Abstract
Immunohistochemical analysis of platelet-derived growth factor receptor-α (PDGFR-α) was performed on human skin wounds obtained from forensic autopsy cases. Thirty human skin wounds were collected at different post-infliction intervals as follows: Group I, 4 h to 3 days (n = 16); Group II, 4 to 7 days (n = 7); Group III, 9 to 10 days (n = 3); and Group IV, 14 to 20 days (n = 4). Immunopositive reactions for PDGFR-α were not observed in the uninjured human skin specimens. In a semi-quantitative morphometrical analysis, the number of PDGFR-α-positive cells was observed increased in Group II, with the average number of PDGFR-α-positive cells being the highest in Group II. Additionally, in Group II, all specimens showed PDGFR-α-positive cells, with an average number of > 200 cells in five fields of view, suggesting a wound age of 4 to 7 days. Taken together, the immunohistochemical detection of PDGFR-α in human skin wounds can be a useful tool for wound age determination.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The crucial role of forensic wound examinations in evaluating the connection between injuries and the cause of death has been well established. Moreover, forensic pathologists have to also ascertain the amount of time that has elapsed since a wound was is incurred, a process known as wound age determination [1,2,3,4,5]. Currently, histopathological and immunohistochemical alterations occurring during various stages of wound healing have been used for wound age determination [6, 7].
Wound healing occurs in three stages: inflammation, proliferation, and maturation [8, 9]. Numerous biological elements such as growth factors, cytokines, and adhesion molecules have been recognized to participate in every stage of the wound healing [10]. From a forensic standpoint, multiple strands of accumulating evidence indicate the potential utility of these substances as markers for wound age determination using immunohistochemical methods [11,12,13,14,15,16,17,18,19,20,21].
Platelet-derived growth factor (PDGF) plays a significant role in the cell division of fibroblasts, and is particularly prevalent in wound healing [22]. It can also promote cell proliferation, migration, and angiogenesis. PDGF also stimulates chemotaxis of fibroblasts, neutrophils, and macrophages [23,24,25,26,27]. Furthermore, PDGF facilitates the contraction of collagen matrices during wound healing, which affects wound contraction [28, 29]. The distinct variations of PDGF isoforms exert their biological effects on target cells by interacting with platelet-derived growth factor receptor (PDGFR)-α and β. Actually, reduced expression of PDGFs and their receptors in diabetic or glucocorticoid-treated mice resulted in the retardation of skin wound healing [30, 31], indicating that PDGFs was essential for skin wound healing. Moreover, over-production of PDGFs would cause hypertrophic scar or keloid formation.
In this study, we conducted an immunohistochemical analysis of PDGFR-α expression in human skin wounds at various stages of healing and investigated the potential applicability of PDGFR-α as an indicator of wound age.
Materials and methods
Human skin wound specimens
A total of 30 human skin wounds (two stab wounds, three incised wounds, 14 surgical wounds, nine lacerations, and two gunshot wounds), with wound ages ranging from a few hours to 20 days, were obtained from forensic autopsies at Department of Forensic Medicine, Wakayama Medical University, Japan. The ages of the victims ranged from 18 to 94 years (mean age, 66.5 years), and the postmortem interval was < 3 days in each case. None of the case had severe malnutrition, malignant diseases, or metabolic disorders, and no substances such as cytostatic agents or glucocorticoids that could have influenced wound repair were administrated during medical treatment. Based on the wound ages, the wound specimens were classified into four groups as follows: Group I (inflammatory phase), 4 h to 3 days (n = 16); group II (early proliferative phase), 4 to 7 days (n = 7); group III (late proliferative phase), 9 to 10 days (n = 3); and group IV (maturation phase), 14 to 20 days (n = 4). Uninjured skin from the same individuals were use as control.
Immunohistochemistry
Immunostaining of PDGFR-α was performed using Ventana Discovery XT (Ventana Medical Systems, Inc., AZ, USA) using rabbit anti-PDGFR-α monoclonal antibody (#3174, clone D1E1E, Cell signaling technology, Danvers, MA). After incubating the specimens with an HRP-conjugated anti-rabbit multimer (518-102128, OmniMap HRP Multimer, Roche Diagnostics K.K., Tokyo, Japan), the immune complexes were visualized using the Chromomap kit DAB (518-100803, Roche Diagnostics K.K.) according to the manufacturer’s instructions. As a negative control, sections were incubated with normal rabbit serum instead of the primary antibody, and no positive signal was detected, indicating the specificity of the antibody.
Morphometrical analysis
Morphometrical analysis was performed for the semi-quantitative evaluation of the immunohistochemical findings by two investigators without prior knowledge, as described previously [18, 32,33,34,35]. Five high-power microscopic fields (magnification: x 200) were randomly selected in each section, and the number of PDGFR-α-positive cells were counted in each microscopic field. The average of the five selected microscopic fields was used as the PDGFR-α expression score for each wound specimen.
Statistical analysis
In each group, the means of the PDGFR-α-positive cell numbers and the standard error (SE) were calculated. Statistical analyses were performed using a one-factor analysis of variance to determine whether differences existed among the group means, followed by Scheffe’s F test to identify significantly different means. P < 0.05 was considered as significant. All statistical analyses were performed using the Statcel3 software under the supervision of a medical statistician.
Results
Immunohistochemical analysis
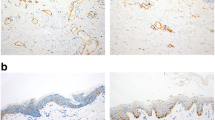
Only a small number of PDGFR-α-positive cells were found in intact skin and wounds which were < 1 day old. In contrast, PDGFR-α positive signals were detected in dermal cells of wounds > 2 days (Fig. 1).
Morphometrical analysis
Morphometric analysis was performed to semi-quantitatively evaluate the immunohistochemical findings. Figure 2a shows the distribution of the PDGFR-α-positive cells with respect to wound age. The average number of PDGFR-α positive cells were lowest in wound specimens from group I (wound age 4 h to 3 day), compared to the other groups (mean ± SE: 76.3 ± 51.3) (Fig. 2b). Group II (wound age 4 to 7 days) had the highest mean PDGFR-α-positive cell count among all the groups (mean ± SE: 370.9 ± 148.6). Moreover, all wounds in Group II showed PDGFR-α-positive cells, with an average number of > 200 cells in five fields of view (Fig. 2a). The average number of PDGFR-α-positive cells were decreased significantly in Group III (wound age 9 to 10 days; mean ± SE: 128.7 ± 51.8) and Group IV (wound age 14 to 20 days; mean ± SE: 97.6 ± 51.9) compared with that of Group II. Statistical analysis revealed significant differences between Group II and the other three groups individually (Fig. 2b).
Morphometrical analysis of PDGFR-α-positive cells. (a) The distribution of immunopositive cells in relation to wound ages. (b) Group I, 4 h to 3 days (n = 16); group II, 4 to 7 days (n = 7); group III, 9 to 10 days (n = 3); and group IV, 14 to 20 days (n = 4). Mean value and standard error of PDGFR-α-positive cell numbers in each wound group. *p < 0.05, **p < 0.01
Discussion
In forensic practices, forensic pathologists must consistently establish diagnoses regarding the correlation between wounds and the cause of death and determine factors such as wound vitality or post-infliction intervals. In particular, the estimation of the wound ages can provide significant scientific information for the investigation of criminal cases. PDGFR-α is a receptor for the PDGF family, which regulates cellular processes through the activation of specific signaling pathways, and plays an important regulatory role in cell growth, migration, angiogenesis and collagen synthesis [23,24,25,26,27,28,29]. Therefore, we hypothesized that PDGFR-α could be used as an effective marker for the estimation of wound ages and performed this study. We found that the average number of positive infiltrated cells was the highest at 4 to 7 days of wound age, imply that PDGFR-α could be utilized in wound age determination.
The process of wound healing is carefully coordinated across three distinct stages: inflammation, proliferation, and maturation. PDGF can promote the formation of new blood vessels, which is an important aspect of tissue repair [36], and induce the increased formation of granulation tissue during wound healing [37, 38]. PDGF is a major factor for the mitogen of fibroblasts, smooth muscle cells, and other cells, and can stimulate contraction of collagen matrices in vitro which can increase the rate of reepithelialization and of neovascularization [28, 29, 39, 40].
Furthermore, in a study of mice lacking PDGFR-α, it was suggested that PDGFRα signaling supported proliferation of fibroblast progenitors and increased their numbers during the early stages of wound healing, followed by downregulation of PDGFR-α to promote differentiation from fibroblasts to myofibroblasts [41]. Individuals with diabetes experience hindered skin wound healing, which can progress into persistent ulcers. Yu et al. discovered that lncRNA-H19 (lncH19) expressed in cutaneous PDGFR-α+ fibroblasts expedite the wound healing process by enhancing dermal fibroblasts proliferation and macrophage infiltration into the wounded skin [42]. These studies suggest that PDGFR-α plays essential roles in wound healing, especially in the early healing process.
In the inflammation stage of wound healing, platelets secrete growth factors, for example: epidermal growth factor (EGF), TGF-β, PDGF, and several chemokines [3]. These substances can recruit leukocytes to the wound sites. PDGF accelerates the proliferation and migration of various cell types, such as fibroblasts, smooth muscle cells, and endothelial cells to fill wound defects and promote tissue repair [43]. The functions and roles of PDGF are highly coordinated during wound healing. After wound formation, PDGF is released and initiates cell signaling pathways, such as PI3K-Akt, MAPK, and Ras, by binding to PDGFR-α and/or -β, which in turn promotes the processes of cell proliferation, migration, angiogenesis, and collagen synthesis [44,45,46]. In this study, we have found that the PDGFR-α expressed strongly during the 4-7days after wound formation, and weakly in the stages of 9–20 days.
Considering its relevance to forensic pathology, the current study demonstrates that PDGFR-α proves to be a suitable indicator for wound age determination. All of wound samples in Group II showed PDGFRα-positive cells of > 200 PDGFR-α-positive cells, suggesting a wound age of 4–7 days (early proliferative phase). Several lines of accumulating evidence indicated that the determination of wound vitality is sometimes more relevant issue in forensic practices [20, 21, 47]. Based on our observations, PDGFRα is unlikely to be available as an indicator for determining wound vitality or extremely short interval after injury. Notably, growing evidence to suggests that IL-1α may be a valuable tool in determining wound ages within a time frame of 4 h to 1 day (inflammatory phase) [20, 21]. In the hierarchy of PDGF expression control, inflammatory cytokines such as IL-1α play pivotal roles as upstream regulators [48, 49]. Collectively, we propose that the immunohistochemical identification of PDGFRα combined with additional indicators can enhance the dependability of wound age determination.
Finally, it is needless to say that the estimation of wound vitality or ages is indispensable in forensic pathology. The results obtained from only animal experiments would be important but sometimes be insufficient from the aspects of forensic application. Thus, we would like to emphasize to perform practical study using human samples as well as animal experiments.
Data availability
The authors declare that all data are available in the article file, or available from the corresponding author (Toshikazu Kondo) upon reasonable request.
References
Raekallio J (1972) Determination of the age of wounds by histochemical and biochemical methods. Forensic Sci Int 1(1):3–16
Kondo T (2007) Timing of skin wounds. Legal Med (Tokyo) 9(2):109–114
Kondo T, Ishida Y (2010) Molecular pathology of wound healing. Forensic Sci Int 203(1–3):93–98
Betz P (1994) Histological and enzyme histochemical parameters for the age estimation of human skin wounds. Int J Legal Med 107(2):60–68
Gauchotte G, Wissler MP, Casse JM, Pujo J, Minetti C, Gisquet H, Vigouroux C, Plénat F, Vignaud JM, Martrille L (2013) FVIIIra, CD15, and tryptase performance in the diagnosis of skin stab wound vitality in forensic pathology. Int J Legal Med 127(5):957–965
Betz P, Eisenmenger W (1996) Morphometrical analysis of hemosiderin deposits in relation to wound age. Int J Legal Med 108(5):262–264
Laiho K (1998) Myeloperoxidase activity in skin lesions. I. Influence of the loss of blood, depth of excoriations and thickness of the skin. Int J Legal Med 111(1):6–9
Singer AJ, Clark RA (1999) Cutaneous wound healing. N Engl J Med 341(10):738–746
Reinke JM, Sorg H (2012) Wound repair and regeneration. Eur Surg Res 49(1):35–43
Patel S, Maheshwari A, Chandra A (2016) Biomarkers for wound healing and their evaluation. J Wound Care 25(1):46–55
Khalaf AA, Hassanen EI, Zaki AR, Tohamy AF, Ibrahim MA (2019) Histopathological, immunohistochemical, and molecular studies for determination of wound age and vitality in rats. Int Wound J 16(6):1416–1425
Wang YN, Yamamoto Y, Kuninaka Y, Kondo T, Furukawa F (2015) Forensic potential of MMPs and CC chemokines for Wound Age determination. J Forensic Sci 60(6):1511–1515
Abd-Elhakim YM, Omran BHF, Ezzeldein SA, Ahmed AI, El-Sharkawy NI, Mohamed AA (2022) Time-dependent expression of high-mobility Group box-1 and toll-like receptors proteins as potential determinants of skin wound age in rats: forensic implication. Int J Legal Med 136(6):1781–1789
Birincioğlu İ, Akbaba M, Alver A, Kul S, Özer E, Turan N, Şentürk A, İnce İ (2016) Determination of skin wound age by using cytokines as potential markers. J Forensic Leg Med 44:14–19
Gao Y, Cai LW, Li DY, Li LL, Wu YL, Ren WJ, Song YR, Zhu LW, Wu YZ, Xu H, Luo CL, Wang T, Lei ZG, Tao LY (2023) Extended characterization of IL-33/ST2 as a predictor for wound age determination in skin wound tissue samples of humans and mice. Int J Legal Med 137(4):1287–1299
Fronczek J, Lulf R, Korkmaz HI, Witte BI, van de Goot FR, Begieneman MP, Schalkwijk CG, Krijnen PA, Rozendaal L, Niessen HW, Reijnders UJ (2015) Analysis of inflammatory cells and mediators in skin wound biopsies to determine wound age in living subjects in forensic medicine. Forensic Sci Int 247:7–13
Grellner W, Vieler S, Madea B (2005) Transforming growth factors (TGF-α and TGF-β) in the determination of vitality and wound age: immunohistochemical study on human skin wounds. Forensic Sci Int 153(2–3):174–180
Ishida Y, Kimura A, Takayasu T, Eisenmenger W, Kondo T (2009) Detection of fibrocytes in human skin wounds and its application for wound age determination. Int J Legal Med 123(4):299–304
Kondo T, Ohshima T, Mori R, Guan DW, Ohshima K, Eisenmenger W (2002) Immunohistochemical detection of chemokines in human skin wounds and its application to wound age determination. Int J Legal Med 116(2):87–91
Kondo T, Ohshima T (1996) The dynamics of inflammatory cytokines in the healing process of mouse skin wound: a preliminary study for possible wound age determination. Int J Legal Med 108(5):231–236
Kondo T, Ohshima T, Eisenmenger W (1999) Immunohistochemical and morphometrical study on the temporal expression of interleukin-1α (IL-1α) in human skin wounds for forensic wound age determination. Int J Legal Med 112(4):249–252
Alvarez RH, Kantarjian HM, Cortes JE (2006) Biology of platelet-derived growth factor and its involvement in disease. Mayo Clin Proc 81(9):1241–1257
Deuel TF, Senior RM, Huang JS, Griffin GL (1982) Chemotaxis of monocytes and neutrophils to platelet-derived growth factor J. Clin Invest 69(4):1046–1049
Grotendorst GR, Seppä HE, Kleinman HK, Martin GR (1981) Attachment of smooth muscle cells to collagen and their migration toward platelet-derived growth factor, Proc. Natl. Acad. Sei. USA 78(6):3669–3672
Senior RM, Griffin GL, Huang JS, Walz DA, Deuel TF (1983) Chemotactic activity of platelet alpha granule proteins for fibroblasts J. Cell Biol 96(2):382–385
Seppä H, Grotendorst G, Seppä S, Schiffmann E, Martin GR (1982) Platelet-derived growth factor is chemotactic for fibroblasts J. Cell Biol 92(2):584–588
Siegbahn A, Hammacher A, Westermark B, Heldin CH (1990) Differential effects of the various isoforms of platelet-derived growth factor on chemotaxis of fibroblasts, monocytes, and granulocytes. J Clin Invest 85(3):916–920
Clark RA, Folkvord JM, Hart CE, Murray MJ, McPherson JM (1989) Platelet isoforms of platelet-derived growth factor stimulate fibroblasts to contract collagen matrices. J Clin Invest 84(3):1036–1040
Gullberg D, Tingström A, Thuresson AC, Olsson L, Terracio L, Borg TK, Rubin K (1990) Beta 1 integrin-mediated collagen gel contraction is stimulated by PDGF. Exp Cell Res 186(2):264–272
Beer HD, Fässler R, Werner S (2000) Glucocorticoid-regulated gene expression during cutaneous wound repair. Vitam Horm 59:217–239
Beer HD, Longaker MT, Werner S (1997) Reduced expression of PDGF and PDGF receptors during impaired wound healing. J Invest Dermatol 109(2):132–138
Ishida Y, Kimura A, Takayasu T, Eisenmenger W, Kondo T (2008) Expression of oxygen-regulated protein 150 (ORP150) in skin wound healing and its application for wound age determination. Int J Legal Med 122(5):409–414
Hayashi T, Ishida Y, Kimura A, Takayasu T, Eisenmenger W, Kondo T (2004) Forensic application of VEGF expression to skin wound age determination. Int J Legal Med 118(6):320–325
Ishida Y, Kimura A, Nosaka M, Kuninaka Y, Takayasu T, Eisenmenger W, Kondo T (2012) Immunohistochemical analysis on cyclooxygenase-2 for wound age determination. Int J Legal Med 126(3):435–440
Ishida Y, Kimura A, Nosaka M, Kuninaka Y, Shimada E, Yamamoto H, Nishiyama K, Inaka S, Takayasu T, Eisenmenger W, Kondo T (2015) Detection of endothelial progenitor cells in human skin wounds and its application for wound age determination. Int J Legal Med 129(5):1049–1054
Raines EW, Ross R (1992) Compartmentalization of PDGF on extracellular binding sites dependent on exon-6-encoded sequences. J Cell Biol 116(2):533–543
Grotendorst GR, Martin GR, Pencev D, Sodek J, Harvey AK (1985) Stimulation of granulation tissue formation by platelet-derived growth factor in normal and diabetic rats. J Clin Lnvest 76(6):2323–2339
Sprugel KH, McPherson JM, Clowes AW, Ross R (1987) Effects of growth factors in vivo. I. Cell ingrowth into porous subcutaneous chambers. Am J Pathol 129(3):601–613
Heldin CH, Westermark B (1999) Mechanism of action and in vivo role of platelet-derived growth factor. Physiol Rev 79(4):1283–1316
Bauer EA, Cooper TW, Huang JS, Altman J, Deuel TF (1985) Stimulation of in vitro human skin collagenase expression by platelet-derived growth factor. Proc Natl Acad Sei USA 82(12):4132–4136
Yao LB, Rathnakar BH, Kwon HR, Sakashita H, Kim JH, Rackley A, Tomasek JJ, Berry WL, Olson LE (2022) Temporal control of PDGFRα regulates the fibroblast-to-myofibroblast transition in wound healing. Cell Rep 40(7):111192
Yu PJ, Guo J, Li JJ, Shi X, Xu N, Jiang YK, Chen W, Hu Q (2022) lncRNA-H19 in fibroblasts promotes Wound Healing in Diabetes. Diabetes 71(7):1562–1578
Leask A (2010) Potential therapeutic targets for cardiac fibrosis: TGFβ, angiotensin, endothelin, CCN2, and PDGF, partners in fibroblast activation. Circ Res 106(11):1675–1680
Wu LW, Chen WL, Huang SM, Chan JY (2019) Platelet-derived growth factor-AA is a substantial factor in the ability of adipose-derived stem cells and endothelial progenitor cells to enhance wound healing. FASEB J 33(2):2388–2395
Hinton DR, He S, Graf K, Yang D, Hsueh WA, Ryan SJ, Law RE (1998) Mitogen-activated protein kinase activation mediates PDGF-directed migration of RPE cells. Exp Cell Res 239(1):11–15
Li TY, Sun YJ, Wang JQ, Zhang CN, Sun YK (2023) Promoted skin Wound Healing by tail-amputated Eisenia foetida proteins via the Ras/Raf/MEK/ERK Signaling Pathway. ACS Omega 8(15):13935–13943
Kimura A, Ishida Y, Nosaka M, Shiraki M, Hama M, Kawaguchi T, Kuninaka Y, Shimada E, Yamamoto H, Takayasu T, Kondo T (2015) Autophagy in skin wounds: a novel marker for vital reactions. Int J Legal Med 129:537–541. doi: 10. 1007/s00414-015-116
Kawaguchi Y, Hara M, Wright TM (1999) Endogenous IL-1α from systemic sclerosis fibroblasts induces IL-6 and PDGF-A. J Clin Invest 103(9):1253–1260
Raines EW, Dower SK, Ross R (1989) Interleukin-1 mitogenic activity for fibroblasts and smooth muscle cells is due to PDGF-AA. Science 243(4889):393–396
Acknowledgements
This study was financially supported by Challenging Research (Pioneering, 22K19676, Y. I.) and Challenging Research (Exploratory, 23K17445, T. K.) from Japan Society for the Promotion of Science.
Author information
Authors and Affiliations
Contributions
Conceptualization: YI and TK; Formal Analysis: WZ, MN, and YK; Funding Acquisition: YI and TK; Investigation: WZ, AI, HY, and AK; Project Administration: TK; Validation: WZ, TK, and UG, YI, and TK; Writing - Original Draft Preparation: WZ and YI; Writing - Review and Editing: TK.
Corresponding authors
Ethics declarations
Ethical approval
This study was approved by the Research Ethics Committee of Wakayama Medical University (No. 3229). All the procedures were performed in accordance with the Declaration of Helsinki Principles. Moreover, this study was conducted using autopsy records from the past, and we could not obtain informed consent from the bereaved family for the use of these records. Therefore, we conducted this study in accordance with the “Ethical Guidelines for Medical Research Involving Human Subjects (enacted by the Ministry of Health, Labor, and Welfare in Japan), Sects. 12–1(2) (a) (c).” This was a de-identified study using archived tissue obtained from judicial autopsy cases, and the information on the implementation of the study was posted on our website (https://www.wakayama-med.ac.jp/dept/igakubu/160420/index.html). If there was a request to refuse the use of the samples for research, they were excluded from samples of this study. In addition, the review board of the Research Ethics Committee of Wakayama Medical University waived the need for written informed consent from the relatives of the individuals studied in accordance with the national legislation and the institutional requirements.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, W., Ishida, Y., Nosaka, M. et al. Immunohistochemical analysis of PDGFR-α for wound age determination. Int J Legal Med 138, 1351–1356 (2024). https://doi.org/10.1007/s00414-024-03208-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00414-024-03208-0