Abstract
Although radiotherapy, especially carbon-ion radiotherapy, is an effective treatment modality against non-small-cell lung cancer (NSCLC), studies using radiation combined with sensitizer for improving the efficacy of radiotherapy are still needed. In this work, we aimed to investigate in NSCLC A549 and H1299 cell lines the effects of different linear energy transfer (LET) radiations combined with diverse sensitizing compounds. Cells pretreated with the CHK1/CHK2 inhibitor AZD7762, Honokiol or Tunicamycin were irradiated with low-LET X-rays and high-LET carbon ions. Cell survival was assessed using the clonogenic cell survival assay. Cell cycle distribution and apoptosis were measured with flow cytometry, and DNA double strand break (DSB) and repair were detected using γ-H2AX immunofluorescence staining. Our results revealed that AZD7762, Honokiol and Tunicamycin demonstrated low cytotoxicity to NSCLC cells and a pronounced radiosensitizing effect on NSCLC cells exposed to carbon ions than X-rays. Unrepaired DNA DSB damages, the abrogation of G2/M arrest induced by irradiation, and finally apoptotic cell death were the main causes of the radiosensitizing effect. Thus, our data suggest that high-LET carbon ion combined with these compounds may be a potentially effective therapeutic strategy for locally advanced NSCLC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lung cancer is one of the most prevalent malignancies around the world. In China, the morbidity and mortality due to lung cancer ranked first among all the tumors, which occupied 20% and 27% of the total cancer cases, respectively (Zheng et al. 2015). Lung cancer was classified into two categories according to the histopathological typing: small-cell lung cancer (SCLC, 20%) and non-small-cell lung cancer (NSCLC) which accounts for more than 80 ~ 85% of all lung tumors. Approximately 35% of patients with NSCLC are presented with locally advanced non metastatic disease (Auperin et al. 2010). Radiochemotherapy is an essential module of the multimodal treatment of locally advanced NSCLC patients. Despite significant progress of radiotherapy delivery (Schwartz et al. 2009; McAvoy et al. 2013) and the schemes with novel chemotherapy combinations (Shen et al. 2014; van den Heuvel et al. 2014), 5-year survival rate is merely 10 ~ 30% (Jalal et al. 2012; Billiet et al. 2014).
In 1994, the National Institute of Radiological Sciences (NIRS), Japan, began to explore carbon-ion radiotherapy (CIRT) as a treatment modality for NSCLC. Compared with conventional radiations (X- and γ-rays), carbon-ion beams have unique biological and physical advantages, including the delivery of high local doses to the tumor and low dose deposition in normal tissues due to inverted dose distribution, the high linear energy transfer (LET) and relative biological effectiveness (RBE) throughout the spread-out Bragg peak (Kanai et al. 1997). As reported previously, local control and overall survival rates at 5 years are 80 ~ 90% and 40 ~ 50%, respectively, in early NSCLC after hypofractionated CIRT, which are comparable to those of surgery or SBRT, with markedly low toxicities (Miyamoto et al. 2007; Sugane et al. 2009; Takahashi et al. 2015).
Only few data demonstrate the effect of combination of chemotherapy with high-LET carbon ions, although based on various preclinical and clinical data, low-LET photons combined with chemotherapy has been successfully used in the clinical practice. However, due to different radiobiological effects of carbon ions, especially on DNA damage, cell cycle control, etc., the molecular mechanisms of combined effects might be different between photons and carbon ions. A study by Combs and colleagues investigated the effect of combination of CIRT and different chemotherapies in glioblastoma cells and found that additive effects with several drugs, whereby paclitaxel and camptothecin displayed the strongest additive effects (Combs et al. 2012). A retrospective study for locally advanced pancreatic cancer also showed that chemotherapy combined with protons or carbon ions resulted in improved 1-year overall survival significantly as compared to other treatment schedules (Durante et al. 2015). However, another study did not reveal any decreased clonogenic survival of several tumor cell lines treated by combinations of different chemotherapeutics with photons or with carbon ions (Schlaich et al. 2013).
AZD7762 (AZD) is an ATP competitive inhibitor of checkpoint kinases and can abrogate the G2 checkpoint activation induced by DNA double strand breaks (DSB) (Isono et al. 2017). Honokiol (HNK), a natural flavonoid, exhibits relatively wide-ranging anticancer capabilities and favorable safety profile (Wang et al. 2018). It has been reported that Tunicamycin (TM) might have radiosensitizing effects on tumor cells, but not on normal cells (Contessa et al. 2008). The present analysis focuses on the evaluation of the effects of X-rays and carbon ions combined with these compounds as radiosensitizer in two NSCLC cell lines.
Materials and methods
Cell culture
Human NSCLC cell lines A549 and H1299 were purchased from the Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China). Cells were maintained in RPMI-1640 medium with 100 U/ml penicillin, 100 μg/ml streptomycin and 10% (v/v) fetal bovine serum and kept at 37 °C, 5% CO2 in incubators.
Treatment with AZD, HNK and TM
AZD, HNK and TM were purchased from Selleck Chemicals Ltd (USA). The molecular structure of these compounds is provided in Fig. 1. Cells were treated with various concentrations of each drug for 24 h, and then the CCK8 kit was used to measure cell proliferation. Non-cytotoxic or mildly cytotoxic concentrations were used in the following experiments (Fig. 1). Cells were pre-treated with AZD, HNK or TM for 2 h before irradiation and then incubated with the respective compound for a maximum period of 24 h after irradiation.
Irradiations
Irradiation using low-LET X-rays was performed as single exposure at doses of 1, 2, 4, and 6 Gy at room temperature. X-rays were delivered by a biological irradiator (Pxinc X-Ray, USA) operated at 225 kV with an average dose rate of 2 Gy/min (current: 13.30 mA and filter: 2 mm Al). The dose was monitored by dosimeter installed in the beam and it shut off the machine when the preset dose was achieved.
Carbon ion irradiation was performed in the superficially-placed tumor heavy-ion therapy terminal of the Heavy Ion Research Facility in Lanzhou (HIRFL) at the Institute of Modern Physics (IMP), Chinese Academy of Sciences, China. The monoenergetic carbon ion beam was delivered at the initial value of 80.55 MeV/u. The beam formed a circular radiation field with 5 cm diameter using a zigzag scanning method. Dose-averaged LET value of the carbon ion beam reached on cell samples was adjusted to be 50 keV/mm using energy absorbers of 6 mm PMMA. Single carbon ion doses of 1, 2, 4, and 6 Gy were applied with an average dose rate of 2 Gy/min (Dai et al. 2007).
Clonogenic cell survival assay
After irradiation, cell survival was detected using the colony formation assay as described previously (Jin et al. 2018).
Analysis of cell cycle
At 8, 12, and 24 h after irradiation, cells were fixed with 70% ethyl alcohol/PBS in 4 °C. After 48 h, cells were resuspended in 1 ml of fluorochrome solution (PI at 0.5 mg/ml in 0.2 mg/ml RNAase and 0.1% Triton X-100) and incubated at room temperature for 30 min in darkness. Cells were measured using a flow cytometer (CyFlow Cube 6, Sysmex Company, Japan). Cells arrested at G2/M (4N) were quantified using the cell-cycle data analysis software (Modfit 3.1).
Detection of apoptosis
Forty-eight hours after irradiation, apoptotic cells were quantified by flow cytometric measurements of Annexin V-FITC and PI (Keygentec, China), as reported previously (Jin et al. 2018).
Immunofluorescence staining
At the indicated post-irradiation times, cells seeded on coverslips were fixed with 4% paraformaldehyde/PBS for 20 min at room temperature. After permeabilization with 0.5% Triton X-100/PBS for 15 min at room temperature, cells were treated with PBST containing 5% BSA for 1.5 h at room temperature. Subsequently, they were incubated with anti-γ-H2AX antibody (1:400 CST) in PBST for 2 h at room temperature. The cells were then incubated in the dark with Alexa Fluor 488-conjugated anti-rabbit secondary antibody at a 1:250 dilution for 1.5 h, followed by DAPI staining. Coverslips were mounted with Prolong Gold antifade reagent (Invitrogen). Fluorescent microscopic analysis was performed using an Olympus BX51 microscope (Olympus, Japan) with a reflected light fluorescence. At least 100 cells were analyzed and a cell with 10 foci or more was marked as positive.
Statistics
Data are represented as the mean ± standard deviation (SD). Statistical analysis was conducted using the unpaired Student’s t test. A difference was considered significant when p < 0.05.
Results
Evaluation of the proliferation of cells treated with the compounds
To assess the effects of the compounds on cell proliferation, NSCLC A549 and H1299 cells were treated with AZD (0 ~ 100 μM), HNK (0 ~ 80 μM) and TM (0 ~ 10 μg/ml) and the proliferation was determined 24 h after treatment. All reagents suppressed the cell proliferation in a concentration-dependent manner (Fig. 1a–c). Treatments with 25 μM (AZD), 20 μM (HNK) and 2 μg/ml (TM) showed to be non-cytotoxic or mildly cytotoxic to the cells, so these concentrations were used in the following experiments.
The radiosensitizing effect of the compounds on cells for X-rays and carbon ions
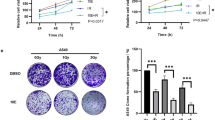
We next evaluated the effect of AZD, HNK and TM on A549 and H1299 cells exposed to X-rays and carbon ions using the clonogenic survival assay. As shown in Fig. 2a, b, the irradiations decreased the clonogenic ability in both NSCLC cell lines in a dose-dependent manner. The AZD pre-treatment represented the most significant sensitizing effect among the three reagents in A549 cells after X-ray irradiation, whereas HNK showed a slight radio-protective effect. For carbon ion irradiation, TM exhibited the most significant sensitizing effect among the reagents on A549 cells and HNK had a slightly lower effect than TM. TM and AZD displayed similar sensitizing effects on H1299 cells to X-rays of low doses while both inducing a radio-protective effect at the high dose of 6 Gy. However, HNK pre-treatment always caused a slight radio-protective effect on H1299 cells after X-ray irradiation (Fig. 2b). Compared with AZD and TM, the most obvious sensitization was presented for HNK in H1299 cells irradiated with carbon ions. The sensitizer enhancement ratios (SER), calculated using the radiation doses at 10% survival level for AZD, HNK and TM, are summarized in Table 1. The data indicate that all the reagents enhanced the sensitivity of NSCLC cells more significantly to carbon ions than to X-rays, TM exhibited the most pronounced radiosensitizing effect on NSCLC cells to carbon ion irradiation.
Effect of AZD, HNK or TM on cellular radiosensitivity. Cellular radiosensitivity was examined using the clonogenic survival assay. a A549 cells were pre-treated with AZD, HNK or TM before the irradiation with X-rays (left) and carbon ions (right). b H1299 cells were pre-treated with AZD, HNK or TM before the irradiation with X-rays (left) and carbon ions (right)
Effect of the compounds on cell cycle distribution after irradiation
As G2/M checkpoint activation is a key event after irradiation and influences the radiosensitivity of cells (Hu et al. 2014), we analyzed the cycle phase distribution of cells co-treated with the reagents and radiation. As shown in Fig. 3b, c, radiation caused an increase in cell proportion at G2/M phase at 8 and 12 h post-irradiation in both the cell lines, suggesting that G2/M checkpoint was activated after irradiation. In A549 cells, the reagent treatment did not significantly alter the cell cycle distribution after carbon ion irradiation. Conversely, the reagent pre-treatment resulted in a decrease in cell proportion at G2/M phase after exposure to carbon ions in H1299 cells, which might be largely due to the different p53 status in the two cell lines (A549, p53 wild-type; H1299, p53-null) (Benada and Macurek 2015). Moreover, the cell cycle distribution returned to normal except in cells pre-treated with HNK at 24 h after carbon ion irradiation (Fig. 3b). In addition, the results derived from X-ray irradiation showed that a relief of G2/M arrest was observed in H1299 cells pre-treated with AZD. Furthermore, HNK or TM pre-treatment had an obvious influence on the distribution of cells in the cell cycle 12 h after irradiation (Fig. 3c). These results suggest that radiation-induced G2/M arrest was relieved by these reagents in H1299 cells but not in A549 cells.
Effect of AZD, HNK or TM on cell cycle distribution after irradiations. a Flow cytometric profiles of DNA content in cells pre-treated with the reagents at 8 h after carbon ion irradiation at 2 Gy. b, c: the G2/M-phase percentages in A549 (left) and H1299 (right) cells after irradiation with carbon ions and X-rays at 2 Gy, respectively. **p < 0.01 versus the irradiation alone
Effect of the compounds on apoptosis after irradiation
Apoptosis is considered to be one of the main cell death mechanisms after exposure to radiations, depending on the radiation dose and p53 status in cancer cells (Debije et al. 2000). Therefore, we analyzed the effects of AZD, HNK or TM pre-treatment on apoptosis induction after irradiation. As shown in Fig. 4, after X-ray irradiation, apoptotic rate increased from 3% to 5% in A549 cells and from 3% to 6% in H1299 cells. Compared to X-ray irradiation alone, the apoptotic rate was slightly increased after irradiation in combination with the reagents. Interestingly, apoptotic rate was significantly elevated after carbon ion irradiation (13.24% in A549 cells and 11.76% in H1299 cells). Furthermore, an enhanced induction of apoptosis was observed in the cells pre-treated with each reagent (HNK: 22.32%, TM: 31% in A549 cells; AZD: 22.42%, HNK: 25.1% and TM: 28.64% in H1299 cells) except A549 cells pre-treated with AZD (11.78%). Our results suggest that although the reagents have no effect on apoptosis induction for X-ray irradiation, they aggravated carbon-induced apoptosis.
Effect of AZD, HNK or TM on induction of apoptosis after irradiation. a Typical flow cytometric profiles of apoptosis detected by the Annexin-FITC and PI double staining assay. A549 cells were analyzed 48 h after 2 Gy irradiation. b, c the corresponding statistical results in A549 and H1299 cells, respectively. *p < 0.05, **p < 0.01 versus the irradiation alone
Effect of the compounds on radiation-induced DSBs
To examine whether the reagents enhance the radiosensitivity of NSCLC cells by inhibiting DSB repair, we measured cellular DSB levels using the γ-H2AX foci formation assay. As shown in Fig. 5, after cells were exposed to X-rays or carbon ions at 2 Gy, the mean number of γ-H2AX foci positive cells was comparable 2 h after irradiation, suggesting none of the reagents influenced the kinetics of γ-H2AX foci formation post-irradiation. The number of cells with high foci numbers gradually decreased over time although the cells sustained more foci dots at 24 h after irradiation with carbon ions than with X-rays. Moreover, AZD pre-treatment inhibited the reduction of foci numbers in A549 and H1299 cells after X-ray irradiation and in H1299 cells after carbon ion irradiation. HNK pre-treatment suppressed the decline of foci numbers in A549 and H1299 cells after carbon ion irradiation. TM pre-treatment also repressed the decrease of foci numbers in A549 and H1299 cells after carbon ion irradiation and in H1299 cells after X-ray irradiation. Furthermore, we counted the average foci numbers in cells after the various treatments and found that there was a similar trend (data not shown). These results were consistent with the results obtained from the radiosensitizing experiments and indicate that DSB repair rather than DSB formation was likely involved in the apoptosis induction and radiosensitizing effects.
Discussion
Our results clearly indicate the pre-treatment with AZD, HNK or TM modulated the sensitivity of NSCLC cells to low-LET X-rays and high-LET carbon ions and the reagents exacerbated the cytotoxicity of carbon ion to NSCLC cells.
AZD is a potent CHK1/CHK2 inhibitor, which has chemosensitizing activity in vitro and in vivo (Zabludoff et al. 2008). Several extracellular stresses lead to DNA damage, including ion radiation. When DSBs are created, telangiectasia mutated (ATM)/CHK2 pathway is activated by the MRE11 sensor complex which is recruited at DNA damage sites, activates the ATM/CHK2 pathway and promotes S-phase cell cycle arrest and the p53-associated G1/S-phase checkpoint. DNA single-strand breaks (DNA SSBs) generated at sites of DNA damage activate the ataxia telangiectasia and RAD3-related (ATR) kinase which phosphorylates CHK1 at Ser-317 and Ser-345. Activated CHK1 triggers the intra-S and G2/M-phase checkpoints (Sancar et al. 2004; Qiu et al. 2018). Cell cycle arrest induced by DNA damage is abolished by suppression activity of the checkpoint kinases CHK1/CHK2 and thus allows cells with damaged DNA to enter mitosis, finally leading to cell death. In the present study, in H1299 cells, the effect of AZD was associated with abrogation of the G2/M arrest induced by X-rays and carbon ions, especially with the persistence of unrepaired DNA damage, as indicated by our findings that AZD inhibited the repair of radiation-induced DSBs evidenced by the sustained expression of γH2AX foci. However, the enhanced cell killing may mostly contribute to the unrepaired DNA DSBs in A549 cells, which did not exhibit the abolition of G2/M arrest because of the wild-type p53 status (Chen et al. 2003).
HNK is a bioactive compound isolated from the bark of Magnolia tree, which is widely applied in traditional Chinese and Japanese medicine to treat various diseases (Lee et al. 2011). Recent studies have shown that it has potent anticancer activities in various cancer models including skin, lung, breast, ovarian, prostate, gastrointestinal and other cancers with low toxicity (Wang et al. 2014; Leeman-Neill et al. 2010; Arora et al. 2012). HNK also has the sensitizing effect on tumor cells to ionizing radiation. For example, the work by Hu et al. demonstrated that co-treatment with liposomal HNK and X-ray irradiation caused a synergistic antitumor efficacy in lung cancer in vitro and in vivo (Hu et al. 2008). The results from Ponnurangam et al. showed that HNK in combination with radiation inhibited the proliferation and promoted apoptosis in colorectal cancer cells (Ponnurangam et al. 2012). Our present study displayed a similar effect of HNK on NSCLC cells and the HNK pre-treatment enhanced cell death induced by carbon ions through reducing DNA DSB repair and promoting apoptosis. This conclusion was also drawn by Wang et al. (Wang et al. 2018). They found that radiation-induced nuclear accumulation of survivin which interacted with the non-homologous end-joining (NHEJ) DNA repair complex after irradiation. This process enhanced DNA DSB repair and tumor radioresistance, but HNK treatment effectively down-regulated survivin expression so as to increase the radiosensitivity in squamous cell carcinoma of the head and neck. Additionally, we also found that the change of the cycle phase distribution of cells pre-treated with HNK might be another reason for the radiosensitization. Similar to the AZD pre-treatment, HNK released the G2/M arrest induced by carbon ions and thus promoted cells with unrepaired DNA damage to enter mitosis, thereby leading to apoptosis in H1299 cells. In addition, in both cell lines, the compounds themselves did not impair the cell cycle transition, but did so in combination with carbon ion irradiation. More systematic analyses of the transition of cells through the cell cycle using checkpoint-related markers will be carried in the future.
In this study, TM, as a classic endoplasmic reticulum (ER) stress inducer, was also tested whether it increases the radiosensitivity of A549 and H1299 cells to X-rays and carbon ions. We revealed that TM had significantly enhanced radiation effects on NSCLC cells except for A549 cells exposed to X-rays. The central mechanism of TM-induced radiosensitization was the sustained DSB-caused apoptosis. This mechanism is similar to that described by Yamamori et al. (Yamamori et al. 2013). They verified that TM stimulates the proteasomal degradation of Rad51, thereby impairing DSB repair and enhancing the radiosensitivity of tumor cells. Interestingly, TM showed the same remission of G2/M arrest as AZD pre-treatment did in H1299 cells, suggesting that G2/M arrest relief might be another mechanism of radiosensitization.
In conclusion, the present study showed that, at minimally toxic concentrations, AZD, HNK and TM exerted radiosensitizing effects on NSCLC cells exposed to low-LET X-rays and high-LET carbon ions. Importantly, radiosensitization by the compounds could be achieved more significantly for irradiation with carbon ions than with X-rays, and TM had the most apparent radiosensitizating effect among the three compounds after carbon ion irradiation. Our data suggest that the persistence of DNA DSB, the abrogation of G2/M arrest induced by irradiation, and finally apoptotic cell death are the main causes of the radiosensitization.
References
Arora S, Singh S, Piazza GA, Contreras CM, Panyam J, Singh AP (2012) Honokiol: a novel natural agent for cancer prevention and therapy. Curr Mol Med 12(10):1244–1252
Auperin A, Le Pechoux C, Rolland E, Curran WJ, Furuse K, Fournel P, Belderbos J, Clamon G, Ulutin HC, Paulus R, Yamanaka T, Bozonnat MC, Uitterhoeve A, Wang X, Stewart L, Arriagada R, Burdett S, Pignon JP (2010) Meta-analysis of concomitant versus sequential radiochemotherapy in locally advanced non-small-cell lung cancer. J Clin Oncol 28(13):2181–2190. https://doi.org/10.1200/JCO.2009.26.2543
Benada J, Macurek L (2015) Targeting the checkpoint to kill cancer cells. Biomolecules 5(3):1912–1937. https://doi.org/10.3390/biom5031912
Billiet C, Decaluwe H, Peeters S, Vansteenkiste J, Dooms C, Haustermans K, De Leyn P, De Ruysscher D (2014) Modern post-operative radiotherapy for stage III non-small cell lung cancer may improve local control and survival: a meta-analysis. Radiother Oncol 110(1):3–8. https://doi.org/10.1016/j.radonc.2013.08.011
Chen Z, Xiao Z, Chen J, Ng SC, Sowin T, Sham H, Rosenberg S, Fesik S, Zhang H (2003) Human Chk1 expression is dispensable for somatic cell death and critical for sustaining G2 DNA damage checkpoint. Mol Cancer Ther 2(6):543–548
Combs SE, Zipp L, Rieken S, Habermehl D, Brons S, Winter M, Haberer T, Debus J, Weber KJ (2012) In vitro evaluation of photon and carbon ion radiotherapy in combination with chemotherapy in glioblastoma cells. Radiat Oncol 7:9. https://doi.org/10.1186/1748-717X-7-9
Contessa JN, Bhojani MS, Freeze HH, Rehemtulla A, Lawrence TS (2008) Inhibition of N-linked glycosylation disrupts receptor tyrosine kinase signaling in tumor cells. Cancer Res 68(10):3803–3809. https://doi.org/10.1158/0008-5472.CAN-07-6389
Dai ZY, Li Q, Xiao GQ, Jin XD, Yan Z (2007) Capability verification of the beam delivery system in the superficially-placed tumor therapy terminal at HIRFL. High Energy Phys Nucl Phys 31(7):655–659. https://doi.org/10.3321/j.issn:0254-3052.2007.07.011(Chinese)
Debije MG, Strickler MD, Bernhard WA (2000) On the efficiency of hole and electron transfer from the hydration layer to DNA: an EPR study of crystalline DNA X-irradiated at 4 K. Radiat Res 154(2):163–170
Durante M, Tommasino F, Yamada S (2015) Modeling combined chemotherapy and particle therapy for locally advanced pancreatic cancer. Front Oncol 5:145. https://doi.org/10.3389/fonc.2015.00145
Hu J, Chen LJ, Liu L, Chen X, Chen PL, Yang G, Hou WL, Tang MH, Zhang F, Wang XH, Zhao X, Wei YQ (2008) Liposomal Honokiol, a potent anti-angiogenesis agent, in combination with radiotherapy produces a synergistic antitumor efficacy without increasing toxicity. Exp Mol Med 40(6):617–628. https://doi.org/10.3858/emm.2008.40.6.617
Hu Y, Hellweg CE, Baumstark-Khan C, Reitz G, Lau P (2014) Cell cycle delay in murine pre-osteoblasts is more pronounced after exposure to high-LET compared to low-LET radiation. Radiat Environ Biophys 53(1):73–81. https://doi.org/10.1007/s00411-013-0499-0
Isono M, Hoffmann MJ, Pinkerneil M, Sato A, Michaelis M, Cinatl J Jr, Niegisch G, Schulz WA (2017) Checkpoint kinase inhibitor AZD7762 strongly sensitises urothelial carcinoma cells to gemcitabine. J Exp Clin Cancer Res 36(1):1. https://doi.org/10.1186/s13046-016-0473-1
Jalal SI, Riggs HD, Melnyk A, Richards D, Agarwala A, Neubauer M, Ansari R, Govindan R, Bruetman D, Fisher W, Breen T, Johnson CS, Yu M, Einhorn L, Hanna N (2012) Updated survival and outcomes for older adults with inoperable stage III non-small-cell lung cancer treated with cisplatin, etoposide, and concurrent chest radiation with or without consolidation docetaxel: analysis of a phase III trial from the Hoosier Oncology Group (HOG) and US Oncology. Ann Oncol 23(7):1730–1738. https://doi.org/10.1093/annonc/mdr565
Jin X, Li F, Liu B, Zheng X, Li H, Ye F, Chen W, Li Q (2018) Different mitochondrial fragmentation after irradiation with X-rays and carbon ions in HeLa cells and its influence on cellular apoptosis. Biochem Biophys Res Commun 500(4):958–965. https://doi.org/10.1016/j.bbrc.2018.04.214
Kanai T, Furusawa Y, Fukutsu K, Itsukaichi H, Eguchi-Kasai K, Ohara H (1997) Irradiation of mixed beam and design of spread-out Bragg peak for heavy-ion radiotherapy. Radiat Res 147(1):78–85
Lee YJ, Lee YM, Lee CK, Jung JK, Han SB, Hong JT (2011) Therapeutic applications of compounds in the Magnolia family. Pharmacol Ther 130(2):157–176. https://doi.org/10.1016/j.pharmthera.2011.01.010
Leeman-Neill RJ, Cai Q, Joyce SC, Thomas SM, Bhola NE, Neill DB, Arbiser JL, Grandis JR (2010) Honokiol inhibits epidermal growth factor receptor signaling and enhances the antitumor effects of epidermal growth factor receptor inhibitors. Clin Cancer Res 16(9):2571–2579. https://doi.org/10.1158/1078-0432.CCR-10-0333
McAvoy SA, Ciura KT, Rineer JM, Allen PK, Liao Z, Chang JY, Palmer MB, Cox JD, Komaki R, Gomez DR (2013) Feasibility of proton beam therapy for reirradiation of locoregionally recurrent non-small cell lung cancer. Radiother Oncol 109(1):38–44. https://doi.org/10.1016/j.radonc.2013.08.014
Miyamoto T, Baba M, Sugane T, Nakajima M, Yashiro T, Kagei K, Hirasawa N, Sugawara T, Yamamoto N, Koto M, Ezawa H, Kadono K, Tsujii H, Mizoe JE, Yoshikawa K, Kandatsu S, Fujisawa T, Working Group for Lung C (2007) Carbon ion radiotherapy for stage I non-small cell lung cancer using a regimen of four fractions during 1 week. J Thorac Oncol 2(10):916–926. https://doi.org/10.1097/JTO.0b013e3181560a68
Ponnurangam S, Mammen JM, Ramalingam S, He Z, Zhang Y, Umar S, Subramaniam D, Anant S (2012) Honokiol in combination with radiation targets notch signaling to inhibit colon cancer stem cells. Mol Cancer Ther 11(4):963–972. https://doi.org/10.1158/1535-7163.MCT-11-0999
Qiu Z, Oleinick NL, Zhang J (2018) ATR/CHK1 inhibitors and cancer therapy. Radiother Oncol 126(3):450–464. https://doi.org/10.1016/j.radonc.2017.09.043
Sancar A, Lindsey-Boltz LA, Unsal-Kacmaz K, Linn S (2004) Molecular mechanisms of mammalian DNA repair and the DNA damage checkpoints. Annu Rev Biochem 73:39–85. https://doi.org/10.1146/annurev.biochem.73.011303.073723
Schlaich F, Brons S, Haberer T, Debus J, Combs SE, Weber KJ (2013) Comparison of the effects of photon versus carbon ion irradiation when combined with chemotherapy in vitro. Radiat Oncol 8:260. https://doi.org/10.1186/1748-717X-8-260
Schwartz DL, Lobo MJ, Ang KK, Morrison WH, Rosenthal DI, Ahamad A, Evans DB, Clayman G, Sherman SI, Garden AS (2009) Postoperative external beam radiotherapy for differentiated thyroid cancer: outcomes and morbidity with conformal treatment. Int J Radiat Oncol Biol Phys 74(4):1083–1091. https://doi.org/10.1016/j.ijrobp.2008.09.023
Shen WY, Ji J, Zuo YS, Pu J, Xu YM, Zong CD, Tao GZ, Chen XF, Ji FZ, Zhou XL, Han JH, Wang CS, Yi JG, Su XL, Zhu WG (2014) Comparison of efficacy for postoperative chemotherapy and concurrent radiochemotherapy in patients with IIIA-pN2 non-small cell lung cancer: an early closed randomized controlled trial. Radiother Oncol 110(1):120–125. https://doi.org/10.1016/j.radonc.2013.10.008
Sugane T, Baba M, Imai R, Nakajima M, Yamamoto N, Miyamoto T, Ezawa H, Yoshikawa K, Kandatsu S, Kamada T, Mizoe J, Tsujii H (2009) Carbon ion radiotherapy for elderly patients 80 years and older with stage I non-small cell lung cancer. Lung Cancer 64(1):45–50. https://doi.org/10.1016/j.lungcan.2008.07.007
Takahashi W, Nakajima M, Yamamoto N, Yamashita H, Nakagawa K, Miyamoto T, Tsuji H, Kamada T, Fujisawa T (2015) A prospective nonrandomized phase I/II study of carbon ion radiotherapy in a favorable subset of locally advanced non-small cell lung cancer (NSCLC). Cancer 121(8):1321–1327. https://doi.org/10.1002/cncr.29195
van den Heuvel MM, Uyterlinde W, Vincent AD, de Jong J, Aerts J, Koppe F, Knegjens J, Codrington H, Kunst PW, Dieleman E, Verheij M, Belderbos J (2014) Additional weekly Cetuximab to concurrent chemoradiotherapy in locally advanced non-small cell lung carcinoma: efficacy and safety outcomes of a randomized, multi-center phase II study investigating. Radiother Oncol 110(1):126–131. https://doi.org/10.1016/j.radonc.2013.10.009
Wang X, Beitler JJ, Wang H, Lee MJ, Huang W, Koenig L, Nannapaneni S, Amin AR, Bonner M, Shin HJ, Chen ZG, Arbiser JL, Shin DM (2014) Honokiol enhances paclitaxel efficacy in multi-drug resistant human cancer model through the induction of apoptosis. PLoS ONE 9(2):e86369. https://doi.org/10.1371/journal.pone.0086369
Wang X, Beitler JJ, Huang W, Chen G, Qian G, Magliocca K, Patel MR, Chen AY, Zhang J, Nannapaneni S, Kim S, Chen Z, Deng X, Saba NF, Chen ZG, Arbiser JL, Shin DM (2018) Honokiol radiosensitizes squamous cell carcinoma of the head and neck by downregulation of survivin. Clin Cancer Res 24(4):858–869. https://doi.org/10.1158/1078-0432.CCR-17-0345
Yamamori T, Meike S, Nagane M, Yasui H, Inanami O (2013) ER stress suppresses DNA double-strand break repair and sensitizes tumor cells to ionizing radiation by stimulating proteasomal degradation of Rad51. FEBS Lett 587(20):3348–3353. https://doi.org/10.1016/j.febslet.2013.08.030
Zabludoff SD, Deng C, Grondine MR, Sheehy AM, Ashwell S, Caleb BL, Green S, Haye HR, Horn CL, Janetka JW, Liu D, Mouchet E, Ready S, Rosenthal JL, Queva C, Schwartz GK, Taylor KJ, Tse AN, Walker GE, White AM (2008) AZD7762, a novel checkpoint kinase inhibitor, drives checkpoint abrogation and potentiates DNA-targeted therapies. Mol Cancer Ther 7(9):2955–2966. https://doi.org/10.1158/1535-7163.MCT-08-0492
Zheng RS, Sun KX, Zhang SW, Zeng HM, Zou XN, Chen R, Gu XY, Wei WW, He J (2019) [Report of cancer epidemiology in China, 2015. Zhonghua Zhong Liu Za Zhi 41(1):19–28. https://doi.org/10.3760/cma.j.issn.0253-3766.2019.01.005
Acknowledgements
This work is jointly supported by the National Key Research Program of China (Grant Nos. 2016YFC0904700, 2016YFC0904702), The National Natural Science Foundation of China (Grant Nos. 11875299, U1532264), National Natural Science Foundation of China Academy of Engineering Physics (Grant Nos. U1730133), The Natural Science Foundation of Gansu Province (Grant Nos. 17JRSRA310).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, B., Chen, W., Li, H. et al. Radiosensitization of NSCLC cells to X-rays and carbon ions by the CHK1/CHK2 inhibitor AZD7762, Honokiol and Tunicamycin. Radiat Environ Biophys 59, 723–732 (2020). https://doi.org/10.1007/s00411-020-00867-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00411-020-00867-6