Abstract
Lactate is produced in carbohydrate metabolism under anaerobic conditions. Lactic acidosis occurs when the production of lactate exceeds its removal. In post-mortem (PM) context, the lactic acidosis is difficult to interpret due to unknown pathophysiological factors prior to death and PM changes that may affect the lactate levels. We evaluated 1865 medico-legal autopsy cases where the quantitation of glucose, lactate, and ketone bodies was performed as a part of the cause of death (CoD) investigation. Lactate was shown to ascend in a logarithmic manner as the PM interval increased until a plateau was achieved approximately after 8–10 days PM, and the elevation was caused mainly by PM changes. The lactate level was higher than the mean in cases where the CoD was diabetes mellitus type 2 (DM2) or metformin poisoning. Although there was a correlation between metformin and lactate levels, our findings suggest the DM2 and its complications were the cause for elevated lactate levels rather than metformin, since the lactate levels were similar in DM2-associated deaths where no metformin was detected. Elevated lactate levels in PM samples rather referred to metabolic disturbances often caused by DM2. An assay to detect D-lactate in PM samples was described.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In mammalian cells, lactate is formed from pyruvate in carbohydrate metabolism under anaerobic conditions. If the production of lactate exceeds the removal of it, lactic acidosis may occur. In lactic acidosis, the serum lactate level is more than 5–6 mM and pH less than 7.35 [1].
In post-mortem (PM) context, the lactate levels are elevated as the PM interval (PMI) increases causing a difficulty in diagnosing ante-mortem (AM) lactic acidosis [2]. The elevation has been thought to partly be caused by PM anaerobic glycolysis and therefore reflects the AM glucose concentration [3–5]. The “Traub formula,” where vitreous glucose and lactate are summed, has been used for estimating possible AM hyperglycemia. The use of Traub formula has been recently questioned, and glucose concentration alone is thought to be more reliable than the combined sum in predicting hyperglycemia [6–8]. The Traub value may also be elevated due to excessive alcohol usage leading to alcoholic lactic acidosis, which may also be concomitant with alcoholic ketoacidosis [9]. Although increased Traub sum value may indicate disturbance in glucose metabolism, other markers such as glycated hemoglobin (HbA1c) and ketone bodies need to be analyzed additionally to diagnose the cause of glycemic disturbance [10]. PM changes that elevate the lactate level in addition to anaerobic glycolysis are autolysis and bacterial metabolism products [2, 11].
Distinguishing lactate levels in PM samples that indicate AM lactic acidosis from those that are elevated due to PM changes only is challenging. Lactic acidosis is present in pathological conditions such as diabetes, sepsis, malignancies, cardiogenic shock, and in some poisonings [12]. It has also been suggested that prolonged and/or intense agony related to the mechanism of death elevates lactate levels [13]. One much debated and important topic is the possible association of metformin and lactic acidosis. Metformin is a biguanide widely used in the treatment of type 2 diabetes (DM2). Due to the association of fatal lactic acidosis with another biguanide, phenformin, there have been concerns if similar association exists with metformin [14, 15] Some investigators suggest that lactic acidosis is induced by accumulation of metformin, which inhibits mitochondrial oxidation of glucose and also gluconeogenesis in the liver. On the other hand, in a Cochrane review by Salpeter et al. [16], the conclusion was that there is no significantly higher incidence of lactic acidosis in patients treated with metformin compared to other DM2 treatments. Lactate accumulation could be coincidental rather than causal, caused by diabetes itself or other precipitating factors [17, 18].
In this study, we evaluated PM lactate levels using a large series of cases (n = 1865) to discriminate true disturbance in lactate metabolism from the PM elevation of lactate level. We conducted an assay to measure D-lactate in PM vitreous humor (VH) in order to look for an additional metabolite that could be informative. In addition, we looked for the relation of lactate levels to glucose, ketone bodies, blood alcohol concentration (BAC), PMI, and metformin.
Materials and methods
Autopsy data and post-mortem samples
Data of biochemical analysis from 1865 medico-legal autopsy cases were retrieved to attain average glucose and lactate levels in PM samples where the PMI was known. Another data set of 319 medico-legal autopsy cases was evaluated where glucose, lactate, ketones, BAC, and metformin were analyzed. The PMI in this set was known in 170 (53 %) of the cases, and all 319 deceased were known to have DM2. An additional series of 69 samples from VH that were sent for analysis of glucose, lactate, and ketone bodies were also analyzed for D-lactate concentrations.
The data were attained from the Laboratory Information Management System and from the autopsy records of the National Institute of Health and Welfare. The collection of samples and the use of autopsy data were approved by the National Institute of Health and Welfare (permit number THL/328/5.05.00/2015) and the National Supervisory Authority for Welfare and Health. The study was approved by the Coordinating Ethics Committee of HUCH (statement number 348/13/03/00/2014).
Glucose and lactate assays
Glucose and L-lactate concentrations in VH were measured enzymatically as previously described [4]. The VH was deproteinized by precipitation with trichloroacetic acid. Glucose was measured with hexokinase/glucose-6-phosphate dehydrogenase method and lactate with lactate dehydrogenase method. Glucose values exceeding 7 mM were considered to indicate hyperglycemia, and the value of L-lactate exceeding 35 mM was considered to indicate lactic acidosis [4]. D-Lactate was measured from vitreous humor with Roche Yellow Line enzymatic assay (F. Hoffmann-La Roche Ltd, Switzerland). Values exceeding 3 mM were considered elevated [19].
Ketone body assays
Ketone bodies in VH were measured as previously described [20–22]. With this method, a combined value of β-hydroxybutyrate (BHB), acetoacetate (AcAc), and acetone was measured by first converting BHB to AcAc enzymatically after which the AcAc was in turn converted to acetone by heating the samples leading to decarboxylation. Acetone was quantitated by gas chromatography. Ketone body level >1 mM referred to ketosis, and values exceeding 3 mM indicated ketoacidosis [23].
Metformin measurements
Metformin concentrations were measured in PM blood samples by liquid chromatography–electrospray tandem mass spectrometry using a standard addition method that involved dilution and protein precipitation [24]. Metformin was measured routinely as a part of CoD investigation if the deceased was known to use metformin or other oral antidiabetic medications, or if requested by the forensic pathologist performing the autopsy.
Results
Relation of PMI to glucose and L-lactate levels
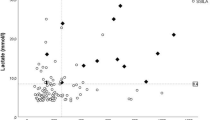
Glucose and lactate levels of 1865 PM cases were evaluated and compared against the PMI of up to 10 days (Table 1 and Fig. 1), with the mean PMI being 5.4 days. Individual values in different PMIs are presented in Online Resources 1 and 2. The mean glucose and lactate values were calculated for all cases and for the following subgroups that were formed based on CoD: DM1, DM2, and metformin poisonings (Table 2 and Fig. 2a, b). Individual values in different PMIs for these subgroups are presented in Online Resources 3 and 4. In addition, a normalized value for lactate was calculated for these groups by dividing the actual lactate value with the mean value of PMI (Table 1). In this data set, there were nine cases where the underlying CoD was metformin poisoning. The detailed data of these cases are presented in Table 3.
a Boxplot presentation of glucose values in all 1865 cases and subgroups DM1 (type I diabetes mellitus, N = 32), DM2 (type II diabetes mellitus, N = 41), and metformin poisonings (N = 9). b Boxplot presentation of lactate values in all 1865 cases and subgroups DM1 (type I diabetes mellitus, N = 32), DM2 (type II diabetes mellitus, N = 41), and metformin poisonings (N = 9)
Correlation between metformin, BAC, metabolic markers, and PMI
Kendall’s tau correlation of glucose, lactate, ketone bodies, metformin, BAC, and PMI in the set of 319 samples was calculated, and the results are presented in Table 4. The values where the significance is at the level p = 0.05 or p = 0.01 (two-tailed) are bolded. Metformin was not detected in 59 of the samples, which indicates that the deceased had not been using it for treatment recently. The mean (±2 standard error (SE)) lactate level in these 59 cases was 37.8 ± 1.9 mM and ketone body level 3.7 ± 2.1 mM. In the remaining 260 cases, the mean metformin concentration (range 1–280 mg/l) was 17.2 ± 3.8 mg/l, lactate level 37.4 ± 0.9 mM, and ketone body level 2.8 ± 0.7 mM.
D-Lactate experiment
D-Lactate was measured from 69 vitreous humor samples. The mean (±2 SE) D-lactate level was 0.42 ± 0.19 mM. No correlation between vitreous D-lactate and L-lactate, glucose, or ketones was detected. PMI and D-lactate had a minor positive correlation with no statistical significance.
In two of the samples, the values exceeded the 3-mM threshold indicating D-lactic acidosis [19]. In the first case, the deceased had advanced post-mortem changes when found and the vitreous was visibly putrefied when sampled. D-Lactate was 5.4 mM, L-lactate 35.7 mM, glucose 1.9 mM, and ketones 0.71 mM. The deceased had no known history of diabetes. In the second case, the death was witnessed and the deceased had been transferred to +4 °C right after the death. D-Lactate was 3.5 mM, L-lactate 47.1 mM, glucose 13.6 mM, and ketones 0.21 mM. The deceased had DM1.
Discussion
L-Lactate levels in post-mortem samples
The lactate level is known to elevate as the PMI increases. This was confirmed by our data of 1865 cases, as the lactate levels elevated until a plateau phase was achieved when PMI was around 8 to10 days. Glycolysis may explain some of the post-mortem elevation of lactate levels, although it is suggested to be limited to the early post-mortem phase [8], and the use of Traub formula indicating the combined sum of glucose and lactate has recently been thought to overestimate diabetic deaths [6, 7]. It has also been suggested that, although the Traub sum alone cannot be used for diagnosing hyperglycemia, it is often elevated in diabetics and may indicate metabolic disturbances [10]. When evaluating the glucose and lactate levels in DM1 and DM2 as the CoD (Table 2), it can be seen that in DM2, the lactate levels explain the elevated Traub sum, whereas in DM1, the lactate levels are lower but the glucose levels significantly elevated. To avoid the confusion of elevated lactate levels to indicate AM hyperglycemia instead of metabolic disturbances, the Traub sum should be omitted and lactate and glucose interpreted independently in CoD investigation.
If glycolysis is restricted to the early PM phase, the elevation as PMI increases is due to autolysis and bacterial metabolism. The lactate elevation is logarithmic and hence similar to the bacterial growth curve (Fig. 1). Although the bacterial growth in VH is less significant than in PM blood [25], the metabolism of micro-organisms seems to have an influence on the lactate levels and therefore the PMI needs to be considered in the interpretation of the results. A normalized value for lactate is preferred due to the effect of PMI for more reliable interpretation than a fixed value. Normalization requires the laboratory to assess a sufficient number of samples with different known PMIs for reference values. Some uncertainty still remains if the exact PMI of samples to be analyzed is not known, which is the case in approximately a half of the medico-legal cases in our institute.
Metformin and L-lactate
In this study, there were nine metformin poisonings in the 1865 cases (Table 3) in which the metformin concentration varied from 7 to 280 mg/l. The metformin poisoning was confirmed to be intentional only in one case; therefore, the impaired kidney function most likely explains the high concentrations in the remaining cases [26]. In four of the metformin poisonings, other precipitating factors for possible lactic acidosis existed: alcohol (cases 1, 4, and 9), hemorrhage (case 5), and hepatic failure (case 9). In addition, in case 1, the normalized lactate value was less than 1. Normalized lactate values are approximately at the same level when the CoD is DM2 or metformin poisoning (Table 2). In cases 2–3, 5, 7, and 8 (Table 3), the ketone body levels refer to ketoacidosis. As lactate is often elevated in ketoacidosis [27], this raises a question if the actual cause for acidosis in some of the metformin poisoning cases is rather the elevated ketone body level while the elevated lactate is only a consequence of ketoacidosis. The clinical laboratory findings in both cases are very similar, including lowered pH and increased anion gap [28]. If ketones are not measured, or as often only the acetoacetate and acetone are measured without the major component beta-hydroxy butyrate (BHB) [29, 30], the ketoacidosis may be missed. Even blood glucose level does not necessarily reveal diabetic ketoacidosis, since the DM2 patients may be close to normoglycemic [31]. Distinguishing in PM context if the lactic acidosis is metformin-induced or metformin-associated [26] is rather complicated if through medical history and both AM and PM laboratory data is not available. A descriptive case was reported by Bonsignore et al. [32], where the accumulation of metformin and following lactic acidosis was diagnosed as the CoD. They presented comprehensive AM and PM data of metabolic markers and metformin. The metformin concentrations in this case were 42 mg/l (AM) and 47.3 mg/l (PM). Markedly elevated BHB levels (blood 10.5 and VH 9.5 mM) were detected in autopsy, but AM data of ketone body concentration was lacking. However, the cause for ketosis was determined to be fasting and emesis prior to death. The lactic acidosis in this case was reliably detected according to AM analysis only, as the PM value of 30 mM was at the range commonly found in PM samples and would have not revealed AM lactic acidosis.
When the sample set of 319 cases was analyzed, it was noticed that the lactate level was similar in cases where no metformin was found compared to the cases where the mean metformin concentration was 17.2 ± 3.8 mg/l (a range from 1 to 280 mg/l). This would suggest that the DM2-related liver complications [33] are the cause for elevated lactate rather than the use of metformin, since lactate is mainly metabolized by the liver. In addition to possibly impaired lactate elimination of the liver, the production of lactate could also be elevated. The prevalence of non-alcoholic fatty liver disease (NAFLD) ranges from 34 to 74 % in DM2 [33]. NAFLD is associated with hypoxia in the liver cells which, in turn, causes the elevation of hypoxia inducible factors (HIFs) [34]. HIF-1 alpha regulates a range of cellular responses including glucose metabolism. It induces the uptake of glucose and cytosolic glycolysis but inhibits the formation of acetyl CoA from pyruvate and promotes the formation of lactate [35]. This could explain the elevated lactate levels in DM2, although the effect of hyperglycemia to HIF expression is controversial [36].
In Table 4, a correlation (Kendall’s tau) was seen between lactate and ketone bodies with metformin (p < 0.01). The limitation in our study is that the PM changes have a greater effect on lactate than ketone bodies, and therefore the threshold limits are not as accurate for diagnosing lactic acidosis as in clinical chemistry. Metformin itself could promote ketoacidosis through activating AMPK in liver, which inhibits gluconeogenesis and promotes the beta-oxidation of fatty acids [37]. On the other hand, the ketone body level is similar in the cases where no metformin was detected compared to the cases where metformin ranged from 1 to 280 mg/l. This would suggest that in diabetics, the metabolic acidosis is often coincidental or coexisting with metformin accumulation due to hepatic and kidney dysfunctions caused by diabetes, although the accumulation may be a precipitating factor increasing the acidosis.
D-Lactate assay
D-Lactic acidosis is very rare and may occur under conditions where malabsorption of carbohydrates is associated with D-lactic acid producing colonial bacteria and impaired D-lactate metabolism [19]. In addition to production in the intestine, D-lactate may be formed in the glyoxylase pathway from methylglyoxal (MG). Elevation of MG and subsequent elevation of D-lactate in diabetes are suggested to be caused by hyperglycemia and ketoacidosis [29], and therefore D-lactate concentrations are higher in diabetics [38]. In this study, we performed a pilot assay to determine D-lactate concentrations of 69 VH samples. In only two of the samples, the level exceeded the 3 mM threshold, which indicates D-lactic acidosis when clinical samples are analyzed [19]. In one of the samples, the reason for the elevated D-lactate was most likely PM bacterial metabolism, since the body showed advanced PM changes and the VH was visibly putrefied. In the other sample, the measured elevation could be explained by diabetic disturbances indicated by elevated L-lactate and glucose. More extensive studies with pre-selected cases (DKA and controls) are needed to explore the suitability of D-lactate analysis for PM samples to resolve the most appropriate method and sample material, and also if the results provide additional information to the assessment of metabolic disturbances.
Conclusions
In PM samples, the lactate level depends largely on the PMI, and this should be considered in the interpretation of the results as the reference values for different PMIs could provide more accurate information of possible AM lactic acidosis. Lactate is often elevated in DM2, indicating metabolic disturbances of the liver caused by diabetes. The relation of metformin to acidosis, according to our results, is rather coexistent than causal in diabetics, although the PM changes on lactate levels cause limitations for interpretation of the results. The usefulness of additional metabolic markers, such as D-lactate, should be studied with a larger PM sample set.
References
Luft D, Deichsel G, Schmulling RM, Stein W, Eggstein M (1983) Definition of clinically relevant lactic acidosis in patients with internal diseases. Am J Clin Pathol 80(4):484–489
Boulagnon C, Garnotel R, Fornes P, Gillery P (2011) Post-mortem biochemistry of vitreous humor and glucose metabolism: an update. Clin Chem Lab Med 49(8):1265–1270. doi:10.1515/CCLM.2011.638
Hess C, Musshoff F, Madea B (2011) Disorders of glucose metabolism-post mortem analyses in forensic cases: part I. Int J Legal Med 125(2):163–170. doi:10.1007/s00414-010-0509-6
Sippel H, Mottonen M (1982) Combined glucose and lactate values in vitreous humour for postmortem diagnosis of diabetes mellitus. Forensic Sci Int 19(3):217–222
Traub F (1969) Method for the detection of lethal glucose metabolism disorders in the corpse (diabetes mellitus and hypoglycemia). Zentralbl Allg Pathol 112(4):390–399
Hess C, Wollner K, Musshoff F, Madea B (2013) Detection of diabetic metabolism disorders post-mortem--forensic case reports on cause of death hyperglycaemia. Drug Test Anal 5(9–10):795–801. doi:10.1002/dta.1479
Palmiere C, Sporkert F, Vaucher P, Werner D, Bardy D, Rey F, Lardi C, Brunel C, Augsburger M, Mangin P (2012) Is the formula of Traub still up to date in antemortem blood glucose level estimation? Int J Legal Med 126(3):407–413. doi:10.1007/s00414-011-0659-1
Zilg B, Alkass K, Berg S, Druid H (2009) Postmortem identification of hyperglycemia. Forensic Sci Int 185(1–3):89–95. doi:10.1016/j.forsciint.2008.12.017
Brinkmann B, Fechner G, Karger B, DuChesne A (1998) Ketoacidosis and lactic acidosis--frequent causes of death in chronic alcoholics? Int J Legal Med 111(3):115–119
Keltanen T, Sajantila A, Palo JU, Partanen T, Valonen T, Lindroos K (2013) Assessment of Traub formula and ketone bodies in cause of death investigations. Int J Legal Med 127(6):1131–1137. doi:10.1007/s00414-013-0917-5
Vivero G, Vivero-Salmeron G, Perez Carceles MD, Bedate A, Luna A, Osuna E (2008) Combined determination of glucose and fructosamine in vitreous humor as a post-mortem tool to identify antemortem hyperglycemia. Rev Diabet Stud 5(4):220–224
Fall PJ, Szerlip HM (2005) Lactic acidosis: from sour milk to septic shock. J Intensive Care Med 20(5):255–271. doi:10.1177/0885066605278644
De Letter EA, Piette MH (1998) Can routinely combined analysis of glucose and lactate in vitreous humour be useful in current forensic practice? Am J Forensic Med Pathol 19(4):335–342
Bailey CJ, Turner RC (1996) Metformin. N Engl J Med 334(9):574–579
Lalau JD (2010) Lactic acidosis induced by metformin: incidence, management and prevention. Drug Saf : Int J Med Toxicol Drug Experience 33(9):727–740. doi:10.2165/11536790-000000000-00000
Salpeter SR, Greyber E, Pasternak GA, Salpeter EE (2010) Risk of fatal and nonfatal lactic acidosis with metformin use in type 2 diabetes mellitus. Cochrane Database Syst Rev 4, CD002967. doi:10.1002/14651858.CD002967.pub4
Scale T, Harvey JN (2011) Diabetes, metformin and lactic acidosis. Clin Endocrinol (Oxf) 74(2):191–196. doi:10.1111/j.1365-2265.2010.03891.x
Stades AME, Heikens JT, Erkelens DW, Holleman F, Hoekstra JBL (2004) Metformin and lactic acidosis: cause or coincidence? A review of case reports. J Intern Med 255(2):179–187. doi:10.1046/j.1365-2796.2003.01271.x
Uribarri J, Oh MS, Carroll HJ (1998) D-lactic acidosis. A review of clinical presentation, biochemical features, and pathophysiologic mechanisms. Medicine 77(2):73–82
Felby S, Nielsen E (1994) Determination of Ketone-Bodies in Postmortem Blood by Headspace Gas-Chromatography. Forensic Sci Int 64(2–3):83–88
Felby S, Nielsen E, Thomsen JL (2008) The postmortem distribution of ketone bodies between blood, vitreous humor, spinal fluid, and urine. Forensic Sci Med Pathol 4(2):100–107. doi:10.1007/s12024-007-9018-4
Thomsen JL, Felby S, Theilade P, Nielsen E (1995) Alcoholic ketoacidosis as a cause of death in forensic cases. Forensic Sci Int 75(2–3):163–171
Laffel L (1999) Ketone bodies: a review of physiology, pathophysiology and application of monitoring to diabetes. Diabetes Metab Res Rev 15(6):412–426. doi:10.1002/(sici)1520-7560(199911/12)15:6<412::aid-dmrr72>3.0.co;2-8
Gergov M, Nenonen T, Ojanpera I, Ketola RA (2015) Compensation of matrix effects in a standard addition method for metformin in postmortem blood using liquid chromatography-electrospray-tandem mass spectrometry. J Anal Toxicol. doi:10.1093/jat/bkv020
Harper DR (1989) A comparative study of the microbiological contamination of postmortem blood and vitreous humour samples taken for ethanol determination. Forensic Sci Int 43(1):37–44
Lalau JD, Arnouts P, Sharif A, De Broe ME (2015) Metformin and other antidiabetic agents in renal failure patients. Kidney Int 87(2):308–322. doi:10.1038/ki.2014.19
Cox K, Cocchi MN, Salciccioli JD, Carney E, Howell M, Donnino MW (2012) Prevalence and significance of lactic acidosis in diabetic ketoacidosis. J Crit Care 27(2):132–137. doi:10.1016/j.jcrc.2011.07.071
Kraut JA, Madias NE (2007) Serum anion gap: its uses and limitations in clinical medicine. Clin J Am Soc Nephrol 2(1):162–174. doi:10.2215/CJN.03020906
Lu J, Zello GA, Randell E, Adeli K, Krahn J, Meng QH (2011) Closing the anion gap: contribution of D-lactate to diabetic ketoacidosis. Clin Chim Acta 412(3–4):286–291. doi:10.1016/j.cca.2010.10.020
Sheikh-Ali M, Karon BS, Basu A, Kudva YC, Muller LA, Xu J, Schwenk WF, Miles JM (2008) Can serum beta-hydroxybutyrate be used to diagnose diabetic ketoacidosis? Diabetes Care 31(4):643–647. doi:10.2337/dc07-1683
Kitabchi AE, Umpierrez GE, Miles JM, Fisher JN (2009) Hyperglycemic crises in adult patients with diabetes. Diabetes Care 32(7):1335–1343. doi:10.2337/dc09-9032
Bonsignore A, Pozzi F, Fraternali Orcioni G, Ventura F, Palmiere C (2013) Fatal metformin overdose: case report and postmortem biochemistry contribution. Int J Legal Med. doi:10.1007/s00414-013-0927-3
Tolman KG, Fonseca V, Dalpiaz A, Tan MH (2007) Spectrum of liver disease in type 2 diabetes and management of patients with diabetes and liver disease. Diabetes Care 30(3):734–743. doi:10.2337/dc06-1539
Suzuki T, Shinjo S, Arai T, Kanai M, Goda N (2014) Hypoxia and fatty liver. World J Gastroenterol 20(41):15087–15097. doi:10.3748/wjg.v20.i41.15087
Weidemann A, Johnson RS (2008) Biology of HIF-1alpha. Cell Death Differ 15(4):621–627. doi:10.1038/cdd.2008.12
Xiao H, Gu Z, Wang G, Zhao T (2013) The possible mechanisms underlying the impairment of HIF-1alpha pathway signaling in hyperglycemia and the beneficial effects of certain therapies. Int J Med Sci 10(10):1412–1421. doi:10.7150/ijms.5630
Pawlyk AC, Giacomini KM, McKeon C, Shuldiner AR, Florez JC (2014) Metformin pharmacogenomics: current status and future directions. Diabetes 63(8):2590–2599. doi:10.2337/db13-1367
Talasniemi JP, Pennanen S, Savolainen H, Niskanen L, Liesivuori J (2008) Analytical investigation: assay of D-lactate in diabetic plasma and urine. Clin Biochem 41(13):1099–1103. doi:10.1016/j.clinbiochem.2008.06.011
Acknowledgments
Financial support for TK was provided by the Doctoral Programme in Population Health (DocPop), University of Helsinki.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Online resource 1
(PDF 23.9 kb)
Online resource 2
(PDF 18.6 kb)
Online resource 3
(PDF 15.2 kb)
Online resource 4
(PDF 15.4 kb)
Rights and permissions
About this article
Cite this article
Keltanen, T., Nenonen, T., Ketola, R.A. et al. Post-mortem analysis of lactate concentration in diabetics and metformin poisonings. Int J Legal Med 129, 1225–1231 (2015). https://doi.org/10.1007/s00414-015-1256-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00414-015-1256-5