Abstract
Immunostaining for beta-amyloid precursor protein (APP) is recognized as an effective tool for detecting traumatic axonal injury, but it also detects axonal injury due to ischemic or other metabolic causes. Previously, we reported two different patterns of APP staining: labeled axons oriented along with white matter bundles (pattern 1) and labeled axons scattered irregularly (pattern 2) (Hayashi et al. (Leg Med (Tokyo) 11:S171-173, 2009). In this study, we investigated whether these two patterns are consistent with patterns of trauma and hypoxic brain damage, respectively. Sections of the corpus callosum from 44 cases of blunt head injury and equivalent control tissue were immunostained for APP. APP was detected in injured axons such as axonal bulbs and varicose axons in 24 of the 44 cases of head injuries that also survived for three or more hours after injury. In 21 of the 24 APP-positive cases, pattern 1 alone was observed in 14 cases, pattern 2 alone was not observed in any cases, and both patterns 1 and 2 were detected in 7 cases. APP-labeled injured axons were detected in 3 of the 44 control cases, all of which were pattern 2. These results suggest that pattern 1 indicates traumatic axonal injury, while pattern 2 results from hypoxic insult. These patterns may be useful to differentiate between traumatic and nontraumatic axonal injuries.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Beta-amyloid precursor protein (APP) is a transmembrane glycoprotein synthesized in neurons [1]. While the actual physiological role of APP is unclear, it may have roles in cell/cell and cell/matrix interactions, growth promotion, and autocrine functions, as well as acting as a binding site for various substances, including heparin [2]. APP is transported along axons to the synapse by fast anterograde transport and is undetectable in normal axons by immunohistochemistry. However, brain insult induces an increase of APP transcription and interrupts its transport, resulting in localized accumulation of APP to detectable levels in the form of axonal bulbs. The detection of APP-positive axonal bulbs is an effective tool for the diagnosis of diffuse traumatic axonal injury in forensic practice [2–9]. The detection of axonal bulbs using conventional hematoxylin and eosin (H-E) or silver staining requires a survival time of at least 15–18 h following head injury. However, immunostaining for APP can detect axonal bulbs as early as 2–3 h after head injury [2–9]. Moreover, some axonal bulbs can be detected at 35 min [10] or 90 min [11] after head injury depending on the case.
Although diffuse axonal injury has been considered the consequence of head trauma, several investigators have emphasized that axonal bulbs can form in the absence of head injury. These axonal bulbs form usually in the presence of both intracranial and systemic pathology, such as cerebral infarction/hemorrhage, meningitis, bronchopneumonia, hypoglycemia, and drug intoxication as well as in cases of ventilator support [5, 7, 12–15]. In particular, hypoxia and ischemic brain damage without head injury show the formation of axonal bulbs. Posttraumatic edema and cerebral and cerebellar herniation secondary to head injury may also cause axonal bulbs. Therefore, it is necessary to distinguish the detection of APP after traumatic axonal injury from other axonal injuries [7, 12–15].
Previously, we reported a preliminary study that suggested that two different immunostaining patterns of APP may distinguish traumatic from nontraumatic axonal injuries [16]. However, no APP-positive staining was observed in control cases with hypoxia/ischemia in the absence of head injury, and therefore, the pattern of hypoxic axonal injury could not be elucidated in that study. Accordingly, in the present study, we performed further investigation using a larger number of head injury cases and control cases and discuss whether the two different immunostaining patterns depict trauma and hypoxic/ischemic brain damage, respectively.
Materials and methods
Forty-four cases of blunt head injury (age range, 2 to 93 years; mean age, 61.0 years; 34 males and 10 females; range of survival time after injury, short time (<10 min) to 90 days) and the same number of control cases showing hypoxia/brain ischemia without head injury (age range, 2 to 92 years; mean age, 54.9 years; 25 males and 19 females) were collected from the Department of Legal Medicine, Graduate School of Medical and Dental Sciences, Kagoshima University. The details of the head injury cases and control cases are summarized in Tables 1 and 2, respectively. From each case, formalin-fixed, paraffin-embedded sections of corpus callosum were immunostained for APP as described previously [11, 16]. Briefly, after deparaffinization and heating to 95 °C in citrate buffer (10 mM, pH 6.0) for 20 min, the sections were immersed in 0.3 % H2O2-phosphate-buffered saline (PBS; pH 7.2) for 30 min to block endogenous peroxidase activity. The sections were then rinsed with PBS and incubated in PBS containing 1 % normal goat serum and 1 % bovine serum albumin (BSA) to reduce nonspecific reactions and with mouse anti-APP monoclonal antibody (clone 22C11, Millipore, Darmstadt, Germany; 1:50) at 4 °C for 14 h. Thereafter, the sections were rinsed three times for 5 min in PBS and incubated with biotinylated goat anti-mouse IgG (1:100) at 18 °C for 1 h. After rinsing in PBS, the sections were incubated with LSAB2 (labeled streptavidin-biotin; DakoCytomation) at 18 °C for 30 min, and positive signals were visualized using 0.02 % 3,3′-diaminobenzidine. The sections were dehydrated and mounted without counterstaining.
Semiquantitation of APP-positive axonal bulbs was performed under ×200 magnification using a light microscope (field area calculated as 0.64 mm2). All sections were examined independently and “blind” to the original diagnosis by two of the authors (T. H. and M. O.). Axonal bulbs were counted as an average from ten fields in each case. According to our previous study [16], the stainings of labeled injured axons, such as varicose axons (sinusoidally swollen axons) and waving axons, were classified into two patterns: labeled axons oriented along with white matter bundles (pattern 1) and axons scattered irregularly (pattern 2). All sections were then reexamined by both observers to reach a consensus.
Results
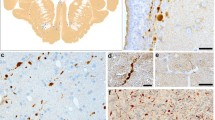
In 24 of the 44 cases of head injury that survived for ≥3 h, at least one axonal bulb per ×200 microscopic field (0.64 mm2) was labeled by APP (Fig. 1 and Table 1). Injured axons, including varicose and waving axons, were also labeled by APP in head injury case, and usually associated with APP-positive axonal bulbs (Fig. 1). Similar to the results of our previous study [16], the staining patterns of labeled injured axons could be divided into two types in 21 of the 24 APP-positive cases (Fig. 1). As shown in Table 1, pattern 1 alone was observed in 14 cases, pattern 2 alone was not observed, and both patterns were detected in 7 cases. In the cases with both patterns, more than five APP-labeled axonal bulbs per ×200 microscopic field (0.64 mm2) were observed and the survival time after injury ranged from 1 to 14 days. In the remaining 3 of the 24 cases, clear patterns were not determined because, in those cases, there were no labeled varicose and/or waving axons which were needed for classification.
Immunostaining for APP in a head injury case with 5-day survival (case 12 in Table 1). a Representative image of pattern 1 showing that APP-labeled axons oriented along with white matter bundles. b Representative image of pattern 2 showing that APP-labeled axons scattered irregularly in white matter. Original magnification, ×200
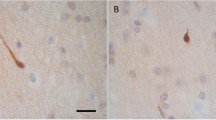
APP-labeled injured axons were also detected in 3 of the 44 control cases (Fig. 2). In those cases, causes of death were sepsis, myocardial infarction, and delayed asphyxia due to ligature ligation, respectively, with survival times from 2 to 14 days (Table 2). In all three cases, the staining patterns of labeled axons were pattern 2 alone (Fig. 2).
APP-positive control cases (a case 1; c case 4; b case 7 in Table 2). In all cases, APP-labeled axons are scattered irregularly in white matter, indicating pattern 2. Original magnification, ×200
Discussion
Several studies have indicated different types of APP immunostaining between traumatic and hypoxic axonal injuries. Graham et al. [8] suggested multifocal and “Z”-shaped patterns, while linear and geographical patterns were described recently by Davceva et al. [17]. These patterns of APP immunostaining indicated the outline of an infarct and, therefore, resulted from vascular complications of an elevated intracranial pressure. Oehmichen et al. [18] suggested that a wave-like pattern may be produced in response to impulsive head rotation or other type of mechanical impact, while the irregularly aggregated pattern may result from hypoxic insult. In the cases reported here, the Z- shaped pattern was not observed. Our results are appropriate since no histological findings of secondary infarction were observed in any cases. In the wave-like pattern, the injured axons were scattered but confined to individual white matter bundles, so that pattern 1 in our study may correspond to the wave-like pattern. In addition, the irregularly aggregated pattern resembles pattern 2 observed in our study. However, Oehmichen et al. [18] did not demonstrate the irregularly aggregated pattern in fatal hypoxic brain without mechanical impact [18].
In the present study, APP-labeled injured axons were detected in 3 of the 44 control cases with hypoxia/brain ischemia in the absence of head injury. Harrington et al. [2] demonstrated that 11 of 20 cases with histological changes of hypoxia without head injury showed positive APP staining and survival times from 1 h to 3 months. Kaur et al. [14] reported that 12 of 25 cases of hypoxia without head injury showed positive APP staining and survival times from a few hours to 68 days. However, specific staining patterns of the labeled axons were not mentioned in their investigations [2, 14]. In our study, pattern 2 (irregularly scattered pattern) alone was detected in the three APP-positive control cases, in which more than one APP-labeled axonal bulb per ×200 microscopic field was detected. Moreover, pattern 1 (oriented along with white matter bundle pattern) alone was detected in 14 of the 44 head injury cases, while cases of pattern 2 alone were not observed. These results suggest that pattern 1 indicates traumatic axonal injury, and pattern 2 results from hypoxic insult. In head injury cases showing both patterns (cases 5, 7, 13, 14, 18, 21, and 22 in Table 1), pattern 2 axons may have resulted from hypoxia, ischemic brain damage, or edema complicated with the head injury.
Local cytoskeletal disruption due to shearing injury may be the initial and main cause of traumatic axonal injuries [14]. Therefore, it seems likely that the injured axons were confined along with the white matter bundles observed in the first pattern of our study, which corresponds to the direction of mechanical impact. On the other hand, a variety of factors, such as metabolic disturbances, loss of cellular membrane integrity, and ultrastructural changes as well as stretching effect due to edema, may associate with hypoxic axonal injuries [2]. Thus, it appears that the injured axons were scattered irregularly depending on a point of weakness to those factors, as observed in pattern 2 of our study.
In clinical and forensic fields, traumatic axonal injury is very difficult to diagnose because the brain appears quite normal on conventional computed tomography (CT) and magnetic resonance imaging (MRI) when the survival period is short. Recently, advances in neuroimaging and laboratory techniques have allowed for more subtle lesions to be detected earlier. Diffusion tensor imaging (DTI) has shown some advantages for detecting axonal injury by acquiring water diffusion in different directions to provide microstructural information about axons or myelin [19–22]. This imaging technique can detect axonal injury within 24 h after head trauma, which was unremarkable in conventional MRI imaging [23, 24]. Moreover, Li et al. [25] demonstrated significant decrease of DTI parameters, including fraction anisotropy and axial diffusivity, corresponding to the axonal damage from 3 h after head trauma in a rat model. DTI is applicable to post mortem imaging in forensic practice, although facilities are limited at present. Additionally, our results suggest that it may be possible to differentiate between traumatic and nontraumatic axonal injuries by the patterns of decrease of DTI parameters.
In conclusion, our results suggest that two different patterns of APP staining in the corpus callosum may distinguish traumatic from nontraumatic injured axons. Several previous studies described differences in the anatomical distribution (corpus callosum, lentiform and caudate nuclei, thalamus, subthalamic regions, medulla, and pons) of APP-positive axons between traumatic and nontraumatic cases [9, 14, 15]. Accordingly, our results have a distinct advantage in enabling the differentiation using only one sample, corpus callosum. Further investigations are required to confirm whether similar results are observed in other anatomical sites of the brain.
References
Selkoe DJ (1994) Normal and abnormal biology of the beta-amyloid precursor protein. Annu Rev Neurosci 17:489–517
Harrington D, Rutty GN, Timperley WR (2000) β-Amyloid precursor protein positive axonal bulbs may form in non-head-injured patients. J Clin Forensic Med 7:19–25
Sherriff FE, Bridges LR, Sivaloganathan S (1994) Early detection of axonal injury after human head trauma using immunocytochemistry for β-amyloid precursor protein. Acta Neuropathol 87:55–62
Sherriff FE, Bridges LR, Gentleman SM, Sivaloganathan S, Wilson S (1994) Markers of axonal injury in post mortem human brain. Acta Neuropathol 88:433–439
Gentleman SM, Roberts GW, Gennarelli TA, Maxwell WL, Adams JH, Kerr S, Graham DI (1995) Axonal injury: a universal consequence of fatal closed head injury? Acta Neuropathol 89:537–543
Blumbergs PC, Scott G, Manavis J, Wainwright H, Simpson DA, McLean AJ (1995) Topography of axonal injury as defined by amyloid precursor protein and the sector scoring method in mild and severe closed head injury. J Neurotrauma 12:565–572
McKenzie KJ, McLellan DR, Gentleman SM, Maxwell WL, Gennarelli TA, Graham DI (1996) Is beta-APP a marker of axonal damage in short-surviving head injury? Acta Neuropathol 92:608–613
Graham DI, Smith C, Reichard R, Leclercq PD, Gentleman SM (2004) Trials and tribulations of using beta-amyloid precursor protein immunohistochemistry to evaluate traumatic brain injury in adults. Forensic Sci Int 146:89–96
Ogata M (2007) Early diagnosis of diffuse brain damage resulting from a blunt head injury. Leg Med (Tokyo) 9:105–108
Hortobágyi T, Wise S, Hunt N, Cary N, Djurovic V, Fegan-Earl A, Shorrock K, Rouse D, Al-Sarraj S (2007) Traumatic axonal damage in the brain can be detected using beta-APP immunohistochemistry within 35 min after head injury to human adults. Neuropathol Appl Neurobiol 33:226–237
Ogata M, Tsuganezawa O (1999) Neuron-specific enolase as an effective immunohistochemical marker for injured axons after fatal brain injury. Int J Legal Med 113:19–25
Oehmichen M, Meissner C, Schmidt V, Pedal I, König HG, Saternus KS (1998) Axonal injury—a diagnostic tool in forensic neuropathology? A review. Forensic Sci Int 95:67–83
Geddes JF, Whitwell HL, Graham DI (2000) Traumatic axonal injury: practical issues for diagnosis in medicolegal cases. Neuropathol Appl Neurobiol 26:105–116
Kaur B, Rutty GN, Timperley WR (1999) The possible role of hypoxia in the formation of axonal bulbs. J Clin Pathol 52:203–209
Oehmichen M, Meissner C, Schmidt V, Pedal I, König HG (1999) Pontine axonal injury after brain trauma and nontraumatic hypoxic-ischemic brain damage. Int J Legal Med 112:261–267
Hayashi T, Ago K, Ago M, Ogata M (2009) Two patterns of beta-amyloid precursor protein (APP) immunoreactivity in cases of blunt head injury. Leg Med (Tokyo) 11:S171–S173
Davceva N, Janevska V, Ilievski B, Spasevska L, Popeska Z (2012) Dilemmas concerning the diffuse axonal injury as a clinicopathological entity in forensic medical practice. J Forensic Leg Med 19:413–418
Oehmichen M, Meissner C, von Wurmb-Schwark N, Schwark T (2003) Methodical approach to brain hypoxia/ischemia as a fundamental problem in forensic neuropathology. Leg Med (Tokyo) 5:190–201
Neil J, Miller J, Mukherjee P, Hüppi PS (2002) Diffusion tensor imaging of normal and injured developing human brain—a technical review. NMR Biomed 15:543–552
Sundgren PC, Dong Q, Gómez-Hassan D, Mukherji SK, Maly P, Welsh R (2004) Diffusion tensor imaging of the brain: review of clinical applications. Neuroradiology 46:339–350
Huisman TA, Schwamm LH, Schaefer PW, Koroshetz WJ, Shetty-Alva N, Ozsunar Y, Wu O, Sorensen AG (2004) Diffusion tensor imaging as potential biomarker of white matter injury in diffuse axonal injury. AJNR Am J Neuroradiol 25:370–376
Mori S, Zhang J (2006) Principles of diffusion tensor imaging and its applications to basic neuroscience research. Neuron 51:527–539
Arfanakis K, Haughton VM, Carew JD, Rogers BP, Dempsey RJ, Meyerand ME (2002) Diffusion tensor MR imaging in diffuse axonal injury. AJNR Am J Neuroradiol 23:794–802
Field AS, Hasan K, Jellison BJ, Arfanakis K, Alexander AL (2003) Diffusion tensor imaging in an infant with traumatic brain swelling. AJNR Am J Neuroradiol 24:1461–1464
Li S, Sun Y, Shan D, Feng B, Xing J, Duan Y, Dai J, Lei H, Zhou Y (2013) Temporal profiles of axonal injury following impact acceleration traumatic brain injury in rats—a comparative study with diffusion tensor imaging and morphological analysis. Int J Legal Med 127:159–167
Acknowledgments
This study was financially supported by Grants-in-Aids for Scientific Research (C) (grant no. 19590677 to M. Ogata) and for Young Scientists (B) (grant no. 22790599 to T. Hayashi) from the Ministry of Education, Culture, Science, and Technology of Japan.
Conflict of interest
The authors have no conflict of interest to declare.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hayashi, T., Ago, K., Nakamae, T. et al. Two different immunostaining patterns of beta-amyloid precursor protein (APP) may distinguish traumatic from nontraumatic axonal injury. Int J Legal Med 129, 1085–1090 (2015). https://doi.org/10.1007/s00414-015-1245-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00414-015-1245-8