Abstract
For locally advanced cervical cancer, the standard therapeutic approach involves concomitant chemoradiation therapy, supplemented by a brachytherapy boost. Moreover, an external beam radiotherapy (RT) boost should be considered for treating gross lymph node (LN) volumes. Two boost approaches exist with Volumetric Intensity Modulated Arc Therapy (VMAT): Sequential (SEQ) and Simultaneous Integrated Boost (SIB). This study undertakes a comprehensive dosimetric and radiobiological comparison between these two boost strategies. The study encompassed ten patients who underwent RT for cervical cancer with node-positive disease. Two sets of treatment plans were generated for each patient: SIB-VMAT and SEQ-VMAT. Dosimetric as well as radiobiological parameters including tumour control probability (TCP) and normal tissue complication probability (NTCP) were compared. Both techniques were analyzed for two different levels of LN involvement – only pelvic LNs and pelvic with para-aortic LNs. Statistical analysis was performed using SPSS software version 25.0. SIB-VMAT exhibited superior target coverage, yielding improved doses to the planning target volume (PTV) and gross tumour volume (GTV). Notably, SIB-VMAT plans displayed markedly superior dose conformity. While SEQ-VMAT displayed favorable organ sparing for femoral heads, SIB-VMAT appeared as the more efficient approach for mitigating bladder and bowel doses. TCP was significantly higher with SIB-VMAT, suggesting a higher likelihood of successful tumour control. Conversely, no statistically significant difference in NTCP was observed between the two techniques. This study’s findings underscore the advantages of SIB-VMAT over SEQ-VMAT in terms of improved target coverage, dose conformity, and tumour control probability. In particular, SIB-VMAT demonstrated potential benefits for cases involving para-aortic nodes. It is concluded that SIB-VMAT should be the preferred approach in all cases of locally advanced cervical cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cervical cancer is the fourth most common malignancy in women and also the fourth most common cause of death from cancer in women worldwide (Sung et al. 2021). For locally advanced diseases, concomitant Cisplatin-based chemoradiation therapy is considered the standard of care with a brachytherapy boost to the local disease (Green et al. 2001; Hsu et al. 2015; Shrivastava et al. 2018). Additionally, an external beam radiotherapy (EBRT) boost should be given to nodal volumes in patients with lymph node (LN) involvement (Bacorro et al. 2018; Wujanto et al. 2019; Kim et al. 2020). Administration of this boost dose to involved LNs is associated with increased normal tissue toxicity because of a larger irradiated volume exposed to higher radiotherapy (RT) doses. This toxicity is further augmented due to the use of concomitant chemotherapy during RT. Therefore, an intensity-modulated technique such as Volumetric Intensity Modulated Arc therapy (VMAT) is favored as it allows for dose intensification while mitigating normal tissue toxicity (Gaffney et al. 2011; Knapp et al. 2019). There are two possible ways of nodal boost during EBRT: Sequential (SEQ) or Simultaneous Integrated Boost (SIB). A sequential boost can be delivered to gross nodes which allows dose intensification with low treatment-related toxicities (Shewalkar et al. 2022). This modality was always considered to be an acceptable option keeping in mind the concern for normal tissue tolerance. Nevertheless, SEQ-RT increases the total treatment time, particularly if a boost is delivered before brachytherapy which could potentially worsen local tumour control (Tanderup et al. 2016; Lin et al. 2017; Hong et al. 2017). On the other hand, the non-homogeneous irradiation of the tumour and lymph nodes to different doses with SIB delivers the initial and boost doses together in a smaller number of fractions and, thus, in a shorter overall treatment time (OTT). However, SIB with increased dose per fraction to involved LN raises theoretical concerns of amplified toxicity (Jensen et al. 2021). Thus, a comprehensive analysis of these two techniques taking into account dosimetric and radiobiological considerations could provide true insight.
Consequently, the present study was carried out including a dosimetric and radiobiological comparison of single-phase SIB-VMAT with two-phases SEQ-VMAT in terms of target coverage, dose to the organs at risk (OARs), tumour control probability (TCP), and normal tissue complication probability (NTCP). The second objective of the present study was to compare these two treatment strategies in different clinical scenarios of only pelvic LNs involvement or pelvic LNs with para-aortic LN involvement.
Materials and methods
The study included ten patients who received radiation therapy between January 2022and December 2022. A written, informed consent was taken from each patient enrolled in the study. The study was approved by the institute’s ethical committee before its commencement.
Patient selection
The study included histologically confirmed cervical cancer patients who underwent definitive chemo-radiation therapy. Patients with FIGO stage IIIC, as detected in fluorodeoxyglucose (FDG) based Positron Emission Tomography-Computed tomography (PET-CT), were included. Five of the ten patients included in this study had pelvic LNs only while the remaining five had pelvic LNs with para-aortic LN).
Treatment planning
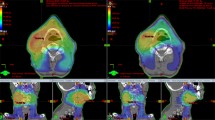
All patients underwent CT imaging in the supine position with a custom thermoplastic immobilization cast. Constant bowel and bladder filling protocols were followed at the time of the procedure. CT images were acquired with a 3 mm slice thickness and were transferred to the treatment planning system. Clinical target volumes (CTVs) were contoured as CTV nodal (CTV 1) and CTV primary (CTV 2 and 3) (Bansal et al. 2013). CTV 1 included involved nodes and relevant draining nodal groups (common iliac, internal iliac, external iliac, obturator, and presacral). CTV 2 included the uterine corpus, the entire cervix, and the vagina depending upon the vaginal involvement, whereas CTV 3 encompassed the parametrium. Appropriate internal target volume (ITV) and planning target volume (PTV) margins were generated. Additionally, para-aortic LN CTV was contoured according to the guidelines by Keenan et al. for delineation of para-aortic lymph node region in cervical cancer (Keenan et al. 2018). Positron emission tomography (PET) avid LNs were contoured with a 1-centimeter isotropic expansion to generate boost volumes. Dose constraints for targets and OARs were given as per the EMBRACE II protocol (Potter et al. 2018). Two sets of plans were generated for each patient - SIB-VMAT and SEQ-VMAT.
For the SIB plans, the prescribed dose was 50.4 Gy to the pelvis (primary tumour, uterus, and the parametrium and elective nodal volume) and 58.8 Gy to the FDG-avid nodes. These doses were delivered in 28 fractions over 5.6 weeks. For the SEQ-VMAT plans, the prescribed dose was 50.4 Gy to the pelvis in 28 fractions (primary tumour, uterus, and the parametrium) followed by a boost of 9 Gy in five fractions to the involved LN. Plans were optimized to achieve adequate target coverage with maximal sparing of the OARs.
Dosimetric and radiobiological analysis
For both SIB-VMAT and SEQ-VMAT plans, dose-volume histograms (DVHs) were generated for the target volumes and OARs. The DVHs were analyzed for the following parameters: mean dose, maximum dose, minimum dose, D95(Dose received by 95% of the PTV), and V95(Volume of PTV receiving 95% of the prescribed dose) for the targets along with the dose constraints met in the case of OARs. The Conformity Index (CI) for the target volume was calculated using Paddick’s conformity index (Paddick et al. 2000) formula as follows:
CIPaddick = TV2RI / TV x VRI.
Where TVRI is the target volume covered by reference isodose line, and VRI is the volume of reference isodose line.
Also, the Homogeneity Index (HI) for either plan was calculated according to ICRU Report 83 (ICRU 2010) as follows:
HI = (D2 – D98)/ DP.
Where D2 and D98 are the doses to 2% and 98% volume of PTV, and DP is the prescribed dose.
The TCP and NTCP were calculated by an in-house program named RBMODELV1 developed in MATLAB (2016b) software (Patel et al. 2022). It is designed for Windows-based computers and includes a menu-driven user interface. The framework of the application is simple: The programme accepts cumulative DVH files in .txt format. The model parameters need to be entered manually from the database provided with the software. TCP or NTCP calculations are performed based on input parameter values for different radiobiological models embedded into the application. In the present study, TCP was calculated by the Poisson model (Tucker et al. 1990) and NTCP by the Lyman-Kutcher-Burman (LKB) model (Kutcher et al. 1991). The biological parameters used for the calculations were taken from published literature and are shown in Table 1. The radiobiological models employed are briefly discussed below.
Poisson TCP model.
This model is based on Poisson statistics and generally relies on the assumption that tumour control requires the killing of all tumour clonogens. Poisson statistics predict the probability of this occurrence as:
TCP = exp (− N p(D)).
Where N is the initial number of clonogens, and p(D) is the cell survival fraction after exposure to dose D.
It is assumed that cell survival can be described by single-hit mechanics:
p (D) = exp (−αD)
This equation can be reformulated by including two parameters: \(\gamma\)50 and D50, describing normalized slope and dose at a 50% probability of control.
Lyman-Kutcher-Burman NTCP model.
Lyman’s model includes a sigmoid dose-response (SDR) curve of NTCP as a function of dose (D) to a uniformly irradiated fractional reference volume (Vref) (Kutcher et al. 1991). In that model, NTCP can be expressed as:
The SDR curve is described by three parameters: n, m, and TD50; n determines the dose-volume dependence of tissue and thus accounts for differences in tissue architecture; m controls the slope of the dose-response curve (in the case of homogeneous irradiation); and TD50 represents the dose at which there is a 50% chance of complication, and thus determines the position of the dose-response curve.
Statistical analysis
Statistical analysis was performed using the SPSS software, version 25.0. Test for normality was done for all the parameters to be compared using the Shapiro-Wilk test. The paired-t test was used to analyze all the normally-distributed parameters whereas the Wilcon signed rank test was applied to compare all the parameters having non-normal distributions. A p-value of less than 0.05 was interpreted as statistical significance.
Results
The dosimetric analysis compared two EBRT techniques, SIB-VMAT and SEQ-VMAT, for ten patients of cervical cancer with involved LN in terms of target coverage, OARs sparing, and radiobiology. The patient and treatment characteristics are shown in Table 2. The comparison of the two techniques showed that SIB-VMAT exhibited superior target coverage compared to SEQ-VMAT although both techniques achieved the pre-specified target volume coverage. Specifically, SIB-VMAT plans achieved significantly higher mean percentages of the PTV receiving at least 95% prescribed dose (V95) and gross tumour volume receiving at least 100% (V100) of the prescribed dose. SIB-VMAT plans also demonstrated significantly better dose conformity over SEQ-VMAT plans. In contrast, the HI was better for SEQ-VMAT as compared to SIB-VMAT (0.09 vs. 0.19, p < 0.01). Table 3 shows the comparison of the two planning techniques in terms of planning targets achieved.
In terms of OAR sparing, SIB-VMAT plans demonstrated notable advantages over SEQ-VMAT plans as shown in Table 4. The bladder and bowel received significantly lower mean doses (Dmean) in SIB-VMAT plans. SIB-VMAT also resulted in lower volumes of the bladder and bowel receiving specific dose thresholds (V40 and V30). However, SEQ-VMAT plans outperformed SIB-VMAT plans in achieving lower doses to the femoral heads.
Additionally, radiobiological parameters were analyzed to assess TCP and NTCP. The TCP was significantly higher in SIB-VMAT plans, indicating a potentially higher likelihood of tumour control with this technique. However, there were no statistically significant differences in the NTCP for OARs between the two techniques, suggesting similar risks of radiation-induced toxicities despite a higher percentage of NTCP observed in SEQ-VMAT plans (Table 5).
Further subset comparative analyses were performed for patients with different nodal station involvement as shown in Tables 3, 4 and 5. For patients with pelvic nodes only (IIIC1), there were no significant differences in the mean percentages of target coverage for GTV or PTV between the two techniques. In contrast, better bladder preservation along with a higher TCP was noted with SIB-VMAT plans. SIB-VMAT demonstrated a trend towards better target coverage and significantly better bladder and bowel preservation with SIB-VMAT plans for patients with both pelvic and para-aortic nodes (IIIC2).
Discussion
This is one of the few studies that address a critical issue of nodal boost in locally advanced cervical cancers (LACCs) incorporating physical as well as radiobiological endpoints. To the best of the authors’ knowledge, this is the only study that has compared the dose distribution and radiobiological parameters in two different clinical scenarios based on the involvement of pelvic and/or para-aortic LNs.
While analyzing all ten patients together, SIB-VMAT was observed to have been superior to SEQ-VMAT in terms of doses achieved in PTV V95 (99.4% vs. 98.9%, p = 0.03) and GTV V100 (99.7% vs. 98.2%, p = 0.01) with lesser areas of high dose region in PTV i.e., V110 (19.5 vs. 33.3%, p < 0.01) along with a better CI (Table 3). In contrast to these findings, a significantly better homogeneity index was attained with SEQ-VMAT planning (0.19 vs. 0.09, p < 0.01; Table 3). A plausible explanation for this finding could be the overlap of dose distributions from the plans of two sequential phases when the HI was being calculated for the plan sum in SEQ-VMAT plans. In plan sum, average dose distributions of two plans were observed where the presence of hot spots and cold spots changed the final dose distribution resulting in a more homogeneous dose as compared to SIB plans.
While analyzing the subset of IIIC1 and IIIC2 separately, SIB-VMAT was found to be more advantageous in patients of IIIC2, having pelvic and para-aortic nodes as compared to IIIC1, pelvic nodes alone (Table 3). This finding underscores the superiority of SIB-VMAT in larger volume diseases where it is challenging to achieve optimum doses along with meeting normal tissue constraints. It was also observed that the mean and volumetric doses of the bladder were lesser in IIIC2 as compared to IIIC1 cases, for both SIB-VMAT and SEQ-VMAT (Table 4). This was observed due to the fact that nodal boost volume (high dose volume) was nearer to the bladder in patients with pelvic nodes alone. Theoretically, another situation whereby multiple pelvic nodes are present could also lead to increased bladder doses. However, there was no significant difference in rectal and bone marrow sparing among both techniques and in both subsets of patients. This finding was in contrast to the finding by Feng et al., where they observed significantly superior sparing of the rectum (Feng et al. 2016). Interestingly, better sparing of the femoral head was observed with SEQ-VMAT. The femur was a non-overlapping structure with PTV as compared to the bladder, rectum, and bowel. Consequently, in sequential planning, where both the PTV and OARs underwent dual optimization, planners gained an added degree of freedom for optimizing femur dosage. Typically, it is observed that, in scenarios involving non-overlapping structures, the optimization algorithm can more effectively restrict dosage against overlapping structures (Hussein et al. 2018).
In their study, Guerrero et al. performed a dosimetric and radiobiological analysis on data from a single patient to explore the possibility of SIB-IMRT to replace conventional two-phase treatment in patients of LACC where brachytherapy was not feasible (Guerrero et al. 2005). The proposed SIB-IMRT with 45 Gy to pelvic nodes and three different SIB prescription doses of 60 Gy or 70 Gy or 80 Gy in 25 fractions to the GTV provided significant sparing to normal structures including the bladder, rectum, and small bowel, leading to smaller irradiated volumes and lower equivalent uniform doses for these organs compared to conventional whole pelvic irradiation with high-dose-rate brachytherapy. The target coverage ranged from 94 to 95.5% and it was inferior to that of the present study (more than 95% for all the patients). However, in the present study, two techniques of VMAT-based nodal boost were compared which is more appropriate in the current practice wherein there is an inclination towards the incorporation of advanced conformal radiation in the treatment of gynecological malignancies.
Sukhikh et al. compared in their study SIB-VMAT and SEQ-VMAT with respect to the dose delivered to the tumour and to OARs, expected TCP, and NTCP (Sukhikh et al. 2020) They found that both techniques allowed good coverage of the target and high-quality dose delivery with better OAR sparing with SIB-VMAT. The SIB-VMAT plans also offered the advantage of OTT shortening by a week compared to SEQ-VMAT plans. Their results also demonstrated that SIB-VMAT and SEQ-VMAT treatment plans had comparable TCP when considering TCD50 values in the range of 60 to 70 Gy. The study concluded that SIB-VMAT was an effective and feasible technique for the radical treatment of LACC, especially in cases where brachytherapy is not feasible or not preferred by patients.
Feng et al. investigated the dosimetric parameters of SIB-IMRT compared to SEQ-IMRT in patients with LACC and PET-avid lymph involvement (Feng et al. 2016). The study included a total of ten patients who received either SIB-IMRT or SEQ-IMRT. They found that SIB-IMRT provided comparable target coverage to SEQ-IMRT while significantly reducing the volumes of doses higher than the prescribed dose to the PTV. SIB-IMRT also demonstrated improved sparing of OARs, particularly for high doses to small volumes of the rectum and small bowel. Despite the higher fractional dose delivered by SIB-IMRT, the equivalent biological doses (EQD2) to OARs were comparable to those of SEQ-IMRT. The study concluded that SIB-IMRT planning is a promising approach for boosting PET-avid nodal targets in LACC, providing improved OAR sparing and comparable target coverage without increasing toxicity. However, this study did not take into account any radiobiological endpoints while comparing these two techniques.
In the study by Jensen et al., 83 patients of gynecological cancers with gross nodal disease treated with SIB at 2.25 Gy per fraction to PET avid LNs were retrospectively reviewed. At a median follow-up of 12.6 months, nodal control was 97.6% in the SIB field area while 90.4% in the non-SIB field area (p = 0.01) with a 100% gross nodal control rate in primary cervical cancer patients. Cervical cancer patients in the definitive setting had a 2-year progression-free survival and overall survival of 67% and 72%, respectively. No acute or late grade ≥ 3 genitourinary toxicity was seen. Acute and late grade ≥ 3 gastrointestinal toxicity rates were 7.2% and 12.0%, respectively. It was concluded that dose-escalated SIB to PET avid lymphadenopathy results in excellent local control with acceptable toxicity (Jensen et al. 2021). The results of these and other studies are summarized in Table 6.
The results of the multi-centered EMBRACE II study accentuated the use of SIB across all the patients of cervix cancer and assessed its clinical outcomes (Potter et al. 2018). However, the present study adds to the available literature by providing a new perspective on choosing the optimum treatment technique for an individual patient based on the region of nodal disease– pelvic and/or para-aortic, while comparing the benefits as well as demerits of the two planning techniques. The major strength of the present study lies in the fact that both the techniques of nodal boost were comprehensively analyzed considering dosimetric and radiobiological parameters.
SIB-VMAT offers the advantage of decreased OTT, but it raises theoretical concerns of increased normal tissue toxicity given the increased dose per fraction. In this sense, radiobiologically it is a double-edged sword (Jensen et al. 2021). Therefore, any comparison should include both dosimetric and radiobiological parameters to provide a complete picture of this technique, as was done in the present study. It was found here that SIB-VMAT outperformed SEQ-VMAT in the majority of dosimetric parameters analyzed, and it was found to achieve higher TCP (82% vs. 80%, p < 0.01) with no difference in NTCP. In addition, the present study also tried to identify the better technique in the context of different clinical scenarios involving pelvic nodes alone or with para-aortic nodes. The result of this novel comparison was that SIB-VMAT performed better in IIIC2 patients in terms of GTV V100 and PTV V110 with improved CI; also, the doses to the bladder and small bowel were reduced. Consequently, an important conclusion drawn from this finding is that SIB-VMAT should be the preferred approach in all cases of LACC.
In terms of radiobiological endpoints, both techniques offer efficient sparing of the surrounding OARs thereby decreasing the chances of acute as well as late complications (Knapp et al. 2019). There was no significant difference in the NTCP observed for the bladder, rectum, kidney, or bowel among the two techniques. For SIB-VMAT plans, the calculated NTCP for the bladder and rectum were 35.8% and 42.6% respectively. The NTCP predicted for the bladder and rectum in the current study was validated by the findings of clinical studies. In a recent study, Jayatilakebanda et al. prospectively evaluated high-dose SIB in node-positive cervical cancer patients and observed late genitourinary complications in 35% of the patients who were treated with chemoradiotherapy (Jayatilakebanda et al. 2021). Similarly, rectal toxicities were observed in 43.9% and bladder toxicities in 39% of the cervical cancer patients treated with high-dose SIB-VMAT in a prospective study of 41 patients by Perumareddy et al. (Perumareddy et al. 2023). These complication rates corresponded with the NTCP predicted by the model employed in the current study. However, the NTCP predicted by this model did not agree with the complication rates observed in clinical studies. While the model used in the present study predicted an NTCP of 17.6% for SIB and 18.3% for the upper gastrointestinal system (Table 5), these complications were actually in the range of 38.5–75.6% in prospective clinical studies (Perumareddy et al. 2023; Cihoric et al. 2014).
Although the number of cases studied was rather low in the present study (i.e., ten) a comprehensive statistical approach was adopted to find out any significant differences between SIB-VMAT and SEQ-VMAT. The paired-t test was used to analyze all the parameters with normal distributions whereas the Wilcoxon signed-rank test was used for variables with non-normal distributions. These tests are appropriate and robust; and can be used in small sample sizes without compromising the statistical significance. To overcome the potential differences in doses achieved due to multiple planners, a single medical physicist performed a duplicate set of planning for all the patients. Also, the suitability of plans was determined by a single radiation oncologist to avoid inter-observer bias.
The main limitation of the present study is the retrospective design with a relatively small number of patients. Data regarding brachytherapy were not included in the analysis as simply combining the results of both brachytherapy and VMAT was considered scientifically inappropriate. Radiobiologically both these treatment modalities are entirely different and simple dose addition is unjustifiable.
The present study confirmed the superiority of SIB-VMAT over SEQ-VMAT in node-positive cervical cancer. It paves the way for a future prospective clinical study considering tumour regression and adaptive RT along with volume-based image-guided brachytherapy to further escalate dose alongside decreasing NTCP.
Conclusion
SIB-VMAT was advantageous over SEQ-VMAT in terms of target coverage, dose conformity, and TCP. Notably, SIB-VMAT showed potential benefits for cases involving para-aortic nodes. It is concluded that SIB-VMAT should be the preferred approach in all cases of LACC.
Data availability
No datasets were generated or analysed during the current study.
References
Bacorro W, Dumas I, Escande A, Gouy S, Bentivegna E, Morice P, Haie-Meder C, Chargari C (2018) Dose-volume effects in pathologic lymph nodes in locally advanced cervical cancer. Gynecol Oncol 148(3):461–467
Bansal A, Patel FD, Rai B, Gulia A, Dhanireddy B, Sharma SC (2013 Oct-Dec) Literature review with PGI guidelines for delineation of clinical target volume for intact carcinoma cervix. J Cancer Res Ther 9(4):574–582
Boyle JM, Dorth J, Craciunescu O, Light K, Roper JR, Chino JP (2014) Simultaneous Integrated Boost to pelvic and para-aortic nodes from Cervical Cancer improves the dosimetric therapeutic ratio. Int J Radiat Oncol Biol Phys 90:S482–S483
Chang JH, Gehrke C, Prabhakar R, Gill S, Wada M, Lim Joon D, Khoo V (2016) RADBIOMOD: a simple program for utilising biological modelling in radiotherapy plan evaluation. Phys Med 32(1):248–254
Cihoric N, Tapia C, Krüger K, Aebersold DM, Klaeser B, Lössl K (2014) IMRT with 18FDG-PET\CT based simultaneous integrated boost for treatment of nodal positive cervical cancer. Radiat Oncol 9:83
Feng CH, Hasan Y, Kopec M, Al-Hallaq HA (2016) Simultaneously integrated boost (SIB) spares OAR and reduces treatment time in locally advanced cervical cancer. J Appl Clin Med Phys 17(5):76–89
Figueredo Negron CI, Gamboa Garay O, Pabón Girón A, Esguerra Cantillo JA, Guerrero Lizcano E (2022) A comparison of intensity-modulated Radiotherapy with Simultaneous Integrated Boost with three-Dimensional Conformal Radiotherapy with Sequential Boost for locally Advanced Cervical Cancer: a dosimetric study. Cureus 14(12):e32940
Gaffney DK, Erickson-Wittmann BA, Jhingran A, Mayr NA, Puthawala AA, Moore D, Rao GG, Small W Jr, Varia MA, Wolfson AH, Yashar CM, Yuh W, Cardenes HR (2011) ACR Appropriateness Criteria on advanced cervical cancer expert panel on radiation oncology-gynecology. Int J Radiat Oncol Biol Phys 81:609–614
Green JA, Kirwan JM, Tierney JF, Symonds P, Fresco L, Collingwood M, Williams CJ (2001) S.urvival and recurrence after concomitant chemotherapy and radiotherapy for cancer of the uterine cervix: a systematic review and meta-analysis. Lancet 358(9284):781–786
Guerrero M, Li XA, Ma L, Linder J, Deyoung C, Erickson B (2005) Simultaneous integrated intensity-modulated radiotherapy boost for locally advanced gynecological cancer: radiobiological and dosimetric considerations. Int J Radiat Oncol Biol Phys 62(3):933–939
Hong JC, Foote J, Broadwater G, Sosa JA, Gaillard S, Havrilesky LJ, Chino JP (2017) Data-derived treatment duration goal for cervical Cancer: should 8 weeks remain the target in the era of concurrent chemoradiation? JCO Clin Cancer Inf 1:1–15
Hsu HC, Li X, Curtin JP, Goldberg JD, Schiff PB (2015) Surveillance epidemiology and end results analysis demonstrates improvement in overall survival for cervical cancer patients treated in the era of concurrent chemoradiotherapy. Front Oncol 5:81
Hussein M, Heijmen BJM, Verellen D, Nisbet A (2018) Automation in intensity modulated radiotherapy treatment planning-a review of recent innovations. Br J Radiol 91(1092):20180270
ICRU (2010) Prescribing, Recording, and Reporting Intensity-Modulated Photon-Beam Therapy (IMRT). (ICRU Report 83), J. ICRU, Volume 10(1). Oxford University Press, Oxford, UK
Jayatilakebanda I, Tsang YM, Hoskin P (2021) High dose simultaneous integrated boost for node positive cervical cancer. Radiat Oncol 16(1):92
Jensen GL, Mezera MA, Hasan S, Hammonds KP, Swanson GP, El-Ghamry MN (2021) Dose escalated simultaneous integrated boost of gross nodal disease in gynecologic cancers: a multi-institutional retrospective analysis and review of the literature. Radiat Oncol J 39(3):219–230
Keenan LG, Rock K, Azmi A, Salib O, Gillham C, McArdle O (2018) An atlas to aid delineation of para-aortic lymph node region in cervical cancer: design and validation of contouring guidelines. Radiother Oncol 127(3):417–422
Kim H, Park W, Cho WK (2020) Who can benefit from a lymph node boost in definitive chemoradiotherapy for node-positive cervical cancer: an evaluation of nodal failure in patients without nodal boost. J Radiat Res 61(3):479–486
Knapp P, Eva B, Reseigh G, Gibbs A, Sim L, Daly T, Cox J, Bernard A (2019) The role of volumetric modulated arc therapy (VMAT) in gynaecological radiation therapy: a dosimetric comparison of intensity modulated radiation therapy versus VMAT. J Med Radiat Sci 66(1):44–53
Kutcher GJ, Burman C, Brewster L, Goitein M, Mohan R (1991) Histogram reduction method for calculating complication probabilities for three-dimensional treatment planning evaluations. Int J Radiat Oncol Biol Phys 21(1):137–146
Lin SM, Ku HY, Chang TC, Liu TW, Hong JH (2017) The prognostic impact of overall treatment time on disease outcome in uterine cervical cancer patients treated primarily with concomitant chemoradiotherapy: a nationwide Taiwanese cohort study. Oncotarget 8(49):85203–85213
Okunieff P, Morgan D, Niemierko A, Suit HD (1995) Radiation dose-response of human tumors. Int J Radiat Oncol Biol Phys 32(4):1227–1237
Paddick I (2000) A simple scoring ratio to index the conformity of radiosurgical treatment plans. Technical note. J Neurosurg 93(Suppl 3):219–222
Patel G, Mandal A, Bharati A, Choudhary S, Mishra R, Mourya A (2022 Oct-Dec) Development and validation of an indigenous, radiobiological model-based tumor control probability and normal tissue complication probability estimation software for routine plan evaluation in clinics. J Cancer Res Ther 18(6):1697–1705
Perumareddy V, Shivananjappa R, Geeta SN, Tiwari R, Mandal SK (2023) An Indian data on response of positive pelvic lymph nodes in carcinoma cervix patients treated with simultaneous integrated boost using volumetric modulated arc radiation therapy. Asian J Oncol 9:9
Pötter R, Tanderup K, Kirisits C, de Leeuw A, Kirchheiner K, Nout R, Tan LT, Haie-Meder C, Mahantshetty U, Segedin B, Hoskin P, Bruheim K, Rai B, Huang F, Van Limbergen E, Schmid M, Nesvacil N, Sturdza A, Fokdal L, Jensen NBK, Georg D, Assenholt M, Seppenwoolde Y, Nomden C, Fortin I, Chopra S, van der Heide U, Rumpold T, Lindegaard JC, Jürgenliemk-Schulz I, EMBRACE Collaborative Group (2018) The EMBRACE II study: the outcome and prospect of two decades of evolution within the GEC-ESTRO GYN working group and the EMBRACE studies. Clin Transl Radiat Oncol 9:48–60
Shewalkar B, Khan A, Yerlekar D, Patel J, Khadilkar H, Sakthivel R, Kataruka R (2022) Dose-escalated intensity-modulated Radiotherapy for the management of locally Advanced Cervical Cancer. Cureus 14(6):e25965
Shrivastava S, Mahantshetty U, Engineer R, Chopra S, Hawaldar R, Hande V, Kerkar RA, Maheshwari A, Shylasree TS, Ghosh J, Bajpai J, Gurram L, Gulia S, Gupta S, Gynecologic Disease Management Group (2018) Cisplatin Chemoradiotherapy vs Radiotherapy in FIGO Stage IIIB squamous cell carcinoma of the uterine cervix: a Randomized Clinical Trial. JAMA Oncol 4(4):506–513
Sukhikh ES, Sukhikh LG, Lushnikova PA, Tatarchenko MA, Abdelrahman AR (2020) Dosimetric and radiobiological comparison of simultaneous integrated boost and sequential boost of locally advanced cervical cancer. Phys Med 73:83–88
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F (2021) Global Cancer statistics 2020: GLOBOCAN estimates of incidence and Mortality Worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71(3):209–249
Tanderup K, Fokdal LU, Sturdza A, Haie-Meder C, Mazeron R, van Limbergen E, Jürgenliemk-Schulz I, Petric P, Hoskin P, Dörr W, Bentzen SM, Kirisits C, Lindegaard JC, Pötter R (2016) Effect of tumor dose, volume and overall treatment time on local control after radiochemotherapy including MRI guided brachytherapy of locally advanced cervical cancer. Radiother Oncol 120(3):441–446
Tucker SL, Thames HD, Taylor JM (1990) How well is the probability of tumor cure after fractionated irradiation described by Poisson statistics? Radiat Res 124(3):273–282
Wujanto C, Choo BA, Tan D, Ilancheran A, Ng J, Low JJH, Shen L, Tang J, Koh V (2019) Does external beam radiation boost to pelvic lymph nodes improve outcomes in patients with locally advanced cervical cancer? BMC Cancer 19(1):385
Acknowledgements
Not applicable.
Author information
Authors and Affiliations
Contributions
RM: Conceptualisation, Methodology, Validation, Formal analysis and Investigations, Resources, Data curation, Writing original draft, Preparation of Tables, Review and Editing, SupervisionSS: Methodology, Validation, Formal analysis and Investigations, Resources, Data curation, Writing original draft, Preparation of Tables, Review and EditingGP and AM: Software, Validation, Formal analysis and Investigations, Data curation, Writing original draft, Review and EditingHM: Formal analysis and Investigations, Data curation, Resources, Review and Editing, Supervision, GuarantorAP, BB, PKS, SS and MT: Formal analysis and Investigations, Review and Editing All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Ethical approval
The study was approved by the institute’s ethical committee before its commencement.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mishra, R., Singh, S., Patel, G. et al. Comparative analysis of simultaneous integrated boost and sequential boost radiotherapy in node-positive cervical cancer: dosimetric and radiobiological considerations. Radiat Environ Biophys 63, 297–306 (2024). https://doi.org/10.1007/s00411-024-01069-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00411-024-01069-0