Abstract
The aim of this study was to investigate the olfactory bulb (OB) and sulcus (OS) in a large group of patients who have been well-characterized in terms of olfactory function, with a specific focus on the comparison between patients with olfactory loss due to chronic rhinosinusitis, head trauma, or acute infections. A retrospective study of 378 patients with olfactory loss was performed. Orthonasal olfactory function was assessed with the“Sniffin’ Sticks” test kit, including tests for odor threshold, odor discrimination, and odor identification. Magnetic resonance imaging analyses were focused on OB volume and OS depth. Major results of the present study included the (1) demonstration of a correlation between olfactory function and OB volume across the various pathologies in a very large group of subjects; (2) the three functional tests exhibited a similar degree of correlation with OB volume. (3) The right, but not the left OS correlated with olfactory function; in addition, (4) OS was negatively correlated with age. In contrast to OS, (5) no side differences were found for the OB. Finally, (6) the three different causes of olfactory loss exhibited different patterns of results for the three olfactory tests. The present data suggest that the morphological assessment of the OB volume and OS depth produces useful clinical indicators of olfactory dysfunction.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Since the groundbreaking work of Yousem et al. [26, 29] magnetic resonance imaging (MRI)-based measurements of the olfactory bulb (OB) have become an established tool in the investigation of olfactory function. It has been used in healthy subjects, people with isolated congenital anosmia, patients with olfactory dysfunction due to chronic rhinosinusitis (CRS), head trauma (TR), postinfectious olfactory dysfunction (PIOD), patients with epilepsy, schizophrenia, Parkinson’s disease, Alzheimer’s disease or children after chemotherapy (for review see [22]). At the same time, the olfactory sulcus (OS) has also been mentioned as a correlate of olfactory function, although it has been studied less rigorously [21, 23].
Aim of this study was to investigate the OB and OS in a large group of patients who were well-characterized in terms of olfactory function, with a specific focus on the comparison between patients with olfactory loss due to CRS, TR, or PIOD, as these are the most frequent causes of olfactory loss apart from age [2, 3]. The dataset from the Department of Otorhinolaryngology at the Brussels’ St. Luc Hospital provided ideal prerequisites to conduct such a study as patients have been investigated since years with the same diagnostic tools and, importantly, the same MR scanner/software.
Materials and methods
Subjects
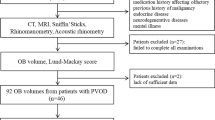
This study was conducted at the Department of Otorhinolaryngology of the Saint Luc University Hospital in Brussels. Data from 378 consecutive patients were included. All of them had received scans of the olfactory bulb (OB) and/or olfactory sulcus (OS) and/or brain using magnetic resonance imaging, they had received an otorhinolaryngological investigation including nasal endoscopy, had a detailed interview with an experienced otorhinolaryngologist (PR, CH), went through psychophysical tests of orthonasal olfactory function.
Psychophysical testing of olfaction performance
Psychophysical testing of olfactory function was performed with the validated “Sniffin’ Sticks” test [10]. Odors were presented to the patients in felt-tip pens. For birhinal stimulation, the pen’s tip is placed approximately 2 cm in front of both nostrils. This test encompasses three different approaches. First, odor thresholds (T; Threshold) are assessed for n-butanol with stepwise dilutions in a row of 16 dilutions. Thresholds are determined using the single staircase technique based on a 3-alternative forced choice (3-AFC) task. Second, patients are asked to discriminate odors. For each discrimination task (D; Discrimination), three pens are presented, two containing the same odor and the third containing the target odorant (3-AFC task). The target odors should be recognized in a row of 16 trials. Thirdly, a row of 16 odors was presented to the patients together with a list of 4 verbal descriptors (I; Identification). Subjects were asked to identify the odors using this multiple forced choice approach. For healthy subjects, the TDI score at the 10th percentile is 30.5 for ages from 15 to 35 years. Functional anosmia (further termed “anosmia”) is diagnosed if the TDI score is less than 16.5. With a TDI score between 16.5 and 30.5, patients are considered hyposmic.
Magnetic resonance imaging (MRI) protocol and measurements
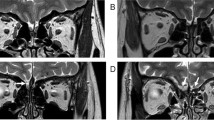
Patients were examined on a 1.5 Tesla MRI system (Signa Echospeed, GEMS, Milwaukee, WI, USA) using a standardized protocol for OB analysis (see Figs. 1, 2). The protocol included: (1) 4-mm-thick standard T2-weighted fast spin-echo images covering the whole brain without interslice gap to rule out any organic brain disorder; (2) 4-mm-thick standard FSE fluid-attenuated inversion recovery images covering the whole brain and 4-mm-thick T2-weighted images gradient-echo images using the Echo-Planar imaging technique (EPI-GRE-T2*) covering the whole brain to rule out the presence of any parenchymal or meningeal post-traumatic hemosiderin deposit; and (3): 1.5-mm-thick T1- and T2-weighted fast spin-echo images without interslice gap in the coronal plane covering the anterior and middle segments of the base of the skull. The analysis followed a standardized method [16]. In summary, OB volumes were calculated by planimetric manual contouring (surface in mm2) and all surfaces were added and multiplied by appropriate factors due to the varying slice thickness to obtain a volume in mm3.
Statistical analyses
All statistics were performed using SPSS software vs. 23.0 (SPSS Inc., Chicago, Ill., USA). Results were submitted to analyses of variance (ANOVA) for repeated measures. Correlations (Pearson) were computed between volumetric measures of the OB and functional measures. The level of significance was set at 0.05.
Results
A total of 378 people were included in the study: 99 had chronic rhinosinusitis (CRS), 201 had post-traumatic olfactory loss (TR), and 78 had postinfectious olfactory loss (PIOD); 193 patients were women, 185 were men. Average age was 49 years (SD 14 years). A detailed list of the measured parameters separately for the causes of olfactory loss is presented in Table 1. Results from partial correlations controlling for age between the obtained parameters are shown in Table 2. Interestingly, depth of the right, but not the left OS correlated with odor thresholds (threshold: r 297 = 0.15, p = 0.012).
When comparing the OB volume between left and right side in relation to etiology (CRS, TR, and PIOD), analyses did not show side-related differences [F(1,348) = 1.90; p = 0.17] but OB volumes differed significantly in relation to the cause of the olfactory disorder [F(2,348) = 9.07; p < 0.001]. Posthoc tests showed that OBs were smallest in TR patients compared to CRS and PIOD patients (p < 0.007). In contrast to OB, OS depth exhibited side differences [F(1,325) = 19.5; p < 0.001] with the right OS being deeper than the left OS. Again in contrast to OB measures, the OS did not vary in relation to the cause of olfactory loss [F(2,325) = 0.15; p = 0.86].
The average OS was 6.7-mm deep on the left and 7.0-mm deep on the right side; we also found that, across all subjects, the left OS depth decreased with the subjects’ age; for the right side only a tendency for such a relation was seen (left OS: r 332 = −0.12, p = 0.024; right OS: r 340 = −0.09, p = 0.093).
We also compared results from the three olfactory tests between the three major etiologic groups. TR patients performed worst compared to CRS and PIOD patients [F(2,364) = 14.9, p < 0.001; posthoc tests: TR vs CRS/PIOD: p < 0.002]. Importantly, there was also a significant interaction between factors “olfactory test” and “cause of olfactory loss” [F(2,364) = 3.77, p = 0.024] indicating that patients of different groups performed differently with the tests. TR patients performed worst for all three tests; SND and PIOD patients had—on average—identical scores at threshold level while their scores were most different at odor identification with CRS patients performing worse than PIOD patients.
To judge the quality of the technique used, we performed a number of additional measurements. In one instance, we looked at the inter-observer reliability. These measurements were made in 50 bulbs by the authors AU and CH. The inter-observer reliability was very high, for the left OB: r 48 = 0.98, p < 0.001; and the right OB: r 50 = 0.98, p < 0.001. Average differences between measures by the two observers were 0.27 mm3 (SD 3.04) for the right OB and 0.07 mm3 (SD 2.46) for the left OB. With regard to intra-observer reliability repeat measures in 224 OBs by one observer produced the following results: left OB—r 224 = 0.945, p < 0.001; right OB—r 224 = 0.949, p < 0.001. The average differences between measures by one observer were 0.84 mm3 (SD 4.77) for the right side and 0.61 mm3 (SD 4.36) for the left side.
In an additional analysis, the question should be answered whether two observers select the same number of slices for measurements, or, in other words, whether two observers use the same criteria for separating OBs and OTs. Results obtained from measurements by AU and CH in 50 OBs with a median number of 7 slices (range 3–10 slices) indicated that the number of slices chosen for measurements for the right OB was the same in 52 %, differed by 1 slice in 34 %, and differed by 2 slices in 14 %; for the left OB this was the same in 54 %, differed by 1 slice in 36 %, by 2 slices in 8 % and 3 slices in 2 %.
Discussion
Major results of the present study in patients with olfactory loss included the (1) demonstration of a correlation between olfactory function and OB volume across the various pathologies in a very large group of subjects; (2) the three functional olfactory tests exhibited a similar degree of correlation with OB volume. (3) The right, but not the left OS correlated with olfactory function; in addition, (4) OS was negatively correlated with age. In contrast to OS, (5) no side differences were found for the OB. Finally, (6) the three different causes of olfactory loss exhibited different patterns of results for the three olfactory tests used. A limitation of the present study was that olfactory testing has been performed in both nostrils simultaneously, so differences in olfactory function between right/left nostril cannot be ruled out.
Magnetic resonance imaging-based volumetric measures of the OB have been studied in various groups of patients with different causes of olfactory loss (e.g., [18, 20, 24, 25]). Overall, they indicated that OB volume is a morphological indicator of olfactory function. The present study confirmed this view in a large group of subjects with mixed causes of olfactory loss. Also, across this group of patients a correlation was found between OB volume and psychophysical tests of olfactory function, again strongly confirming previous work. This analysis also showed that none of the three olfactory tests used, odor threshold, odor discrimination or odor identification exhibited a “best” correlation with OB volume. This corresponds to a recent review analysis showing, across numerous studies from various laboratories, that OB volume is correlated to scores from odor thresholds and odor identification in a similar strength [22]. Thus, OB volume is an indicator of general olfactory function.
At the level of the OS, but not at the level of the OB, side differences were found, with the right OS being deeper than the left OS. In addition, a correlation between odor thresholds and odor discrimination was found for the right OS, not the left OS. Why was this seen for the right side? One idea relates to the potentially higher significance of the right hemisphere in terms of olfactory function which has been indicated by numerous studies [7, 9, 12, 28, 30].
Another question relates to the fact that this side difference was not seen for the OB. However, here it has to be kept in mind that the OB is subject to relatively short-term changes following changes in olfactory function [4]. Such changes have not been observed for the OS [19]. It appears as if the depth of the OS was more determined by long-term changes, e.g., aging and corresponding changes such as brain atrophy. This negative correlation with age (which also has been shown recently by [5]) may also explain that the average OS depth was below the depth limit of 8 mm that has been suggested in a group of younger subjects to separate people with isolated congenital anosmia form healthy controls [1, 10]. Because mostly older people have been investigated in the present study, the OS probably became shallower with age. Accordingly, normative data for the OS depth should be related to age.
As an additional outcome, the present study showed that the cause of olfactory loss is related to different patterns in scores for odor threshold, odor discrimination, and odor identification (compare [13]). TR patients had the lowest scores which are congruent with the fact that TR patients typically have relatively low recovery rates, considering that recovery is related to residual olfactory function [8, 14]. In contrast, SND and PIOD patients had—on average—identical scores at threshold level while their scores were most different at odor identification with CRS patients performing worse than PIOD patients. This seems to indicate in a clinical setting that the three olfactory tests tap into different dimensions of olfactory function, as already shown previously [6, 15]. Although the current findings are presented at a group level, still it seems to be worth the while to examine the patients with olfactory dysfunction with different tools to obtain comprehensive information at different levels.
The current study also showed that both inter- and intra-observer reliability are very high (r = 0.98 and r = 0.94, respectively). Considering these high coefficients of correlations it seems negligible whether observers agree only in about 50 % of the cases on the number of slices to be included in the volumetric measure; obviously the volumes at the proximal end of the OB (in patients with olfactory loss!) are too small to produce a major difference in overall OB volume, regardless whether they are included or not. Interestingly, the current results compare very well to previous research. Yousem et al. [27] reported inter-observer coefficients of correlation of r = 0.97 and intra-observer coefficients of correlation of r = 0.92; similar figures were reported a few years later with coefficients of correlation between measures (inter-observer reliability) of r = 0.96 for the right OB, and r = 0.98 for the left-sided OB [17].
Changes in relation for the duration of olfactory loss have not been investigated in the present study. However, previous work already showed that there is a negative correlation between duration of olfactory loss and OB [20] and changes of OB. Specifically, in 23 patients there was a negative correlation between OB volumes and duration of the olfactory loss (left OB: r = −0.57, p = 0.003; right OB: r = −0.59, p = 0.002). In contrast, no such correlation has been reported for the depth of the OS.
In the present study, the olfactory sulcus has been found to be deeper on the right side than on the left. This has already been observed in people with isolated congenital anosmia [1], and also in healthy, normosmic subjects [11]. However, as this is not found in all studies (e.g., [20]) it is difficult to speculate about possible causes of such lateralized differences.
To summarize, the present data suggest that the morphological assessment of the OB volume and OS depth is a relatively simple but very useful clinical measure to assess olfactory dysfunction.
References
Abolmaali ND, Hietschold V et al (2002) MR evaluation in patients with isolated anosmia since birth or early childhood. AJNR Am J Neuroradiol 23:157–164
Doty RL (1979) A review of olfactory dysfunctions in man. Am J Otolaryngol 1:57–79
Gentry LR, Godersky JC et al (1988) MR imaging of head trauma: review of the distribution and radiopathologic features of traumatic lesions. AJR Am J Roentgenol 150:663–672
Gudziol V, Buschhuter D et al (2009) Increasing olfactory bulb volume due to treatment of chronic rhinosinusitis—a longitudinal study. Brain 132(Pt 11):3096–3101
Hebbal GV, Mysorekar VR (2003) Anatomical and behavioural asymmetries in right and left handers from India. Ann Anat 185:267–275
Hedner M, Larsson M et al (2010) Cognitive factors in odor detection, odor discrimination, and odor identification tasks. J Clin Exp Neuropsychol 30:1–6
Heine O, Galaburda AM (1986) Olfactory asymmetry in the rat brain. Exp Neurol 91:392–398
Hummel T, Lötsch J (2010) Prognostic factors of olfactory dysfunction. Arch Otolaryngol Head Neck Surg 136:347–351
Hummel T, Pauli E et al (1995) Chemosensory event-related potentials in patients with temporal lobe epilepsy. Epilepsia 36:79–85
Hummel T, Sekinger B, Wolf SR, Pauli E, Kobal G (1997) 'Sniffin' sticks': olfactory performance assessed by the combined testing of odor identification, odor discrimination and olfactory threshold. Chem Senses 22:39-52
Hummel T, Damm M et al (2003) Depth of olfactory sulcus and olfactory function. Brain Res 975:85–89
Jones-Gotman M, Zatorre RJ (1993) Odor recognition memory in humans: role of right temporal and orbitofrontal regions. Brain Cogn 22(2):182–198
Klimek L, Hummel T et al (1998) Lateralized and bilateral olfactory function in patients with chronic sinusitis compared with healthy control subjects. Laryngoscope 108(1 Pt 1):111–114
London B, Nabet B et al (2008) Predictors of prognosis in patients with olfactory disturbance. Ann Neurol 63:159–166
Lotsch J, Reichmann H et al (2008) Different odor tests contribute differently to the evaluation of olfactory loss. Chem Senses 33(1):17–21
Lübke K, Gottschlich M et al (2012) No effects of handedness on passive processing of olfactory stimuli: a FMRI study. Chemosens Percept 5:22–26
Mueller A, Abolmaali ND et al (2005) Olfactory bulb volumes in patients with idiopathic Parkinson’s disease—a pilot study. J Neural Transm 112:1363–1370
Mueller A, Rodewald A et al (2005) Reduced olfactory bulb volume in post-traumatic and post-infectious olfactory dysfunction. Neuroreport 16:475–478
Nguyen DT, Nguyen-Thi PL et al (2012) How does measured olfactory function correlate with self-ratings of the sense of smell in patients with nasal polyposis? Laryngoscope 122:947–952
Rombaux P, Mouraux A et al (2006) Olfactory function and olfactory bulb volume in patients with postinfectious olfactory loss. Laryngoscope 116:436–439
Rombaux P, Potier H et al (2010) Olfactory bulb volume and depth of olfactory sulcus in patients with idiopathic olfactory loss. Eur Arch Otorhinolaryngol 267(10):1551–1556
Seubert J, Freiherr J et al (2013) Statistical localization of human olfactory cortex. Neuroimage 66:333–342
Wang J, You H et al (2011) Association of olfactory bulb volume and olfactory sulcus depth with olfactory function in patients with Parkinson disease. Am J Neuroradiol 32:677–681
Yousem DM, Geckle RJ et al (1996) MR evaluation of patients with congenital hyposmia or anosmia. Am J Radiol 166:439–443
Yousem DM, Geckle RJ et al (1999) Posttraumatic smell loss: relationship of psychophysical tests and volumes of the olfactory bulbs and tracts and the temporal lobes. Acad Radiol 6:264–272
Yousem DM, Geckle RJ et al (1996) Posttraumatic olfactory dysfunction: MR and clinical evaluation. AJNR Am J Neuroradiol 17:1171–1179
Yousem DM, Geckle RJ et al (1997) Reproducibility and reliability of volumetric measurements of olfactory eloquent structures. Acad Radiol 4:264–269
Yousem DM, Maldjian JA et al (1999) Gender effects on odor-stimulated functional magnetic resonance imaging. Brain Res 818:480–487
Yousem DM, Turner WJD et al (1993) Kallmann Syndroem: MR evaluation of olfactory system. Am J Neuroradiol 14:839–843
Zatorre RJ, Jones-Gotman M et al (1992) Functional localization and lateralization of human olfactory cortex. Nature 360:339–340
Acknowledgments
We would like to thank Cornelia Hummel for helpful comments on the manuscript.
Conflicts of interest
On behalf of all the authors, the corresponding author states that there is no conflict of interest.
Ethical standard
The study design was approved by the local Ethics Committee.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hummel, T., Urbig, A., Huart, C. et al. Volume of olfactory bulb and depth of olfactory sulcus in 378 consecutive patients with olfactory loss. J Neurol 262, 1046–1051 (2015). https://doi.org/10.1007/s00415-015-7691-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-015-7691-x