Abstract
Purpose
Skeletal muscle tissue is proposed as a forensic model tissue with strong potential, as it is easily accessible and its true-to-life state structure and function is well known. Despite this strong potential, skeletal muscle degradation studies are rare. The aim of this study was to test if a skeletal muscle-based protein analysis is applicable to delimitate the time since death.
Methods
Under standard conditions, two pigs were stored either at 22 °C for 5 days or 4 °C for 21 days. Their Mm. biceps femori were sampled periodically for analyses of ten skeletal muscle proteins postmortem.
Results
All analyzed proteins can serve as markers for a delimitation of the time since death. Desmin, nebulin, titin, and SERCA 1 displayed distinct protein patterns at certain points of time. The other five proteins, α-actinin, calsequestrin-1, laminin, troponin T-C, and SERCA 2, showed no degradation patterns within the analyzed postmortem time frame.
Conclusions
Referring to specific skeletal muscle proteins, results showed short-term stabilities for just a minority of analyzed proteins, while the majority of investigated proteins displayed characteristics as long-term markers. Due to specific patterns and the possibility to determine definite constraints of the presence, absence, or pattern alterations of single proteins, the feasibility of porcine skeletal muscle as forensic model tissue is outlined and the potential of skeletal muscle as forensic model tissue is underlined, especially with respect to later postmortem phases, which so far lack feasible methods to delimitate the time since death.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The delimitation of the time since death is one of the central topics in forensic research and a critical step in many death investigations. In the early postmortem phase, the delimitation of the time since death is relatively definite, because of several combinable methods (complex method) [1, 2]. With increasing time since death, its approximation becomes more diffuse as there are just a few comparative methods being available such as determining the potassium concentration in the vitreous humor [2] or other body fluids as the cerebrospinal fluid [3]. Furthermore, the delimitation of the time since death proves to be intricate due to tissue structures already decomposing by internal degradation processes. The methods used so far for the delimitation of the time since death in late postmortem phase are mainly based on degradation processes and, because of insufficient comparative studies in vivo, primarily lack the practical approach in forensic daily routine [2]. However, progress has been made exploring new methods for postmortem interval estimation, and new lines of research focussing on protein [2, 4, 5], RNA [6], and DNA [7] degradation or real-time RT-PCR analysis of gene expression of clock genes [8] potentially provide future, more precise methods for a delimitation of the time since death.
Due to the former mentioned facts, the temporal delimitation can be fixed by a number of typical characteristics in the early postmortem phase, while late postmortem periods bear severe intricacies, and a series of factors such as way of death, body composition, as well as environmental changes of the location of the corpse all hamper a confident and reliable delimitation of the time since death [5]. Particularly sensitive is the period between the end of rigor mortis and start of degradation processes, as this period basically lacks reliably applicable methods. So far, to our knowledge, the validity of chemical methods is still limited. This is underlined by the fact that current methods to estimate the time since death are not always accurate or reproducible [5]. In this context, skeletal muscle tissue is proposed as a forensic model tissue as it has a strong potential for delimitation of the time since death: it is easily accessible; its true-to-life state structure and function is well known, including developmental, sex-specific, and age-dependent characteristics; and finally, depending on the specific cause of death, there are several skeletal muscles to select from.
A variety of meat quality studies reports proteolysis of myofibrillar proteins being a key event during postmortem degradation, with a temporally staggered degradation of the various proteins [9]. Proteins that are degraded during muscle fiber degradation are specific myofibrillar, cytoskeletal, and costamere proteins, such as troponin-I and troponin-T, desmin, vinculin, filamin, dystrophin, titin, and nebulin, and some cleavage was also observed of the major myofibrillar proteins such as actin and myosin [9–15].
Although proposed to have a strong potential as model tissue for the delimitation of the time since death, studies focussing on skeletal muscle degradation processes are rare. Knowledge of alterations of skeletal muscle proteins, gained by meat science studies to contribute to meat quality attributes, may only provide basic knowledge of degradation processes, but are nonnegotiable to forensic science, as these experiments were carried out solely in cold environments after standard slaughtering processes and dressing of the body.
Therefore, the aim of this study was to specifically characterize postmortem alterations of skeletal muscle proteins at standardized low and warm temperature regimes to contribute to forensic research by providing a novel approach for the delimitation of the time since death.
Material and methods
Sampling
Under veterinary guidance, pigs were anesthetized by a bolt apparatus strongly put onto the forehead. By severance of the spinal marrow between the first and second cervical vertebra, the pigs were sacrificed. With the absence of vital signs (no heart beat, no eye lid reflex, no corneal reflex, no rhythmic breathing) and the presence of injury interfering with life, death was confirmed. The carcasses were fixated in supine position on wooden platforms. The upper hind limbs had no contact to the underground and were easily accessible without moving the hind limbs. One pig was stored at 22 ± 2 °C for 5 days postmortem, and the second pig was stored at 4 ± 1 °C for 21 days postmortem. The environmental conditions (room temperature and humidity) as well as the body core temperature were documented digitally. For sampling purposes, the hind limbs were washed with water to remove superficial dirt. The bristles were shaved off and the skin was cleaned finally with 70 % ethanol. The integument was opened carefully without influencing the connective tissue underneath and the epimysium of the skeletal muscle removed with caution at the sampling site. At each point of time postmortem, a superficial (3 cm deep, measured from the surface skin) muscle sample was dissected. Two 100-mg samples were shock frozen in liquid nitrogen and stored in a Dewar. Between two sampling areas approximately 3 cm was left out to avoid artificial structural alterations (caused by previous dissections). After each dissection, the integument was closed carefully by means of surgical sewing to avoid external influences on the site.
Skeletal muscle tissue was homogenized by means of cryogenic grinding with a motor-driven pestle and a mortar (CryoGrinderTM OPS Diagnostics, LLC.). Skeletal muscle powder was transferred into a precooled tube, weighed, and finally solubilized in ten volumes of a freshly produced ice-cold extraction buffer. The extraction buffer was a RIPA buffer (ready-to-use) containing 0.025 M Tris-HCl pH 7.6, 0.15 M sodium chloride, 1 % NP-40, 1 % sodium deoxycholate, and 0.1 % sodium dodecyl sulfate (SDS) with additionally 0.005 M ethylenediaminetetraacetic acid (EDTA) and a protease inhibitor (complete mini protease inhibitor cocktail). The homogenate was further broken down by ultrasonication (Bandelin Sonopuls GM 70) with three bursts of 50 % of maximum cycling. After centrifugation at 1500 rpm for 10 min, the supernatant fraction was collected and split into two single probes.
The protein concentration was determined using Pierce BCA Assay Kit. Protein concentration was determined by using a bovine serum albumin (BSA) standard curve. The samples were stored at −20 °C till use.
SDS-polyacrylamide gel electrophoresis and Western blot
Dependent on the size of the particular protein (25 to 220 kD), discontinuous SDS gels were used with different percentages of the separating gel (8, 10, and 12.5 %). The separation gel was made of a ready-to-use acrylamide to N,N′-bis-methylene acrylamide 37.5:1 (wt/wt) solution, 0.375 M (wt/vol) Tris-HCl pH 8.8, 0.4 % (wt/vol) SDS, 0.05 % N′N′N′N′-tetramethylethylenediamine (TEMED), and 0.05 % (wt/vol) ammonium persulfate (APS). The stacking gel contained 5 % of a ready-to-use acrylamide to N,N′-bis-methylene acrylamide 37.5:1 (wt/wt) solution, 0.125 M (wt/vol) Tris-HCl pH 6.8, 0.4 % (wt/vol) SDS, 0.125 % TEMED, and 0.075 % (wt/vol) APS.
For large proteins (200 to 3000 kD) a 5 % separation gel without stacking gel was used. Besides the 5 % polyacrylamide solution which consisted of the two components acrylamide and N,N′-bis-methylene acrylamide in a ratio of 100:1 (wt/wt), the gel contained 0.1 % SDS, 0.2 M Tris-HCl pH 8.0, and 2 mM EDTA.
The antibody regime used was α-actinin (N-19), calsequestrin 1 (D-10), desmin (DE-R-11), nebulin (N-19), SERCA 1 (N-19), SERCA 2 (N-19), titin (N-20), tropomyosin (CH1), and troponin T-C (C-19). from Santa Cruz Biotechnologies, Inc. as well as laminin (AHP420) from AbD Serotec and μ-calpain (9A4H8D3) from Thermo Scientific.
Gels were run at vertical double gel systems (PEQLAB Biotechnologie GmbH). The running buffer for the discontinuous SDS-polyacrylamide gel electrophoresis (PAGE) contained 0.025 M Tris, 0.192 M glycine, 0.1 % SDS, and 0.002 M EDTA. According to the percentage of each individual skeletal muscle protein in the whole muscle homogenates, the samples were diluted with aqua bidest till achieving the optimum protein concentrations (20–100 μg) for Western blot detection. Additionally, 5 μl ×4 SDS-PAGE sample buffer containing 0.25 M Tris-HCl pH 6.8, 8 % SDS, 20 % 2-mercaptoethanol (MCE), 40 % glycerol, and 0.08 % bromophenol blue was added to each diluted sample for denaturation and negative charge loading. Samples were denatured at 50 °C for 20 min, and immediately afterwards, 20 μl of each sample as well as a prestained protein marker were loaded onto the gels. Gels were run on a 300-V power supply (PEQLAB Biotechnologie GmbH) at constant voltage settings of 150 V at room temperature. The running buffer for the continuous SDS-PAGE contained additionally 0.1 % MCE to aid in mobilization of these large proteins. Gels were loaded with 120 μg of denatured protein and run at 3 mA for approximately 24 h.
Gels used for studying all protein bands were stained overnight in an excess of 0.1 % (wt/vol) Coomassie Brilliant Blue R-250 dye solved in 40 % methanol, 10 % acetic acid, and 50 % aqua bidest. Afterwards, gels were destained in an excess of a solution containing 50 % methanol, 40 % aqua bidest, and 10 % acetic acid till the protein bands were clearly visible and the background destained completely. For documentation purposes, gels were finally dried between two layers of wetted preserving membrane under vacuum conditions in a Gel Dryer Model 583 (Bio-Rad) for 2 h at 60 °C.
Gels used for protein detection were equilibrated for 20 min in ice-cold transfer buffer containing 0.025 M Tris, 0.192 M glycine, 0.002 M EDTA, and 20 % methanol. The gel sandwich was blotted in a PerfectBlue Tank Electroblotter (PEQLAB Biotechnologie GmbH) filled with transfer buffer. The transfer was performed for 75 min under constant circulation of the buffer at 4 °C and 250 mA. The membranes were either stained in 0.1 % (wt/vol) Ponceau S red solved in 1 % acetic acid for 1 min or destained some seconds in aqua bidest to control the success of the protein transfer.
Protein detection
Membranes were blocked in 0.025 M Tris-HCl pH 7.5, 0.15 M sodium chloride, 0.05 % Tween 20, and 1 % BSA. Primary antibodies were incubated for 2 h. Secondary antibodies were labelled with horse radish peroxidase (HRP). Signals were made visible on X-ray films (Amersham HyperfilmTM ECL, high-performance chemiluminescence film, GE Healthcare) after incubation in SuperSignal West Pico Chemiluminescence solution. The documentation of the degradation patterns of the single proteins was done qualitatively by the documentation of protein degradation at 22 and 4 °C, respectively.
In order to avoid false results as well as artifacts, four Western blot repetitions were carried out for each protein with the best blots being shown herein. With respect to all proteins, no artifacts occurred during analyses.
To enable qualitative analysis of protein-specific degradation profiles, specific amounts of the protein homogenate, based upon predetermined protein concentrations, were loaded onto the SDS-PAGE gels, to generate profiles, indicating equivalent protein loading. Slight variations in the amounts occurred seldomly, which were the result of minor application errors during SDS-PAGE procedures, but did not affect the final result.
Results
Postmortem observations
Both pigs had similar features regarding their age, the body weight, the feeding and keeping conditions, the slaughtering method, and the storage position. The body core temperature was similar with 39.6 and 39 °C. At 22 °C storage temperature, the alignment of the body core temperature to the environmental temperature was observed after 30 h postmortem (hpm), while at 4 °C, this circumstance was reached after 96 h.
The pig carcass stored at 22 °C showed classical signs of death with expression of livor mortis at the head and neck region from 12 hpm onwards; greenish autolytical stains at the eye (48 hpm), ear (48 hpm), and inguinal region (36 hpm); gas assembling under the skin in the thorax region (36 hpm); and later postmortem also in the inguinal region (48 hpm), translucency of the venous lattice (36 hpm), protrusion of the rectum (54 hpm) and the eyes (72 hpm) as well as softening of the tissue.
At 4 °C, only faint greenish small stains in the inguinal region were observed from 132 hpm onwards and no further signs of decomposition were observed till 504 hpm.
Protein degradation
At 0 h, all proteins as well as μ-calpain were successfully demonstrated on the Western blot or, in case of titin and nebulin, on the SDS-PAGE gel, due to their high molecular weights.
All proteins were detectable till 5 days at 22 °C and 21 days at 4 °C, respectively. For both temperature regimes, proteins were assigned to three groups, due to their postmortem patterns at Western blot level.
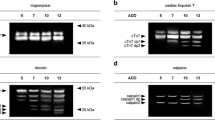
The first group of proteins showed no discernible changes in their patterns during postmortem time courses at 22 and 4 °C (Fig. 1). This group included α-actinin, calsequestrin-1, laminin, and tropomyosin. α-Actinin was resistant to proteolysis, while laminin and calsequestrin-1 displayed slight variations in its detectable amount, but were resistant to proteolysis until 5 days postmortem at 22 °C and 21 days postmortem at 4 °C, respectively (Fig. 1).
Western blot analysis of the first group containing α-actinin, calsequestrin-1, laminin, and tropomyosin during 5 days (120 h postmortem (hpm)) at 22 °C and 21 days (504 hpm) at 4 °C. This group of protein showed no signs of proteolysis on Western blot level. The time lines are displayed horizontally for each protein, while vertically, the molecular size markers for each protein are shown in kilodalton (kD). On the left side of the figure, the protein degradation patterns at 22 °C are displayed, while on the right side their patterns at 4 °C are seen
The second group included the proteins troponin T-C and SERCA 2, which showed clear decreases in their detectable amounts without formation of protein fragments during postmortem time courses at 22 and 4 °C (Fig. 2). At 22 °C, troponin T-C was initially resistant to degradation, until, after 4 days postmortem, its amount decreased strongly, and at 5 days postmortem only a weak band was determined. At 4 °C, troponin T-C degradation progressed at a slower rate, with a minor loss in its amount between 15 and 21 days postmortem. A degradation of SERCA 2 was determined at both storage temperature regimes. At 22 °C, the amount of SERCA 2 varied slightly between 1 and 4 days postmortem, until, after this point of time, a strong decline was observed leading to an almost complete loss of signal at 5 days postmortem. At 4 °C, the degradation of SERCA 2 progressed rather continuously compared to the one at 22 °C (Fig. 2).
Western blot analysis of the second group containing troponin T-C and SERCA 2 during 5 days (120 h postmortem (hpm)) at 22 °C and 21 days (504 hpm) at 4 °C. Both proteins showed a strong decrease at 4 days postmortem at 22 °C, while at 4 °C both proteins displayed no proteolytic fragments till 21 days postmortem. At 21 days postmortem, a red arrow points out the discernible band of SERCA 2 at 21 days postmortem. The time lines are displayed horizontally for each protein, while vertically the molecular size markers for each protein are shown in kilodalton (kD). On the left side of the figure, the protein degradation patterns at 22 °C are displayed, while on the right side their patterns at 4 °C are seen
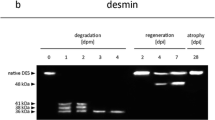
Desmin, nebulin, titin, and SERCA 1 were combined to a third group, reflecting proteins with distinct postmortem patterns (Fig. 3). At both storage temperatures, desmin was very susceptible to proteolysis. At 22 °C, degradation of desmin led to the appearance of a first distinctive protein fragment of approximately 39 kD, which was detected between 1 and 5 days postmortem, followed by two additional smaller sized fragments of about 36 and 35 kD at 5 days postmortem. At 4 °C, three prominent protein fragments of approximately 45, 39, and 35 kD were seen between 10 and 21 days postmortem (Fig. 3).
Western blot analysis of the third group containing desmin and SERCA 1 and two SDS-PAGE gel analyses of nebulin and titin during 5 days (120 h postmortem (hpm)) at 22 °C and 21 days (504 hpm) at 4 °C. All four proteins showed several degradation fragments (red and black arrows) starting at 1 day postmortem at 22 °C and at 10 days postmortem at 4 °C. The time lines are displayed horizontally for each protein, while vertically the molecular size markers for each protein are shown in kilodalton (kD). On the left side of the figure, the protein degradation patterns at 22 °C are displayed, while on the right side their patterns at 4 °C are seen
Titin and nebulin degradation was followed up on the SDS-PAGE gel, displaying a similar degradation pattern. Based on equal analysis procedure as described in the literature, titin and nebulin bands could be identified on the SDS-PAGE gels, despite the absence of an appropriate high molecular weight marker. By reason of this fact, the exact shifts in their molecular weights could not be defined. At 22 °C, both proteins showed their first shifts at 1 day postmortem, followed by the second one being observable at 84 h postmortem. At 4 °C, titin and nebulin displayed only one shift in their molecular weights between 0 and 10 days postmortem, identical to that observed at 22 °C (Fig. 3).
SERCA 1 was susceptible to postmortem proteolysis, showing multiple protein fragments at 22 °C. At 22 °C, proteolysis led to a first distinctive protein fragment with a molecular weight of about 70 kD between 3 and 4 days postmortem, followed by three additional smaller sized protein fragments of approximately 54, 39, and 35 kD between day 4 and 5 postmortem. At 4 °C, a first faint protein fragment of about 70 kD was detectable between 15 and 21 days postmortem (Fig. 3).
The 80-kD subunit of the proteinase μ-calpain did not decrease during 5 days postmortem at 22 °C and 21 days at 4 °C, respectively (Fig. 4). During 5 days postmortem at 22 °C, the 80-kD catalytic subunit of μ-calpain was seen. Between day 1 and 5 at 22 °C, an approximately 70-kD fragment was present next to the catalytic subunit. At 4 °C, the 80-kD subunit was seen during 21 days postmortem.
Western blot analysis of μ-calpain during 5 days (120 h postmortem (hpm)) at 22 °C and 21 days (504 hpm) at 4 °C. The time lines are displayed horizontally, while vertically the molecular size markers are shown in kilodalton (kD). During 5 days postmortem at 22 °C, the 80-kD catalytic subunit of μ-calpain was seen. Between day 1 and 5 at 22 °C, an approximately 70-kD fragment was present next to the catalytic subunit (red arrow). At 4 °C, the 80-kD subunit was seen during 21 days postmortem. On the left side of the figure, degradation patterns at 22 °C are displayed, while on the right side their patterns at 4 °C are seen
Discussion
The aim of this study was to determine proteins with specific postmortem patterns, which may represent feasible markers for a delimitation of the time since death, with focus on the intermediate and late postmortem phase.
As to the present study, degradation patterns of ten proteins, out of the wide range of skeletal muscle proteins, were analyzed.
While four proteins, namely, α-actinin, tropomyosin, laminin, and calsequestrin-1, were highly resistant to proteolysis, six proteins, namely, desmin, nebulin, SERCA 1, SERCA 2, titin, and troponin T-C, were susceptible to degradation.
During the two chosen postmortem experimental periods of time, no complete degradation of any analyzed protein was achieved, highlighting the general resistance of skeletal muscle tissue to degradation and, in particular, of its proteins, and once more demonstrates the high potential of skeletal muscle as means of delimitation of the time since death.
Standardizing protein levels postmortem holds a problem, since standard markers (for example actin, tubulin), that are usually used, may also undergo digestion. To be able to qualitatively analyze degradation patterns of proteins, a loading-specific approach was chosen [5]. For this purpose, a particular amount of cell homogenate, based upon predetermined protein concentrations, was applied to generate equivalent protein loading profiles in SDS-PAGE gels.
Due to this long-term stability during postmortem storage at 22 and 4 °C, laminin, a protein of the basement membrane, may serve as an estimator for the late postmortem phase at cold and warm environmental temperatures.
Based on several histological and fine structural findings, suggesting an early postmortem alteration of the cytoskeleton, desmin, a main constituent of the cytoskeleton, was chosen as representative of cytoskeletal proteins. Several studies report on early postmortem desmin degradation in porcine skeletal muscle [16, 17], which is underlined by the present study. The molecular size range of these fragments is similar to the sequential degradation of desmin that might result in destabilization of the intermediate filament network as described by Baron et al. [9]. With respect to a delimitation of the time since death, desmin may serve as an early postmortem marker at warm environmental temperatures, due to the appearance of a first characteristic protein fragment within the first day, but moreover, desmin has the capability to serve as a marker for the late postmortem phase at both warm and cold environmental temperatures.
The majority of the analyzed proteins belongs to the group of myofibrillar proteins, and various meat science studies report on their degradations [11, 18–20].
α-Actinin, a cross-linking protein of the Z-disc complex, demonstrated its ability as long-term postmortem marker. This finding is supported by α-actinin being detectable throughout a 56-day postmortem period at 4 °C in lamb skeletal muscle [19].
Based on fine structural myofibrillar observations, the I-band region was highly susceptible to postmortem degradation. Three proteins of the thin filament, namely, tropomyosin, troponin T-C, and nebulin, were chosen for examination of this region.
With respect to tropomyosin, a regulatory and strengthening protein, no literature was found so far, while several studies focus on troponin-T and nebulin degradation [18–20].
Postmortem troponin-T studies demonstrated its presence till the late postmortem phase, generally supporting the present results, but in addition, formations of one to two protein fragments are described commonly, which were not observable in the present study, indicating undetected cleavage of the troponin-T subunit.
In accordance to the present results of nebulin degradation, a further strengthening protein of the myofilament, a complete degradation of nebulin was observed till 42 days postmortem at 4 °C in ovine skeletal muscle [19], while in contrast to that, an absence of nebulin after 3 days postmortem at 4 °C was seen in bovine skeletal muscle [18]. Due to the absence of an adequate molecular weight marker, the exact shifts in the molecular weight of nebulin could not be determined, but similarities may be assumed between the bovine, ovine, and porcine model [18–20].
With respect to a determination of the time since death, tropomyosin may serve as long-term postmortem marker, while nebulin may be used as marker for both postmortem phases showing distinct patterns in the early and late postmortem phase. Troponin T-C, in addition, might be helpful as estimator for the beginning of the late postmortem phase in warm environmental settings and for the late postmortem phase in cold settings.
Titin, connecting thick filaments to the Z-disc complex, has been described as susceptible to postmortem proteolysis [18, 19]. As to the present study, the observed degradation patterns are generally in accordance with those of the aforementioned studies, although temporal differences in their traceability, due to different storage proceedings, were observed. Degradation of titin leads to a weakening of the sarcomere structure and contributes to meat tenderness [18]. Due to its pattern and with regard to a delimitation of the time since death, titin may serve as a marker for both postmortem phases, the early and the late one, respectively.
Knowledge of postmortem sarcoplasmic reticulum alterations and of the calcium homoeostasis was gained by the analysis of calsequestrin-1, SERCA 1, and SERCA 2. At both storage temperatures, calsequestrin-1, the major calcium binding protein of the sarcoplasmic reticulum of fast twitch muscle fibers, seemed resistant to proteolysis during 5 and 21 days postmortem. The Ca2+-ATPases are located in the membrane of the sarcoplasmic reticulum of either slow twitch (SERCA 2) or fast twitch (SERCA 1) muscle fibers [21]. Due to the degradation of SERCA, it can be inferred that the membrane of the sarcoplasmic reticulum is broken down postmortem, exposing membrane-enclosed SERCA to proteases. With regard to the differences in their degradation patterns and the fact that both antibodies bind to the same region, it might be deduced that proteases cleave SERCA 1 and 2 differently. With respect to a delimitation of the time since death, calsequestrin-1 may serve as long-term postmortem estimator beyond 5 and 21 days postmortem at both temperature regimes, while SERCA 1 and 2 may be used as markers for the beginning of the late postmortem phase. Especially SERCA 1, which displayed distinct protein fragments, has a high potential as marker.
In the present study, the occurrence of the Ca2+-dependent proteinase μ-calpain was used to determine proteolytic activity, as μ-calpain is the widely proposed main protease in postmortem skeletal muscle [9]. The applied antibody detected the 80-kD catalytic subunit of μ-calpain. As reported by other authors, intact μ-calpain degrades to a 76-kD fragment, due to autolysis in the presence of sustained high postmortem calcium concentrations, ultimately leading to loss of activity [22, 23].
Based on the strong signal intensity of the μ-calpain antibody, the high amount of applied protein homogenate, and its quick appearance during film processing, the autolysis of its 80-kD subunit to 76 kD may be superposed on the film, hampering a conclusion of its assumed initial autolysis.
The early observable 70-kD fragment at 22 °C may indicate subsequent degradation of μ-calpain due to the presence of a sustained high calcium concentration and, moreover, may highlight its degradation through additional proteases, probably caspases [15]. The proteolytic activity of μ-calpain in porcine skeletal muscle is underlined by the findings of desmin, nebulin, and titin degradation patterns, which correspond to specific μ-calpain cleavage profile [18]. At 4 °C, a likewise early μ-calpain activation during the first 2 days postmortem and a longer duration of its activity may be hypothesized, due to pattern persistence of desmin, nebulin, and titin between 10 and 21 days and a previously carried out study [10].
Conclusions
With respect to the skeletal muscle protein-based approach, their analysis displays a great potential for a delimitation of the time since death, due to specific degradation patterns and the possibility to determine definite constraints of the presence, absence, or pattern alterations of single proteins, most notably with respect to the later postmortem phases, which so far lack accurate methods. Moreover, the fact that antibodies are commercially available for a wide range of skeletal muscle proteins and that the methodological approach may be standardized easily emphasizes the potential of this protein-based approach. With regard to particular postmortem interval markers, desmin, nebulin, titin, and SERCA 1 exhibit specific patterns, pointing out to their applicability to precisely delimitate the time since death during both the early and late postmortem phase. All other, so far regarded, proteins as α-actinin, calsequestrin-1, laminin, troponin T-C, and SERCA 2 possess postmortem stability properties, making them ideal markers for the intermediate and late postmortem phase, probably even by displaying specific patterns within this phase, which need to be examined in subsequent studies.
However, the obtained basic science results were shown in an animal study with controlled environmental conditions. To bring the results to daily forensic practice, a clinical trial is needed to test the chosen protein regime on human corpses with known times of death under the consideration of the different influence factors as age, temperature regime, and cause of death. This will point out the applicability of this protein-based approach for daily forensic practice. Furthermore, a subsequent animal study should test further temperature regimes as well as further proteins during extended postmortem storage times. This will give insights on how an increase of the range of available proteins may enhance the accuracy of a skeletal muscle protein-based delimitation of the time since death for forensic practice.
References
Brinkmann B, Madea B (2004) Handbuch gerichtliche Medizin. Springer, Germany
Wehner F (2009) Die Eingrenzung der Leichenliegezeit im spätpostmortalen Intervall. Med Welt 6:1–6
Madea B, Musshoff F (2007) Postmortem biochemistry. Forensic Sci Int 165:165–171
Kang S, Kassam N, Gauthier ML, O’Day D (2003) Post-mortem changes in calmodulin binding proteins in muscle and lung. Forensic Sci Int 131:140–147
Poloz YO, O’Day D (2009) Determining time of death: temperature-dependent postmortem changes in calcineurin A, MARCKS, CaMKII, and protein phosphatase 2A in mouse. Int J Legal Med 123:305–314
Bauer M, Gramlich I, Polzin S, Patzelt D (2003) Quantification of mRNA degradation as possible indicator of postmortem interval—a pilot study. Legal Med 5:220–227
Yi SH, Zhao XH, Liu L (2008) Selection parameters to infer post-mortem interval by detecting DNA degradation using comet assay. Chin J Forensic Med 23:1–4
Kimura A, Ishida Y, Hayashi T, Nosaka M, Kondo T (2011) Estimating time of death based on the biological clock. Int J Legal Med 125:385–391
Koomaraie M, Geesink GH (2006) Contribution of post mortem muscle biochemistry to the delivery of consistent meat quality with particular focus on the calpain system. Meat Sci 74:34–43
Goll DE, Thompson VF, Taylor RG, Christiansen JA (1992) Role of the calpain system in muscle growth. Biochimie 74:225–237
Taylor RG, Geesink GH, Thompson VF, Koomaraie M, Goll DE (1995) Is Z-disc degradation responsible for post-mortem tenderization? J Anim Sci 73:1351–1367
Talor RG, Koomaraie M (1998) Effects of postmortem storage on the ultrastructure of the endomysium and myofibrils in normal and callipyge longissimus. J Anim Sci 76:2811–2817
Hopkins DL, Thompson JM (2002) The degradation of myofibrillar proteins in beef and lamb using denaturing electrophoresis—an overview. J Muscle Foods 13:81–102
Lametsch R, Karlsson A, Rosenvold K, Andersen H, Roepstorff P, Bendixen E (2003) Postmortem proteome changes of porcine muscle related to tenderness. J Agric Food Chem 51:692–700
Kemp CM, Sensky PL, Bardsley RC, Buttery PJ, Parr T (2010) Tenderness—an enzymatic view. Meat Sci 84:248–256
Baron CP, Jacobsen S, Purslow PP (2004) Cleavage of desmin by cysteine proteases: calpains and cathepsin B. Meat Sci 68:447–456
Bee G, Anderson AL, Lonergan SM, Huff-Lonergan E (2007) Rate and extent of pH decline affect proteolysis of cytoskeletal proteins and water-holding capacity in pork. Meat Sci 76:359–365
Huff-Lonergan E, Mitshuhasi T, Parrish FC Jr, Robson RM (1996) Proteolysis of specific muscle structural proteins by mu-calpain at low pH and temperature is similar to degradation in postmortem bovine muscle. J Anim Sci 74:993–1008
Geesink GH, Koomaraie M (1999) Postmortem proteolysis and calpain/calpastatin activity in callipyge and normal lamb biceps femoris during extended postmortem storage. J Anim Sci 77:1490–1501
Melody JL, Lonergan SM, Rowe LJ, Huiatt TW, Mayes MS, Huff-Lonergan E (2004) Early post mortem biochemical factors influence tenderness and water-holding capacity of three porcine muscles. J Anim Sci 82:1195–1205
Sacchetto R, Bertipaglia I, Giannetti S, Cendron L, Mascarello F, Damiani E, Carafoli E, Zanotti G (2012) Crystal structure of sarcoplasmic reticulum Ca2+-ATPase (SERCA) from bovine muscle. J Struct Biol 178:38–44
Goll DE, Thompson VF, Li HQ, Wei W, Cong JY (2003) The calpain system. Physiol Rev 83:731–801
Pomponio L, Lametsch R, Karlsson AH, Costa LN, Grossi A, Ertbjerg P (2008) Evidence for post mortem m-calpain autolysis in porcine muscle. Meat Sci 80:761–4
Acknowledgments
The authors thank Dr. Walter Stoiber, Dr. Peter Steinbacher, and Prof. Edith Tutsch-Bauer for their input.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Foditsch, E.E., Saenger, A.M. & Monticelli, F.C. Skeletal muscle proteins: a new approach to delimitate the time since death. Int J Legal Med 130, 433–440 (2016). https://doi.org/10.1007/s00414-015-1204-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00414-015-1204-4