rate of global BV loss in randomized clinical trials as well as in some open-label studies. Even though not only drug-specific, but also methodological aspects should be taken into consideration when interpreting treatment effects on BV; its well-proven relation to disability progression [69] makes accurate description of such effects very relevant in the definition of the therapeutic profiles of any drugs used in the treatment of MS.
References
Frohman EM, Racke MK, Raine CS (2006) Multiple sclerosis—the plaque and its pathogenesis. N Engl J Med 354:942–955. doi:10.1056/NEJMra052130
Miller DH, Barkhof F, Frank JA et al (2002) Measurement of atrophy in multiple sclerosis: pathological basis, methodological aspects and clinical relevance. Brain J Neurol 125:1676–1695
Bermel RA, Bakshi R (2006) The measurement and clinical relevance of brain atrophy in multiple sclerosis. Lancet Neurol 5:158–170
Smith SM, Zhang Y, Jenkinson M et al (2002) Accurate, robust, and automated longitudinal and cross-sectional brain change analysis. Neuroimage 17:479–489
Zivadinov R, Reder AT, Filippi M et al (2008) Mechanisms of action of disease-modifying agents and brain volume changes in multiple sclerosis. Neurology 71:136–144
Filippi M, Rovaris M, Inglese M et al (2004) Interferon beta-1a for brain tissue loss in patients at presentation with syndromes suggestive of multiple sclerosis: a randomised, double-blind, placebo-controlled trial. Lancet 364:1489–1496
De Stefano N, Comi G, Kappos L et al (2013) Efficacy of subcutaneous interferon -1a on MRI outcomes in a randomised controlled trial of patients with clinically isolated syndromes. J Neurol Neurosurg Psychiatry 85:647–653. doi:10.1136/jnnp-2013-306289
Kappos L, Freedman MS, Polman CH et al (2007) Effect of early versus delayed interferon beta-1b treatment on disability after a first clinical event suggestive of multiple sclerosis: a 3-year follow-up analysis of the BENEFIT study. Lancet 370:389–397
Kappos L, Freedman MS, Polman CH et al (2009) Long-term effect of early treatment with interferon beta-1b after a first clinical event suggestive of multiple sclerosis: 5-year active treatment extension of the phase 3 BENEFIT trial. Lancet Neurol 8:987–997
Rudick RA, Fisher E, Lee J-C et al (2000) Brain atrophy in relapsing multiple sclerosis: relationship to relapses, EDSS, and treatment with interferon -1a. Mult Scler 6:365–372. doi:10.1177/135245850000600601
Hardmeier M, Wagenpfeil S, Freitag P et al (2005) Rate of brain atrophy in relapsing MS decreases during treatment with IFN -1a. Neurology 64:236–240. doi:10.1212/01.WNL.0000149516.30155.B8
Kappos L, Traboulsee A, Constantinescu C et al (2006) Long-term subcutaneous interferon beta-1a therapy in patients with relapsing-remitting MS. Neurology 67:944–953. doi:10.1212/01.wnl.0000237994.95410.ce
Comi G, Martinelli V, Rodegher M et al (2009) Effect of glatiramer acetate on conversion to clinically definite multiple sclerosis in patients with clinically isolated syndrome (PreCISe study): a randomised, double-blind, placebo-controlled trial. Lancet 374:1503–1511
Comi G, Martinelli V, Rodegher M et al (2012) Effects of early treatment with glatiramer acetate in patients with clinically isolated syndrome. Mult Scler J 19:1074–1083. doi:10.1177/1352458512469695
Ge Y, Grossman RI, Udupa JK et al (2000) Glatiramer acetate (Copaxone) treatment in relapsing-remitting MS: quantitative MR assessment. Neurology 54:813–817
Rovaris M, Comi G, Rocca MA et al (2001) Short-term brain volume change in relapsing-remitting multiple sclerosis: effect of glatiramer acetate and implications. Brain J Neurol 124:1803–1812
Sormani MP, Rovaris M, Valsasina P et al (2004) Measurement error of two different techniques for brain atrophy assessment in multiple sclerosis. Neurology 62:1432–1434. doi:10.1212/01.WNL.0000120663.85143.B3
Rovaris M, Comi G, Rocca MA et al (2007) Long-term follow-up of patients treated with glatiramer acetate: a multicentre, multinational extension of the European/Canadian double-blind, placebo-controlled, MRI-monitored trial. Mult Scler 13:502–508. doi:10.1177/1352458506070704
Comi G, Cohen JA, Arnold DL et al (2011) Phase III dose-comparison study of glatiramer acetate for multiple sclerosis. Ann Neurol 69:75–82. doi:10.1002/ana.22316
Mikol DD, Barkhof F, Chang P et al (2008) Comparison of subcutaneous interferon beta-1a with glatiramer acetate in patients with relapsing multiple sclerosis (the REbif vs Glatiramer Acetate in Relapsing MS Disease [REGARD] study): a multicentre, randomised, parallel, open-label trial. Lancet Neurol 7:903–914. doi:10.1016/S1474-4422(08)70200-X
O’Connor P, Filippi M, Arnason B et al (2009) 250 microg or 500 microg interferon beta-1b versus 20 mg glatiramer acetate in relapsing-remitting multiple sclerosis: a prospective, randomised, multicentre study. Lancet Neurol 8:889–897. doi:10.1016/S1474-4422(09)70226-1
Lublin FD, Cofield SS, Cutter GR et al (2013) Randomized study combining interferon and glatiramer acetate in multiple sclerosis: the CombiRx Study. Ann Neurol 73:327–340. doi:10.1002/ana.23863
Miller DH, Soon D, Fernando KT et al (2007) MRI outcomes in a placebo-controlled trial of natalizumab in relapsing MS. Neurology 68:1390–1401
Radue E-W, Stuart WH, Calabresi PA et al (2010) Natalizumab plus interferon beta-1a reduces lesion formation in relapsing multiple sclerosis. J Neurol Sci 292:28–35. doi:10.1016/j.jns.2010.02.012
Kappos L, Radue E-W, O’Connor P et al (2010) A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med 362:387–401
Cohen JA, Barkhof F, Comi G et al (2010) Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N Engl J Med 362:402–415
Calabresi PA, Radue E-W, Goodin D et al (2014) Safety and efficacy of fingolimod in patients with relapsing-remitting multiple sclerosis (FREEDOMS II): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Neurol 13:545–556. doi:10.1016/S1474-4422(14)70049-3
Radue E-W (2012) Impact of Fingolimod Therapy on Magnetic Resonance Imaging Outcomes in Patients With Multiple Sclerosis. Arch Neurol 69:1259. doi:10.1001/archneurol.2012.1051
Cohen JA, Barkhof F, Comi G et al (2013) Fingolimod versus intramuscular interferon in patient subgroups from TRANSFORMS. J Neurol 260:2023–2032. doi:10.1007/s00415-013-6932-0
Khatri B, Barkhof F, Comi G et al (2011) Comparison of fingolimod with interferon beta-1a in relapsing-remitting multiple sclerosis: a randomised extension of the TRANSFORMS study. Lancet Neurol 10:520–529
Arnold DL, Gold R, Kappos L et al (2014) Effects of delayed-release dimethyl fumarate on MRI measures in the phase 3 DEFINE study. J Neurol. doi:10.1007/s00415-014-7412-x
Miller DH, Fox RJ, Phillips JT et al (2015) Effects of delayed-release dimethyl fumarate on MRI measures in the phase 3 CONFIRM study. Neurology 84:1145–1152. doi:10.1212/WNL.0000000000001360
O’Connor P, Wolinsky JS, Confavreux C et al (2011) Randomized trial of oral teriflunomide for relapsing multiple sclerosis. N Engl J Med 365:1293–1303
Wolinsky JS, Narayana PA, Nelson F et al (2013) Magnetic resonance imaging outcomes from a phase III trial of teriflunomide. Mult Scler J 19:1310–1319. doi:10.1177/1352458513475723
Comi G, Jeffery D, Kappos L et al (2012) Placebo-controlled trial of oral laquinimod for multiple sclerosis. N Engl J Med 366:1000–1009
On behalf of the BRAVO Study Group, Vollmer TL, Sorensen PS et al (2014) A randomized placebo-controlled phase III trial of oral laquinimod for multiple sclerosis. J Neurol 261:773–783. doi:10.1007/s00415-014-7264-4
CAMMS223 Trial Investigators, Coles AJ, Compston DAS et al (2008) Alemtuzumab vs. interferon beta-1a in early multiple sclerosis. N Engl J Med 359:1786–1801. doi:10.1056/NEJMoa0802670
Cohen JA, Coles AJ, Arnold DL et al (2012) Alemtuzumab versus interferon beta 1a as first-line treatment for patients with relapsing-remitting multiple sclerosis: a randomised controlled phase 3 trial. Lancet 380:1819–1828
Coles AJ, Twyman CL, Arnold DL et al (2012) Alemtuzumab for patients with relapsing multiple sclerosis after disease-modifying therapy: a randomised controlled phase 3 trial. Lancet 380:1829–1839
Paolillo A, Pozzilli C, Giugni E et al (2002) A 6-year clinical and MRI follow-up study of patients with relapsing–remitting multiple sclerosis treated with Interferon-beta. Eur J Neurol 9:645–655
Frank JA, Richert N, Bash C et al (2004) Interferon-β-1b slows progression of atrophy in RRMS: three-year follow-up in NAb− and NAb+ patients. Neurology 62:719–725. doi:10.1212/01.WNL.0000113765.75855.19
Zivadinov R, Locatelli L, Cookfair D et al (2007) Interferon beta-1a slows progression of brain atrophy in relapsing-remitting multiple sclerosis predominantly by reducing gray matter atrophy. Mult Scler 13:490–501. doi:10.1177/1352458506070446
Calabrese M, Bernardi V, Atzori M et al (2011) Effect of disease-modifying drugs on cortical lesions and atrophy in relapsing-remitting multiple sclerosis. Mult Scler J 18:418–424. doi:10.1177/1352458510394702
Khan O, Bao F, Shah M et al (2012) Effect of disease-modifying therapies on brain volume in relapsing–remitting multiple sclerosis: results of a five-year brain MRI study. J Neurol Sci 312:7–12. doi:10.1016/j.jns.2011.08.034
Rojas JI, Patrucco L, Miguez J et al (2013) Brain atrophy as a non-response predictor to interferon-beta in relapsing-remitting multiple sclerosis. Neurol Res 36:615–618. doi:10.1179/1743132813Y.0000000304
Magraner M, Coret F, Casanova B (2012) The relationship between inflammatory activity and brain atrophy in natalizumab treated patients. Eur J Radiol 81:3485–3490. doi:10.1016/j.ejrad.2012.01.028
Vidal-Jordana A, Sastre-Garriga J, Pérez-Miralles F et al (2013) Early brain pseudoatrophy while on natalizumab therapy is due to white matter volume changes. Mult Scler J 19:1175–1181
Sastre-Garriga J, Tur C, Pareto D et al (2014) Brain atrophy in natalizumab-treated patients: a 3-year follow-up. Mult Scler Houndmills Basingstoke Engl. doi:10.1177/1352458514556300
Rinaldi F, Calabrese M, Seppi D et al (2012) Natalizumab strongly suppresses cortical pathology in relapsing-remitting multiple sclerosis. Mult Scler J 18:1760–1767. doi:10.1177/1352458512447704
Portaccio E, Stromillo ML, Goretti B et al (2013) Natalizumab may reduce cognitive changes and brain atrophy rate in relapsing-remitting multiple sclerosis: a prospective, non-randomized pilot study. Eur J Neurol 20:986–990. doi:10.1111/j.1468-1331.2012.03882.x
Zivadinov R (2005) Reproducibility and accuracy of quantitative magnetic resonance imaging techniques of whole-brain atrophy measurement in multiple sclerosis. J Neuroimaging 15:27–36. doi:10.1177/1051228404271010
Huppertz H-J, Kröll-Seger J, Klöppel S et al (2010) Intra- and interscanner variability of automated voxel-based volumetry based on a 3D probabilistic atlas of human cerebral structures. Neuroimage 49:2216–2224. doi:10.1016/j.neuroimage.2009.10.066
Dalton CM, Chard DT, Davies GR et al (2004) Early development of multiple sclerosis is associated with progressive grey matter atrophy in patients presenting with clinically isolated syndromes. Brain 127:1101–1107. doi:10.1093/brain/awh126
Perez-Miralles F, Sastre-Garriga J, Tintore M et al (2013) Clinical impact of early brain atrophy in clinically isolated syndromes. Mult Scler J 19:1878–1886. doi:10.1177/1352458513488231
Racke MK, Lovett-Racke AE, Karandikar NJ (2010) The mechanism of action of glatiramer acetate treatment in multiple sclerosis. Neurology 74:S25–S30
Thöne J, Ellrichmann G, Seubert S et al (2012) Modulation of autoimmune demyelination by laquinimod via induction of brain-derived neurotrophic factor. Am J Pathol 180:267–274. doi:10.1016/j.ajpath.2011.09.037
Freedman MS, Kaplan JM, Markovic-Plese S (2013) Insights into the Mechanisms of the Therapeutic Efficacy of Alemtuzumab in Multiple Sclerosis. J Clin Cell Immunol 4:152. doi:10.4172/2155-9899.1000152
Colombo E, Di Dario M, Capitolo E et al (2014) Fingolimod may support neuroprotection via blockade of astrocyte nitric oxide. Ann Neurol 76:325–337. doi:10.1002/ana.24217
Groves A, Kihara Y, Chun J (2013) Fingolimod: direct CNS effects of sphingosine 1-phosphate (S1P) receptor modulation and implications in multiple sclerosis therapy. J Neurol Sci 328:9–18. doi:10.1016/j.jns.2013.02.011
Miron VE, Schubart A, Antel JP (2008) Central nervous system-directed effects of FTY720 (fingolimod). J Neurol Sci 274:13–17. doi:10.1016/j.jns.2008.06.031
Coelho RP, Payne SG, Bittman R et al (2007) The immunomodulator FTY720 has a direct cytoprotective effect in oligodendrocyte progenitors. J Pharmacol Exp Ther 323:626–635. doi:10.1124/jpet.107.123927
Kelland EE, Gilmore W, Hayardeny L et al (2014) In vitro assessment of the direct effect of laquinimod on basic functions of human neural stem cells and oligodendrocyte progenitor cells. J Neurol Sci 346:66–74. doi:10.1016/j.jns.2014.07.058
Kerschensteiner M, Gallmeier E, Behrens L et al (1999) Activated human T cells, B cells, and monocytes produce brain-derived neurotrophic factor in vitro and in inflammatory brain lesions: a neuroprotective role of inflammation? J Exp Med 189:865–870
Dhib-Jalbut S, Marks S (2010) Interferon-β mechanisms of action in multiple sclerosis. Neurology 74:S17–S24
Ransohoff RM (2007) Natalizumab for multiple sclerosis. N Engl J Med 356:2622–2629
Linker RA, Lee D-H, Ryan S et al (2011) Fumaric acid esters exert neuroprotective effects in neuroinflammation via activation of the Nrf2 antioxidant pathway. Brain J Neurol 134:678–692. doi:10.1093/brain/awq386
Bar-Or A, Pachner A, Menguy-Vacheron F et al (2014) Teriflunomide and its mechanism of action in multiple sclerosis. Drugs 74:659–674. doi:10.1007/s40265-014-0212-x
De Stefano N, Filippi M, Miller D et al (2007) Guidelines for using proton MR spectroscopy in multicenter clinical MS studies. Neurology 69:1942–1952. doi:10.1212/01.wnl.0000291557.62706.d3
Sormani MP, Arnold DL, De Stefano N (2014) Treatment effect on brain atrophy correlates with treatment effect on disability in multiple sclerosis. Ann Neurol 75:43–49. doi:10.1002/ana.24018
Conflicts of interest
Dr. Vidal-Jordana reports personal fees from Teva, Biogen-Idec, Novartis, and Genzyme, all outside the submitted work. Dr. Sastre-Garriga reports personal fees from Biogen-Idec, Novartis, Almirall, Teva, Roche, Merck-Serono and grants and personal fees from Genzyme, all outside the submitted work. Dr. Rovira serves on scientific advisory boards for Biogen Idec, Novartis, Genzyme, and OLEA Medical, and on the editorial board of the American Journal of Neuroradiology and Neuroradiology, has received speaker honoraria from Bayer, Genzyme, Bracco, Merck-Serono, Teva Pharmaceutical Industries Ltd., OLEA Medical, Stendhal, Novartis and Biogen Idec, receives research support from Bayer, and has research agreements with Siemens AG. Dr. Montalban has received speaking honoraria and travel expense reimbursement for participation in scientific meetings, has been a steering committee member of clinical trials or participated in advisory boards of clinical trials in the past years with Actelion, Almirall, Bayer, Biogen Idec, Genzyme, Merck, Novartis, Receptos, Roche, Sanofi-Genzyme, Teva and Trophos.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vidal-Jordana, A., Sastre-Garriga, J., Rovira, A. et al. Treating relapsing–remitting multiple sclerosis: therapy effects on brain atrophy. J Neurol 262, 2617–2626 (2015). https://doi.org/10.1007/s00415-015-7798-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-015-7798-0