Abstract
Quinoa is one of the crops well-adapted to high altitude regions that can grow relatively well under drought, humid, and high UV radiation conditions. This study was performed to investigate the effects of gamma-radiation on quinoa. Seeds were treated with various doses of 50 Gy, 100 Gy, 200 Gy, 300 Gy, 400 Gy, 600 Gy, 800 Gy, and 1000 Gy. We investigated germination, as well as plant height, chlorophyll content, and normalized difference vegetation index (NDVI) at 0, 30, 44, 58, and 88 days after transplanting (DAT) and panicle weight at 88 DAT. The plants grown from the seeds treated at radiation doses greater than 200 Gy showed reduced values in most growth and physiological characteristics. The germination rate and germination speed were higher in the 50 Gy-treated seeds than in 0 Gy-treated (control) seeds. Plant height and panicle weight were highest in the plants from 50 Gy-treated seeds. Chlorophyll content was higher in all treated samples than in the controls. NDVI value showed the highest value in 0 Gy controls and plants treated with 50 Gy. The antioxidant activity was also higher in the plants from the seeds treated with 50 Gy and 100 Gy, showing a steady increase as the radiation dose increased even at 200 Gy. The plants from seeds treated with 0 Gy showed higher expression of proteins related to photorespiration and tubulin chains. The plants from seeds treated with 50 Gy induced more stress-responsive proteins.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Quinoa (Chenopodium quinoa Willd.) is an annual C3 plant belonging to the Chenopodiaceae family which exhibits extensive environmental adaptability. It grow in various regions with a preference of over 500 m highlands, and even above 3000 m above sea level. Several studies have reported that they show viable characteristics under extreme conditions such as salt stress (Adolf et al. 2013; Li et al. 2016), low-temperature stress (Yang et al. 2016), intense ultraviolet stress (González et al. 2009), and heavy metal stress (Buss et al. 2012). In addition, quinoa can grow under drought conditions of low (< 150 mm per year) precipitation (Martínez et al. 2009).
Living organisms exposed to high-dose ionizing radiation have altered cell cycles, inhibiting or delaying normal cell division. In addition, changes in cytoskeletal proteins result in various unusual changes such as cell deformation, chromatin decondensation, and DNA damage (Zhang et al. 2022). Irradiated plants exhibit changes on various morphological and molecular levels, including changes in chlorophyll content, structure and function of cell wall, chromosome, mitochondria, and cell membrane (Keresztes and Kovács 1991; Kovács and Keresztes 2002; Hilal et al. 2004). These internal changes also lead to the inhibition of germination and a decline in the growth of the irradiated plant (Kon et al. 2007; Marcu et al. 2013). There is also an increase in oxidative stress (Kovács and Keresztes 2002) and secondary metabolites (Mackerness et al. 1999), and a decrease in nutrient absorption through roots (Sosedov and Vakar 1963).
On the other hand, low-dose ionizing radiation has been shown to promote physiological activity in plants (Miller and Miller 1987). It should be noted that the definition of low dose in plants, and especially in dry seeds, differs from the standard definition (Caplin and Willey 2018). This positive effect is associated with hormesis. For plants, it was reported that this hormesis promotes initial growth, germination rate, phytochemical content, and antioxidant activity and increases the resistance to the external environment through the induction of enzymes related to plant growth (Miller and Miller 1987). However, in the case of ionizing radiation, plants exhibit a variety of physiological and physical changes depending on the radiation dose, and in some cases, different mutations occur, so identifying changes induced by ionizing radiation is very important (Khan and Goyal 2009; Dhakshanamoorthy et al. 2011). The application of ionizing radiation at a low dose causes only a slight change in the levels of hydrogen peroxide malondialdehyde (MDA) and O2. Although these changes are not high, they activate antioxidant enzymes, therefore, the plants become more resistant to external stress (Mittler et al. 2004; Zhang et al. 2016).
This study was conducted to investigate how gamma irradiation of seeds affects germination, growth and physiological parameters of quinoa and to determine whether hormesis effects occur at low irradiation doses.
Materials and methods
Plant material and radiation treatments
Quinoa (cv. Challamocko) seeds were irradiated at the 60CO gamma radiation facility (AECL, IR-79, MDS Nordion International Co., Ltd., Ottawa, ON, Canada) of the Advanced Radiation Technology Institute (ARTI) with 50 Gy, 100 Gy, 200 Gy 300 Gy, 400 Gy, 600 Gy, 800 Gy, and 1000 Gy (Hanafy and Akladious 2018). The seeds were treated at room temperature with a dose rate of 1.5 kGy/h. The absorbed dose of seeds in a sample bag was confirmed with an alanine dosimeter (dia. 5 mm, Bruker Instrument, Rheinstetten, Germany). The moisture content of seeds was adjusted to 15% before irradiation.
Germination test
The radiation-treated seeds were placed on the filter paper (Whatman No. 1) in a 9-cm plastic petri dish. For each treatment, four replications of 50 seeds each on the paper moistened with 5 mL water were incubated under dark conditions at 25 °C. The germination test was conducted according to the International Seed Testing Association (ISTA) (Hampton and Tekrony 1995). It was performed for 40 h, and germinated seeds were counted every 4 h. Seeds with a protrusion of the radicle by at least 1 mm were considered as germinated. Germination rate (%) indicates the proportion of germinated seeds over total seeds and germination speed that reflects the germination vigor at each measured time was calculated by dividing the additionally germinated seed number by the period (h). Therefore, based on the germination rate and germination speed, we could evaluate the germination performance quantitatively as well as qualitatively. They were calculated as follows.
Germination speed = (ni/ti), where, ni is the number of additionally germinated seeds for the period, ti is the period (h).
Plant growth and physiological measurement
Quinoa seeds (5 g) irradiated with various radiation doses were imbibed in distilled water for 48 h at room temperature and planted in 48 cell nursery-trays filled with a mixture of soil and peat moss (1:3) at room temperature. After 30 days, the seedlings were transplanted to pots [20 cm (H) × 20 cm (D)] filled with field soil and moved to a greenhouse (27 °C/14 °C, light (12 h)/dark (12 h), relative humidity 65%). The photosynthetic photon flux density in the greenhouse was about 1500 μmol m−2 s−1 at noon. Plant height was measured every week from 30 days after transplanting (DAT) to 88 DAT, and panicle weight was measured at 88 DAT after drying at 70 °C for 48 h.
Chlorophyll content was measured for five plants with five replications on a fifth leaf using a portable chlorophyll meter (Soil Plant Analysis Development, SPAD-502, Minolta, Japan). Normalized difference vegetation index (NDVI) was measured for five plants with five replications on fully expanded leaves at 30, 44, 58, and 88 days after transplanting using Plantpen NDVI 300 (Photon System Instrument, Czech Republic).
Antioxidative activity (ORAC) assay
Five seedlings (30 days old) were collected and macerated in liquid nitrogen and stored at − 80 °C until use. The antioxidant activity of leaves was measured by ORAC (oxygen radical absorbance capacity) assay according to the previously described method of Gillespie et al. (2007). The antioxidant capacity of a substance can be directly estimated by comparison with the standard curve of Trolox.
Protein extraction and two-dimensional electrophoresis
Fully expanded leaves collected from 5 plants at 35 DAT were powdered in liquid nitrogen, and 5 g of powder was put into a 15 ml tube. Protein was extracted by the modified phenol method of Wang et al. (2008). After extraction, lysis buffer [7 M Urea, 2 M thiourea, 4% 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate (CHAPS), 40 mM dithiothreitol (DTT)] was added to the dried protein pellet, and the sample was incubated for 1 h at 25 °C. After centrifugation for 20 min at 20,000g, the supernatant was transferred to a 1.5 ml tube and stored at − 80 °C until use. The extracted protein was quantified by the Bradford method (Bradford 1976) before electrophoresis. Two-dimensional polyacrylamide gel electrophoresis was carried out. Briefly, the extracted protein samples (900 μg) were loaded on an immobilized pH gradient (IPG) strip of 18 cm length and a pH range of 4–7 (Bio-Rad, USA). After rehydrating with rehydration solution [7 M urea, 2 M thiourea, 4% CHAPS, 40 mM DTT, 1% (v/v) IPG buffer, pH 4–7] for 16 h isoelectric focusing was performed using a Protean IEF Cell (Bio-Rad). After isoelectric focusing the strip was placed on a 12.5% SDS gel (185 × 200 × 1.0 mm) and subjected to electrophoresis using a Protean II XI electrophoresis device (Bio-Rad). The proteins were stained for 24 h in Coomassie brilliant blue G-250 (Genomic Base) solution.
Protein identification using mass spectrometry (MALDI-TOF/TOF mass spectrometry)
Protein spots were visualized using an Epson scanner (Epson Perfection V800 Photo, Japan). For the accuracy of the data, the 2-DE analysis was performed three times per sample. The gel image was analyzed using PDQuest version 7.2.0 software (Bio-Rad). Spots with statistically significant differences (P < 0.05, Student’s t-test) of more than 1.5 times were used for protein analysis, and then the protein spots were excised from the gel. In‐gel digestion and tryptic peptide extraction were performed by the method of Shevchenko et al. (1996). The gel pieces were washed and destained using water and acetonitrile repeatedly at 20 °C. The proteins in the gel piece were reduced with 10 mM DTT in 100 mM NH4HCO3 at 56 °C and then incubated with 55 mM iodoacetamide in 100 mM NH4HCO3 for 30 min at 20 °C. For digestion, the gel pieces were rehydrated in 50 mM NH4HCO3 with 10 ng trypsin on ice for 45 min and then incubated overnight at 37 °C. After digestion, peptides were sequentially extracted using 5 µl of 5% (v/v) trifluoroacetic acid (TFA) followed by 50 µl of 50% (v/v) acetonitrile with 2.5% (v/v) TFA. Peptides were desalted using a column of POROS R2 resin (AB Sciex, Foster City, CA), then the samples were spotted on a MALDI target plate. Samples were then analyzed using an AB Sciex 4800 Plus MALDI-TOF/TOF mass spectrometer (Applied Biosystems, Franklin Lakes, NJ, USA). The peptide mass fingerprints (PMFs) obtained were searched against sorghum in the Swissprot and/or NCBI database using the MS-Fit program (http://prospector.ucsf.edu), and PMFs were identified by first searching in the Swissprot database and the NCBI database. The possible functions of identified proteins were determined according to the information from the UniProt (www.uniprot.org).
Statistical analysis of plant variable
The data were statistically analyzed using a PROC ANOVA procedure of SAS software (ver. 9.4, SAS Inst., NC USA), and the mean values were compared by Tukey’s test at p < 0.05 and expressed with standard error.
Results and discussion
Dose-dependent effects on seed germination
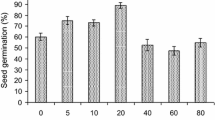
Gamma radiation at low dose has been reported to accelerate seed germination (Kim et al. 2000). In the present study, the germination rate varied with the radiation dose, and low-dose gamma irradiation at 50 Gy showed promoting effects on germination (Fig. 1). After irradiation with 50 Gy the germination rate at 40 h after imbibition (HAI) was 88 ± 2% and thus higher than for the 0 Gy control, which had an 85 ± 2% germination rate. However, the germination rate decreased at doses of 100 Gy and higher, showing 66% at the highest dose of 1000 Gy. The germination began at 16 h after imbibition (HAI), showing the highest germination speed at 20 HAI (0 Gy, 50 Gy, 100 Gy) or 24 HAI (> 100 Gy) depending on the dose. Although the speed did not show significant differences between seeds irradiated at O Gy, 50 Gy, and 100 Gy at the peak time (20 HAI), the speed at the beginning time (16 HAI) was higher in the treatment of 50 Gy than 0 Gy. These results imply that relatively low irradiation (50 Gy) induces rapidly the germination in the irradiated seeds. The germination speed was more sensitive to radiation dose than the germination rate at the early period of germination.
Changes in germination rate (upper) and germination speed (lower) of Chenopodium quinoa seeds treated with various gamma irradiation dose levels (0 Gy, 50 Gy, 100 Gy, 200 Gy, 300 Gy, 400 Gy, 600 Gy, 800 Gy, and 1000 Gy). Data were the mean and standard error (vertical bars) from four replications. Asterisks indicate significant difference among radiation doses within an incubation period (*P < 0.05, **P < 0.01, ***P < 0.001)
Previous results showed that seed irradiation at 300 Gy or higher reduces the germination rate in long bean (Vigna sequencedalis) (Ellyfa et al. 2007), while a low-dose irradiation at 50 Gy showed increased germination in cucumber and okra (Jaipo et al. 2019), similar to our results. The facilitated germination suggests that a low irradiation dose below/around 50 Gy may promote the germination of quinoa seeds. However, since high-dose radiation causes damage to protein and DNA structure during plant growth including germination (Hilal et al. 2004), it is believed that higher-dose radiation of more than 200 Gy in this study inhibited embryo growth due to lowered digestion of stored nutrients, resulting in a decline in germination.
Seeds should absorb water to utilize the stored nutrients for germination (Nonogaki et al. 2010). In our study, the germination promotion by low-dose irradiation had positive effects on germination by enhancing the utilization phase of storage nutrients as the seeds imbibed well in all treatments (data not shown).
Plant height and panicle weight of quinoa plant according to the dose of gamma radiation
Several studies have reported that low-dose ionizing radiation increases the plant height and the panicle weight related to crop yield, for example (Maity et al. 2005). Early after transplanting, the plant height of the plants grown from the seeds treated at 600 Gy was lowest, and the plants from the seeds treated at 50 Gy, 100 Gy, 200 Gy, 300 Gy, and 400 Gy showed greater plant height than that treated at 0 Gy (Fig. 2). At 44 days after transplanting (DAT), plant height was 14.5 ± 0.3, 13.7 ± 0.2, 13.6 ± 0.2, 13.3 ± 0.4, and 11.6 ± 0.8 cm in the samples treated with 50, 100, 200, 300, and 400 Gy, respectively, showing a more remarkable growth than the plants from seeds treated at 0 Gy (11.1 ± 0.4). Plants from seeds treated at 600 Gy showed a smaller plant height of 7.8 ± 1.3 cm and they died before 58 DAT. Plants from the seeds irradiated at 800 Gy and 1000 Gy died at 30 DAT and were not measured. At 88 DAT, all doses except for 50 and 100 Gy resulted in a smaller plant height as compared to the control (0 Gy), but the promotive effect of low-dose radiation of 50 Gy persisted (Fig. 4). The increase in plant height after low-dose gamma irradiation is presumed to be due to hormesis caused by metabolic adjustment (Volkova et al. 2020), improving the energy supply required for stem growth. The stimulation of growth has also been attributed to accelerating cell division (Zaka et al. 2004) and increased activity of auxin (Jan et al. 2012.
Changes in plant height (upper) and panicle weight (lower) in Chenopodium quinoa originated 4 from the seeds treated with various gamma irradiation dose levels (0 Gy, 50 Gy, 100 Gy, 200 Gy, 300 Gy, 400 Gy, 600 Gy, 800 Gy, and 1000 Gy). Panicle weight was measured at 88 days after transplanting. The panicles of plants treated at high doses greater than 400 Gy were not harvested due to abortion. Data were the means ± SE (n = 5). Different letters are significantly different at P < 0.05 by Tukey’s test
Panicle weight was lower in the plants from seeds treated at 0 Gy (4.6 ± 0.2 g) than those treated at 50 (6.5 ± 0.2 g) and 100 Gy (5.3 ± 0.2 g), but the weight became smaller as the irradiation dose increased from 200 Gy (Fig. 2). Similarly, in wheat doses greater than 400 Gy decreased the yield (Abdel and Ali 2006). However, in the study of Hegazi and Hamideldin (2010), gamma radiation treatment to seeds at 0, 200, 300, 400, and 500 Gy in Okra (Abelmoschus esculentus) resulted in an increase in plant length and yield by irradiation with 400 Gy as compared to plant from seeds treated at 0 Gy. The dose range that showed positive results in the previous reports was different from that of our study, which suggests that the beneficial dose range depends on various factors such as plant species and treatment conditions (Agathokleous et al. 2019).
Changes in chlorophyll and NDVI of plants grown from irradiated seeds at different doses of gamma-ray
The SPAD value, which represents the chlorophyll content in the leaves, was the lowest after 0 Gy treatment and highest in the plants treated by irradiation with 400 Gy and 600 Gy at 30 and 44 DAT, respectively (Fig. 3). However, the plants from seeds treated at 600 Gy were not available for SPAD measurement due to no more extended leaf development from 58 DAT. At 58 DAT, the SPAD value of the plant treated at 0 Gy was 41.4 ± 1.4, which was lower by more than 4.0 as compared to the plants from seeds treated at 50–400 Gy. Physiological changes caused by ionizing irradiation include increased photosynthesis, chlorophyll content, and associated enzyme activity (Sah et al. 1996). Our results also reflect that ionizing radiation induces changes in proteins related to chlorophyll production in plants, resulting in an effect on SPAD values that are measured on the viable part of fully expanded leaves. This means that the effects of gamma radiation on chlorophyll content in plants vary depending on the level of dose as inhibition or promotion.
Changes in SPAD value (upper) and NDVI (lower) of the 5th leaf from 30 to 88 DAT in 3 Chenopodium quinoa originated from the seeds treated with various gamma irradiation dose levels (0 4 Gy, 50 Gy, 100 Gy, 200 Gy, 300 Gy, 400 Gy, and 600 Gy). Data were the means ± SE (n = 5). Different letters are significantly different at P < 0.05 by Tukey’s test
NDVI is an indicator that is closely related to the relative distribution and activity of green plants on the land surface, leaf area index, chlorophyll content, and absorbed photosynthetically active radiation. This index is measured based on the reflectances of specific bands of visible light (Gamon et al. 1995). NDVI was higher at 0.71 ± 0.01, 0.73 ± 0.01 in the plants from seeds treated at 0 Gy and 50 Gy, respectively, and lower at 0.68 ± 0.01, 0.64 ± 0.03, and 0.55 ± 0.05 in the plants from seeds treated at 300, 400, and 600 Gy at 30 DAT (Fig. 3). However, at 44 DAT, there was no significant difference in all treatments. At 58 DAT, the plants irradiated at 600 Gy were dead, and the NDVI tended to increase slightly compared to the results at 44 DAT. The NDVI was similar among radiation doses since 58 DAT except for the plants from seeds treated irradiated at 50 Gy that showed higher NDVI than other radiation doses. In this study, the NDVI of plants from seeds treated at 50 Gy was higher throughout the investigated period of quinoa growth than plants treated at other doses as well as control (Fig. 3). The visible light region for estimating NDVI is 680–760 nm, closely related to chlorophyll content (Richardson et al. 2002). In the results, NDVI was more closely related to growth than chlorophyll content (SPAD value). Therefore, NDVI was more appropriate than chlorophyll content as an indicator of the plant response to the level of gamma radiation. NDVI results show that low-dose radiation of 50 Gy has a hormesis effect of a beneficial contribution to plant growth. NDVI and SPAD reflect the status of the pigments involved in the photosynthesis of leaves in plants (Basyouni et al. 2015).
Changes in antioxidant activity of plants grown from irradiated seeds at different doses of gamma-ray
The results of the ORAC assay which measured the free radical scavenging ability of antioxidants showed the lowest antioxidant activity of 6629 ± 308 μΜ Trolox eq./g FW in the plants treated at 0 Gy and the highest activity of 7477 ± 160 μΜ Trolox eq./g FW in the plants from seeds treated at 100 Gy. At a dose range of 200–1000 Gy, antioxidant activity slightly increased with the dose of ionizing radiation (Fig. 4). The changes in antioxidant activity were attributed to the ionizing radiation causing stress on the plant, producing excess active oxygen, and increasing the activity of antioxidant enzymes. Previous studies reported similar results in arabidopsis (Zhang et al. 2016) and rice (Kim et al. 2004). Thus, low-dose ionizing radiation increases antioxidant activity due to the signaling actions of active oxygen rather than as a stress factor, increasing its resistance to abiotic stresses such as drought, cold, and other oxidative stresses (Gygi et al. 1999). In addition, low-dose ionizing radiation has a direct effect on their antioxidant concentration, antioxidant enzyme production involved in the antioxidant activity, and the genes involved in the signaling system between ROS and antioxidative system (Kim et al. 2011).
Antioxidant activity (ORAC) in 30 days old seedlings of Chenopodium quinoa originated from the seeds treated with various gamma irradiation dose levels (0 Gy, 50 Gy, 100 Gy, 200 Gy, 300 Gy, 400 Gy, 600 Gy, 800 Gy, and 1000 Gy). Data were the means ± SE (n = 3). Different letters are significantly different at P < 0.05 by Tukey’s test
In our study, 50 Gy and 100 Gy treatments improved the ability to increase resistance, but doses of radiation above 200 Gy did not show positive effects. Thus, it is not clear whether the signaling result arising from low-dose radiation is essential or the role of metabolic adjustment is necessary. However, since the regulation of different metabolisms also causes antioxidant capacity, it can be deduced that the regulation of metabolism, especially the regulation of antioxidant metabolism, is enhanced by low-dose radiation.
Differences in protein expression in the plant grown from the seeds irradiated with different doses of gamma radiation
Protein changes in plants from quinoa seeds that were gamma-irradiated were identified through two-dimensional electrophoresis and MALDI-TOF-TOF analysis. A total of 49 proteins were found at 35 DAT to differ in their induction depending on the dose of gamma radiation (Fig. 5; Table 1). Furthermore, as a result of categorizing the identified proteins by metabolic type, 51% of the proteins were associated with the primary metabolism, and 12.2% of the proteins were associated with stress response (Fig. 6).
Images of 2-DE of proteins from the 3rd leaves in Chenopodium quinoa at 35 DAT. A, B, and C Indicate 0 Gy, 50 Gy and 100 Gy, respectively. Arrows indicate up- and down-regulated expression by irradiation treatments. Up- and down were compared to the lowest spot intensity between treatments. The numbers beside arrows were presented in Table 1
The proteins in the primary metabolism were those mainly associated with photosynthesis and respiration, carbohydrate metabolism, protein metabolism (spot numbers: 47, 51, 57, 58, 60), and fat metabolism (Table 1). Rubisco activase (spot 75, 76, 77) and PS II BNR domain-containing protein (spot 45, 46) which play an essential role in photosynthesis in plants, were more highly expressed in the plants from seeds treated at 0 Gy than in the plants grown from seeds treated at 50 and 100 Gy. However, carbonic anhydrase (spot 14, 15, 16, 18), which regulates the pH of chloroplasts and the fixation of CO2 while preventing protein denaturation, was higher in plants grown from seeds treated by gamma-rays at 50 and 100 Gy than in plants treated at 0 Gy. In addition, ribulose-phosphate 3-epimerase (spot 13), which is involved in the synthesis of intermediates in the pentose phosphate pathway, showed enhanced induction in the plants from seeds treated at 50 Gy. Phosphoglycolate phosphatase (spot 42, 43), an enzyme involved in photorespiration, was increased in the plants from seeds treated at 0 Gy compared to 50 Gy and 100 Gy-treated plants. Changes in proteins reflected that photosynthesis, internal carbon dioxide concentration, transpiration rate, and stomatal conductance were lower in 0 and 100 Gy-treated plants than in 50 Gy-treated plants. Proteins induced in the plants grown from the seeds irradiated at 50 Gy have roles linked to the acceleration of primary metabolism through metabolic adjustments for improving the fundamental growth of quinoa plants. The promotion of primary metabolism by low dose further improves the ability to supply the energy sources needed to increase resistance to stress, allowing stress response metabolism to be carried out efficiently.
Hayashi et al. (2015) reported the expression of stress-related proteins according to gamma irradiation. Our study also showed similar results that increased the expression of stress-related proteins in the plants grown from the seeds treated by gamma radiation at 50 Gy (spot 19, 40, 56, 61, 62, 82). In particular, the expression of superoxide dismutase [Fe] (FeSOD, spot 1) in the leaves of plants from seeds treated at 50 and 100 Gy was higher than that of plants from seeds treated at 0 Gy. In addition, the expression of ascorbate peroxidase (APX, spot 82), one of the antioxidant enzymes, was also increased in 50 Gy-treated plants. Under environmental stress, such as strong light and drought, plants produce superoxides due to electron accumulation in the thylakoid membrane (Foyer and Noctor 2000), O2·− is a ROS that causes serious injury to the cell membrane, so SOD scavenges O2·− into hydrogen peroxide and oxygen (McCord and Fridovich 1969; Apel and Hirt 2004) and at the same time, one of the antioxidant enzymes, ascorbate peroxidase (spot 82), converts hydrogen peroxide into water (Asada 1999; Pilon et al. 2011). In this study, we showed that these SOD and APX were increased together by low-dose gamma radiation. The stress-related proteins identified in our results were mainly associated with antioxidant systems, consistent with the results of increased antioxidant activity in 50 Gy-treated plants.
Among the proteins associated with cellular structures, tubulin proteins (spot 70, 71, 73, 74) were identified (Fig. 5; Table 1). Structural proteins were more highly expressed in the plants from seeds treated at 0 Gy than those treated with gamma radiation. The main function of tubulin is known to be involved in cell plate formation and cell wall production, and in this study, increased curvature of the stem was observed as the dose of gamma radiation increased, which is attributed to its lower expression than in the controls. Structural proteins like cytoskeleton contribute to enhancing adaptability to the external environment by inducing inhibition of abnormal growth or malformation and mechanical reinforcement (Wang and Mao 2019), thus contributing to the positive effects of low dose radiation as in the results of plant growth.
The hormesis effect of low-dose gamma radiation treated to seeds was identified during vegetative growth, indicating that the hormesis effect of low-dose ionizing radiation was caused by improved photosynthetic efficiency and improved resistance to stress. Furthermore, the occurrence of malformation caused by high-dose of gamma radiation is thought to be due to the inhibition of the synthesis of structural proteins such as tubulin. The hormesis effect is likely to be better if the negative effect on the production of tubulin is reduced.
Conclusions
This study was conducted to investigate the responses that occur after ionizing radiation treatment in quinoa seeds. The results showed that germination rate and germination speed were higher at 50 Gy treatments than in untreated controls and after irradiation at higher doses, and germination rates and germination rates decreased as doses increased from 200 Gy. Plant growth after germination and panicle weight also showed the highest value in the plants from seeds treated at 50 Gy. Physiological parameters such as chlorophyll content, chlorophyll fluorescence, and NDVI were also the highest after 50 Gy treatment of seeds, confirming the beneficial effects of low-dose radiations. Antioxidant capacity was increased by the low-dose ionizing radiation, especially at 100 Gy, indicating that low-dose gamma rays improved antioxidant activity. Protein expression analysis confirmed the induction of stress-related proteins and cellular structural proteins by low-dose ionizing radiation. Therefore, low-dose gamma radiations were found to induce hormesis due to increased stress tolerance and enhanced cell structure. In quinoa, the appropriate dose of gamma radiation to seeds for hormesis induction is 50 Gy, with inhibitory effects occurring if dose was higher than 200 Gy. Hormesis, caused by a low level of irradiation, is believed to be due to enhanced metabolism and antioxidant metabolism.
References
Abdel HMS, Ali ZA (2006) Effect of gamma irradiation on wheat immature culture regenerated plant. Res J Appl Sci 2:310–316
Adolf VI, Jacobsen SE, Shabala S (2013) Salt tolerance mechanisms in quinoa (Chenopodium quinoa Willd.). Environ Exp Bot 92:43–54. https://doi.org/10.1016/j.envexpbot.2012.07.004
Agathokleous E, Kitao M, Calabrese EJ (2019) Hormesis: a compelling platform for sophisticated plant science. Trends Plant Sci 24:318–327. https://doi.org/10.1016/j.tplants.2019.01.004
Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 55:373–399. https://doi.org/10.1146/annurev.arplant.55.031903.141701
Asada K (1999) The water-water cycle in chloroplasts: scavenging of active oxygens and dissipation of excess photons. Annu Rev Plant Biol 50:601–639. https://doi.org/10.1146/annurev.arplant.50.1.601
Basyouni R, Dunn BL, Goad C (2015) Use of nondestructive sensors to assess nitrogen status in potted poinsettia (Euphorbia pulcherrima L. (Willd. ex Klotzsch)) production. Sci Hortic 192:47–53
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1016/j.scienta.2015.05.011
Buss W, Kammann C, Koyro HW (2012) Biochar reduces copper toxicity in Chenopodium quinoa Willd. in a sandy soil. J Environ Qual 41:1157–1165. https://doi.org/10.2134/jeq2011.0022
Caplin N, Willey N (2018) Ionizing radiation, higher plants, and radioprotection: from acute high doses to chronic low doses. Front Plant Sci 9:847. https://doi.org/10.3389/fpls.2018.00847
Dhakshanamoorthy D, Selvaraj R, Chidambaram ALA (2011) Induced mutagenesis in Jatropha curcas L. using gamma rays and detection of DNA polymorphism through RAPD marker. C R Biol 334:24–30. https://doi.org/10.1016/j.crvi.2010
Ellyfa K, Ahmed OH, Shaharudin S, Majid NMN (2007) Gamma radiosensitivity study on long bean (Vigna sesquipedalis). Am J Appl Sci 4:1090–1093. https://doi.org/10.3844/ajassp.2007.1090.1093
Foyer CH, Noctor G (2000) Tansley review No. 112: Oxygen processing in photosynthesis: regulation and signaling. New Phytol 146:359–388. https://doi.org/10.1046/j.1469-8137.2000.00667.x
Gamon JA, Field CB, Goulden ML, Griffin KL, Hartley AE, Joel G, Peñuelas J, Valentini R (1995) Relationships between NDVI, canopy structure, and photosynthesis in three Californian vegetation types. Ecol Appl 5:28–41. https://doi.org/10.2307/1942049
Gillespie KM, Chae JM, Ainsworth EA (2007) Rapid measurement of total antioxidant capacity in plants. Nat Protoc 2:867–870. https://doi.org/10.1038/nprot.2007.100
González JA, Rosa M, Parrado MF, Hilal M, Prado FE (2009) Morphological and physiological responses of two varieties of a highland species (Chenopodium quinoa Willd.) growing under near-ambient and strongly reduced solar UV-B in a lowland location. J Photochem Photobiol B 96:144–151. https://doi.org/10.1016/j.jphotobiol.2009.05.003
Gygi SP, Rochon Y, Franza BR, Aebersold R (1999) Correlation between protein and mRNA abundance in yeast. Nat Rev Mol Cell Biol 19:1720–1730. https://doi.org/10.1128/MCB.19.3.1720
Hampton JG, Tekrony DM (1995) Handbook of Vigor Test Methods, 3rd edn. ISTA, p 117
Hanafy RS, Akladious SA (2018) Physiological and molecular studies on the effect of gamma radiation in fenugreek (Trigonella foenum-graecum L.) plant. J Genet Eng Biotechnol 16:683–692. https://doi.org/10.1016/j.jgeb.2018.02.012
Hayashi G, Moro CF, Rohila JS, Shibato J, Kubo A, Imanaka T, Kimura S, Ozawa S, Fukutani S, Endo S, Ichikawa K (2015) 2D-DIGE-based proteome expression changes in leaves of rice seedlings exposed to low-level gamma radiation at Iitate village, Fukushima. Plant Signal Behav 10:e1103406. https://doi.org/10.1080/15592324.2015.1103406
Hegazi AZ, Hamideldin N (2010) The effect of gamma irradiation on enhancement of growth and seed yield of okra [Abelmoschus esculentus (L.) Moench] and associated molecular changes. J Hortic for 2:38–51
Hilal M, Parrado MF, Rosa M, Gallardo M, Orce L, Massa EM, Prado FE (2004) Epidermal lignin deposition in quinoa cotyledons in response to UV-B radiation. J Photochem Photobiol 79:205–210. https://doi.org/10.1562/0031-8655(2004)079%3c0205:eldiqc%3e2.0.co;2
Jaipo N, Kosiwikul M, Panpuang N, Prakrajang K (2019) Low dose gamma radiation effects on seed germination and seedling growth of cucumber and okra. J Phys Conf Ser 1380:012106. https://doi.org/10.1088/1742-6596/1380/1/012106
Jan S, Parween T, Siddiqi TO, Mahmooduzzafar (2012) Effect of gamma radiation on morphological, biochemical and physiological aspects of plants and plant products. Environ Rev 20:17–39. https://doi.org/10.1139/a11-021
Keresztes Á, Kovács E (1991) Ultrastructural effects of ionizing radiation on plant cells. Scann Microsc 5:287–296. https://doi.org/10.1007/BF03030408
Khan S, Goyal S (2009) Improvement of mungbean varieties through induced mutations. Afr J Plant Sci 3:174–180
Kim JS, Lee YG, Kim DH, Park HS, Back MH (2000) Influence of low dose gamma radiation on the growth of maize (Zea mays L.) varieties. Korean J Environ Agric 19:328–331
Kim JH, Back MH, Chung BY, Wi SG, Kim JS (2004) Alterations in the photosynthetic pigments and antioxidant machineries of red pepper (Capsicum annuum L.) seedlings from gamma irradiated seeds. J Plant Biol 47:314–321. https://doi.org/10.1007/BF03030546
Kim DS, Kim JB, Goh EJ, Kim WJ, Kim SH, Seo YW, Kang SY (2011) Antioxidant response of Arabidopsis plants to gamma irradiation: genome-wide expression profiling of the ROS scavenging and signal transduction pathways. J Plant Physiol 168:1960–1971. https://doi.org/10.1016/j.jplph.2011.05.008
Kon E, Ahmed OH, Saamin S, Majid NM (2007) Gamma radiosensitivity study on long bean (Vigna sesquipedalis). Am J Appl Sci 4:1090–1093. https://doi.org/10.3844/ajassp.2007.1090.1093
Kovács E, Keresztes A (2002) Effect of gamma and UV-B/C radiation on plant cells. Micron 33:199–210. https://doi.org/10.1016/S0968-4328(01)00012-9
Li L, Jiang Q, Niu F, Hu Z, Zhang H (2016) Research progress on salt tolerance mechanisms in quinoa. J Agr Sci Technol (beijing) 18:31–40
Mackerness SAH, Surplus SL, Blake P, John CF, Buchanan-Wollaston V, Jordan BR, Thomas B (1999) Ultraviolet-B-induced stress and changes in gene expression in Arabidopsis thaliana: role of signalling pathways controlled by jasmonic acid, ethylene and reactive oxygen species. Plant Cell Environ 22:1413–1423. https://doi.org/10.1046/j.1365-3040.1999.00499.x
Maity JP, Mishra D, Chakraborty A, Saha A, Santra SC, Chanda S (2005) Modulation of some quantitative and qualitative characteristics in rice (Oryza sativa L.) and mung (Phaseolus mungo L.) by ionizing radiation. Radiat Phys Chem 74:391–394. https://doi.org/10.1016/j.radphyschem.2004.08.005
Marcu D, Damian G, Cosma C, Cristea V (2013) Gamma radiation effects on seed germination, growth and pigment content, and ESR study of induced free radicals in maize (Zea mays). J Biol Phys 39:625–634. https://doi.org/10.1007/s10867-013-9322-z
Martínez EA, Veas E, Jorquera C, San Martín R, Jara P (2009) Re-introduction of Chenopodium quinoa Willd. into arid Chile: cultivation of two lowland races under extremely low irrigation. J Agron Crop Sci 195:1–10. https://doi.org/10.1111/j.1439-037X.2008.00332.x
McCord JM, Fridovich I (1969) Superoxide dismutase: an enzymic function for erythrocuprein (hemocuprein). J Biol Chem 244:6049–6055. https://doi.org/10.1016/S0021-9258(18)63504-5
Melki M, Marouani A (2010) Effects of gamma rays irradiation on seed germination and growth of hard wheat. Environ Chem Lett 8:307–310. https://doi.org/10.1007/s10311-009-0222-1
Miller MW, Miller WM (1987) Radiation hormesis in plants. Health Phys 52:607–616. https://doi.org/10.1097/00004032-198705000-00012
Mittler R, Vanderauwera S, Gollery M, Van Breusegem F (2004) The reactive oxygen gene network in plants. Trends Plant Sci 9:490–498. https://doi.org/10.1016/j.tplants.2004.08.009
Nonogaki H, Bassel G, Bewley JD (2010) Germination—still a mystery. Plant Sci 179:574–581. https://doi.org/10.1016/j.plantsci.2010.02.010
Pilon M, Ravet K, Tapken W (2011) The biogenesis and physiological function of chloroplast superoxide dismutases. Biochim Biophys Acta 1807:989–998. https://doi.org/10.1016/j.bbabio.2010.11.002
Richardson AD, Duigan SP, Berlyn GP (2002) An evaluation of noninvasive methods to estimate foliar chlorophyll content. New Phytol 153:185–194. https://doi.org/10.1046/j.0028-646X.2001.00289.x
Sah NK, Pramanik S, Raychaudhuri SS (1996) Peroxidase changes in barley induced by ionizing and thermal radiation. Int J Radiat Biol 69:107–111. https://doi.org/10.1080/095530096146237
Shevchenko A, Wilm M, Vorm O, Mann M (1996) Mass spectrometric sequencing of proteins from silver-stained polyacrylamide gels. Anal Chem 68:850–858. https://doi.org/10.1021/ac950914h
Sosedov NI, Vakar AB (1963) Effect of gamma-rays on the biochemical properties of wheat. Proc Intern Congr Biochem 13:27–40
Volkova PY, Clement G, Makarenko ES, Kazakova EA, Bitarishvili SV, Lychenkova MA (2020) Metabolic profiling of γ-irradiated barley plants identifies reallocation of nitrogen metabolism and metabolic stress response. Dose Response 18:1559325820914186. https://doi.org/10.1177/1559325820914186
Wang X, Mao T (2019) Understanding the functions and mechanisms of plant cytoskeleton in response to environmental signals. Curr Opin Plant Biol 52:86–96. https://doi.org/10.1016/j.pbi.2019.08.002
Wang W, Tai F, Chen S (2008) Optimizing protein extraction from plant tissues for enhanced proteomics analysis. J Sep Sci 31:2032–2039. https://doi.org/10.1002/jssc.200800087
Yang A, Akhtar SS, Amjad M, Iqbal S, Jacobsen SE (2016) Growth and physiological responses of quinoa to drought and temperature stress. J Agron Crop Sci 202:445–453. https://doi.org/10.1111/jac.12167
Zaka R, Chenal C, Misset MT (2004) Effects of low doses of short-term gamma irradiation on growth and development through two generations of Pisum sativum. Sci Total Environ 320:121–129. https://doi.org/10.1016/j.scitotenv.2003.08.010
Zhang DW, Yuan S, Xu F, Zhu F, Yuan M, Ye HX (2016) Light intensity affects chlorophyll synthesis during greening process by metabolite signal from mitochondrial alternative oxidase in Arabidopsis. Plant Cell Environ 39:12–25. https://doi.org/10.1111/pce.12438
Zhang Y, Fu Q, Huang T, Liu CG, Lin A (2022) Ionizing radiation-induced DNA damage response affect cell compressibility. Biochem Biophys Res Commun 603:116–122. https://doi.org/10.1016/j.bbrc.2022.03.032
Acknowledgements
This study is a part of the results of the research funded by the Korean Ministry of Environment (grant no. 2018002270002).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Song, K.E., Park, C.Y., Hong, S.H. et al. Beneficial effects of gamma-irradiation of quinoa seeds on germination and growth. Radiat Environ Biophys 61, 465–477 (2022). https://doi.org/10.1007/s00411-022-00986-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00411-022-00986-2