Abstract
The purpose of this investigation was to study the effect of acute γ-irradiation of parent adults on the endoreduplication of giant chromosomes in F1 generation of Drosophila melanogaster Meig. A wild-type Oregon-R strain was used as the material. Virgin females and males of Drosophila adults at the age of 3 days were irradiated with doses of 8, 16 and 25 Gy. Giant chromosomes were studied by cytomorphometry on squashed preparations of Drosophila salivary glands stained with acetoorsein. The preparations were obtained at late third instar larvae. The mean values of the polyteny degree of chromosomes (PDC) in males increased after 8 Gy by 10.6%, after 25 Gy by 7.4%, and did not change after the dose of 16 Gy. In females, the PDC did not differ from the control irrespective of the irradiation dose. An increase in endoreduplication was also evidenced by the accelerated development of offsprings of both sexes after irradiation of parents with 25 Gy, and in males also at a dose of 16 Gy. The statistical impact of power of radiation on polyteny was 26.8%, while the impact of sex was 4.9%. The impact of power of radiation on the developmental rate of offspring was 4.4% in males and 7.5% in females. The enhancement of endoreduplication is considered as a consequence of increasing selection pressure after irradiation. The possible involvement of epigenetic effects in the effect of ionizing radiation on endoreduplication is discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It is known that ionizing radiation can influence the genetic apparatus of cells, causing damage to DNA and mutations (Alexander and Bergendahl 1964; Dubrova 2006; Golub and Chernyk 2008; Vasil’eva et al. 2011; Skorobagatko et al. 2015a, b). This is due to direct action of radiation quanta on the DNA molecules, as well as the activity of free radicals that occur in the cells after irradiation as a result of radiolysis of water (Einor et al. 2016).
Ionizing radiation also affects the passage of cells through the cell cycle. A well-known effect is a sharp decrease in the mitotic index, the so-called “radiation-induced mitotic block” (Deckbar et al. 2011). In addition, the delay G1/S transition (G1 block) and the transition from G2 phase to M phase (G2 block) is possible. Sometimes there are opposite effects: an increase in the rate of cell passage through the cycle and an increase in cell proliferation. These effects indicate a violation of the mechanisms of cell cycle regulation as a result of the action of ionizing radiation (Can and Hicks 2006; Deckbar et al. 2011).
The endocycle is an alternative to the mitotic version of the cell cycle and is also referred to as the cell cycle of terminal differentiation (Larkins et al. 2001). The consequence of successive cycles of endoreduplication is the formation of polytene chromosomes in cell nuclei. The polyteny deserves attention as one of the effective mechanisms for enhancing gene expression in eukaryotes. In the literature, various aspects of the adaptive and evolutionary significance of this phenomenon are discussed (Edgar and Orr-weaver 2001; Lee et al. 2009; Nagl 1976; Øvrebø and Edgar 2018).
Endoreduplication is widespread. In various forms (endocycle, endomitosis), this phenomenon occurs both in invertebrate animals and mammals, as well as in plants (Bandura and Zielke 2017). Drosophila is a very important model organism in which significant progress has been made in studying the mechanisms of this specific cell cycle (Zielke et al. 2011; Edgar et al. 2014; Øvrebø and Edgar 2018).
One of the consequences of irradiation in the offspring of exposed parents is an increase in the level of embryonic and post-embryonic mortality. Differential mortality of organisms reflects the differential fitness of genotypes. In the case of radiation, radioresistant individuals survive, and radiosensitive individuals die. As a result, the genetic structure of the population changes. In connection with this, it is of interest to study the features of the functioning of the genome in the progeny of irradiated organisms. Important questions are: (1) whether the effects of ionizing radiation persist in the next generation, and (2) in what way do biological systems (organisms, populations) overcome the effects of radiation damage in subsequent generations after an exposure?
The purpose of the present investigation was to study effect of single-entry acute γ-irradiation of parent adults on the endoreduplication of giant chromosomes in F1 generation of Drosophila melanogaster Meig. The aims were to investigate the dependence of the effects on irradiation dose, sex, and to determine the impact of power of these factors on the degree of chromosome polyteny and developmental rate in the progeny of flies. To evaluate the possible selective effect of irradiation on the studied parameters, we examined embryonic mortality under different experimental conditions.
Materials and methods
Biological material and environmental conditions
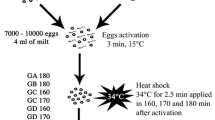
Wild-type strain Oregon-R of Drosophila melanogaster Meig. from the collection of the Department of Genetics and Cytology of VN Karazin Kharkiv National University was used in the experiments. Flies were grown on a standard sugar-yeast nutrient medium at a temperature of 24.0 ± 0.5 °C. Drosophila cultures developed in 60 ml vials with 10 ml of the culture medium. Virgin females and males of Drosophila adults at the age of 3 days were irradiated. Two hours after irradiation, they were placed in pairs in vials with a nutrient medium for mating. In our study of polyteny, females laid eggs for 5 days, being in pairs with the males. Larvae for the experiment were taken during the first 2 days of emergence. Ten larvae in each variant of the experiment were studied. On average, 148–213 nuclei per preparation were studied. In total, between 1484 and 2134 nuclei were analyzed in each experimental variant.
Exposure to γ-radiation
Doses of 8 Gy, 16 Gy and 25 Gy were used in the experiments. Flies were irradiated with a linear electron accelerator LEA-10 (NSC ‘Kharkiv Institute of Physics and Technology’, Kharkiv, Ukraine). Females and males were irradiated separately. The exposure time was 1–3 min, depending on the dose. Irradiation was carried out by bremsstrahlung γ-quanta, formed during the interaction of an electron beam with a thick aluminum target. The electron energy was 9.4 MeV, the current − 810 μA, the thickness of the aluminum converter was 38 mm. The dose rate at the irradiation point was calculated using Harwell Red 4034 detectors (Harwell, UK), and was 0.4 Gy/s. The brake spectrum, taking into account the geometry of the experiment, was calculated using GEANT 4 software package. The brake spectrum was the Bethe–Heitler curve, where 97% of the γ-ray energy was up to 3 MeV, including 70% of energy up to 500 keV.
According to Lindsley and Tokuyasu (1980), Drosophila spermatogenesis at 25 °C lasts about 250 h (more than 10 days) with the following chronology: 0–50 h—mitotic divisions; 50–120 h—spermatocyte growth; 120–140 h—meiotic divisions; 140–250 h—spermiogenesis. McKee et al. (2012) reported that spermatocyte growth occurs at the prophase of meiosis I and lasts 80–90 h. The development of each egg takes about 8 days: eggs reside for half of this time in the germarium for egg chamber formation (also called follicle), and the remaining 4 days are required for egg development, including meiosis divisions (Hudson and Cooley 2014). The oocyte undergoes both developmental maturation and meiosis throughout the course of oogenesis, and these processes are intimately linked (McLaughlin and Bratu 2015). Given this, it becomes apparent that paternal mature sperm cells and gametes at the stage of spermiogenesis were irradiated. As for the maternal germ cells, mature eggs, as well as eggs at the stage of meiosis and maturation, were exposed.
Determination of polyteny degree of chromosomes
The polytene chromosomes were studied on squashed preparations of Drosophila salivary glands, stained with acetoorcin: 2% orcein (Merck KGaA, Darmstadt, Germany) in 45% acetic acid solution (Reahimtrans, Kyiv, Ukraine). The preparations were obtained at the stage of the wandering larva in the late third instar.
Giant chromosomes were examined with a light microscope (MBI-6, “LOMO”, St. Petersburg, Russia). Differences in polyteny degree were determined by the cytomorphometric method (Strashnyuk et al. 1995). Control measurements of the width of chromosomes were carried out in the region of disk 22A of chromosome 2L at 600 × magnification. The ratio of the classes of nuclei with different polyteny degree was studied at 200 × magnification.
We investigated the distribution of nuclei with different levels of polyteny in the total preparations of the salivary glands. Based on these data, we calculated the average polyteny degree of chromosomes in normal conditions and after γ-irradiation exposure. Three independent experiments were carried out.
Determination of developmental rate
To assess the dynamics of endoreduplication in ontogenesis, we correlated the degree of polyteny in F1 offspring after irradiation with the rate of fly development. The rate of development was studied in synchronized cultures of Drosophila. Irradiated virgin females and males mated throughout the day. After mating, four-day-old females laid eggs for 3 h. In each vial, 20 females were placed. The number of adults released was counted every 3 h from the beginning to the end of their exit. Males and females were accounted for separately. Three independent experiments were carried out.
Analysis of embryonic mortality
To assess the selection pressure under different experimental conditions, an indicator of embryonic mortality was used. The analysis was carried out according to a standard method (Tikhomirova 1990). Irradiated 3-day-old virgin females and males were mated throughout the day. Then, egg clutches were prepared on Petri dishes filled with sugar-agar medium (100 g of distilled water, 3 g of agar–agar and 5 g of sugar) with a thin layer of yeast suspension on the surface. After 8 h, the number of eggs laid by ten females on each Petri dish was counted. Forty-eight hours later, the number of undeveloped eggs was counted. Undeveloped eggs were classified as manifestation of early (EEM) and late (LEM) embryonic mortality. Eggs with EEM are white and contain white opaque seals inside. Eggs with LEM are brown or yellow. There are also a small number of unfertilized eggs that are transparent. Embryonic mortality was defined as the proportion of undeveloped eggs of the total number of fertilized eggs. The frequency of early and late embryonic mortality was determined, as well as the total level of embryonic mortality: TEM = EEM + LEM. Three independent experiments were carried out.
Statistical methods
Statistical analysis of the experimental data was carried out. The data are presented as the mean ± standard error.
The verification of data distributions for compliance with the normal law was carried out using the Shapiro–Wilk test. The significance of the differences in the distribution of nuclei with different polyteny degrees of chromosomes was determined by the Chi square test. To distinguish differences in the average degree of polyteny, a two-factor analysis of variance was used with the assessment of statistical impact power of the radiation exposure and the sex. Multiple comparisons were made using the Tukey–Kramer test and Dunnett’s test.
For the analysis of the point parameters of the development rate, the criterion χ2 was used. As point estimates, we used the median time of development. To compare the distributions in different variants, the Kruskal–Wallace test was used, followed by multiple comparisons with the control, using the Dunn test.
Differences in the level of embryonic mortality were analyzed using the χ2 test.
Differences were considered significant at p < 0.05.
Results
In Drosophila, the first cycles of endoreduplication occured already in embryogenesis (Britton and Edgar 1998). By the end of larval development, which ends about 120 h after egg laying, in cell nuclei of the salivary glands are derived from 7 to 10 endoreduplication cycles. As a result, the levels of polyteny 256C, 512C, 1024C and 2048C are reached. Normally, most nuclei (about 70%) have a ploidy of 1024C that corresponds to 9 endocycles (Rodman 1967). ‘C’ indicates total ‘chromatin’ value or DNA content, as a multiple of the haploid genome (Øvrebø and Edgar 2018). According to Rodman (1967), the initiation of new cycles of endoreduplication in polytene chromosomes stops a few hours before larval-prepupal molt.
Each cycle of endoreduplication results in a twofold increase in the number of chromatids in polytene chromosomes. Therefore, nuclei with different levels of polyteny can be easily visually distinguished. In cytological preparations, chromosomes with different polyteny degrees differ in width and intensity of staining (Kiknadze and Gruzdev 1970; Strashnyuk et al. 1995). The thickness of chromosomes of different classes of nuclei in the region of the 22A disk used for control measurements was 1.6, 2.3, 3.2, and 4.6 μm. Chromosomes with greater polyteny were more intensely stained with acetoorsein.
The polyteny degree of chromosomes (PDC) varies in different parts of the salivary gland: in the distal part it is higher than in the proximal (Fig. 1). The correspondence between the cytomorphometric characteristics of polytene chromosomes and their degree of polyteny was demonstrated earlier (Strashnyuk et al. 1995; Dyka et al. 2016). The number of classes of nuclei with different widths of the chromosomes, their location in the gland and percentage showed close compliance with Rodman’s (1967) cytophotometry data.
Figure 2 presents data on the distribution of nuclei with different polyteny degrees of chromosomes in the salivary glands of Drosophila larvae in the F1 generation after γ-irradiation. In males, irradiation at a dose of 8 Gy caused a decrease in the fraction of 256C and 512C nuclei and an increase in the percentage of 1024C and 2048C nuclei. A similar effect occurred at a dose of 25 Gy, with the exception of the fraction of 256C nuclei that did not differ from the control values. At a dose of 16 Gy, on the contrary, an increase in the percentage of nuclei 256C and 516C was observed, while the percentage of 1024C nuclei was lower than in the control. However, the proportion of nuclei with maximum polyteny 2048C increased.
In females, the changes were less significant. At the dose of 8 Gy the portion of 2048C nuclei increased slightly. At the dose of 16 Gy, the content of 512C nuclei was higher, and the number of 1024C nuclei decreased. At the dose of 25 Gy, the distribution of nuclei with different degrees of polyteny did not differ from the control values.
Data on the percentage of nuclei with different genome ploidy were used for calculation of averages of polyteny degree in the salivary glands of Drosophila larvae in the control and in experimental variants. The results are shown in Fig. 3. In males, the mean values of polyteny in F1 generation after γ-irradiation were higher than the control values at the dose of 8 Gy by 10.6%, and at the dose of 25 Gy—by 7.4%. At the dose of 16 Gy, the average PDC in males did not show significant changes. This means that changes in the distribution of nuclei with different polyteny degrees at this dose were compensatory in nature.
The average values of polyteny degree of chromosomes (PDC) in Drosophila melanogaster salivary glands in F1 generation after γ-irradiation: C—total ‘chromatin’ value, as a multiple of the haploid genome. *p < 0.05; **p < 0.01: versus to control group. Error bars represent standard error from three repeats
In females, the mean values of polyteny degree of chromosomes after γ-irradiation of parents did not differ from the control, irrespective of the irradiation dose.
The obtained data indicate that the degree of genome amplification in the salivary glands of Drosophila after γ-irradiation of the parental individuals depends on two factors: sex and radiation dose. To estimate the statistical impact of power of the studied factors on endoreduplication, the variance analysis of two-factor complexes was used. The impact of power of a factor is defined as the fraction of factorial variability in the overall variability of the trait. The results of the analysis are presented in Table 1. According to the data obtained, the impact of power (ή2) of the sex on the polyteny degree of chromosomes was 4.9%, while the radiation impact was 26.8%. The combined effect of the two factors was not significant.
To assess the effect of radiation on endoreduplication, it is important to study not only changes in the degree of polyteny, but also the dynamics of fly development. The data presented in Fig. 4 show that the rate of development did not change in both males and females after irradiation with dose of 8 Gy. In males, development significantly accelerated at 25 Gy: the median decreased by 5.3 h (p < 0.001). Some acceleration of development, although less significant, was also observed after a dose of 16 Gy in males. The median decreased by 1.0 h (p < 0.05). The final polyteny in this case did not differ from the control values. However, this result was achieved in a shorter time. Consequently, endoreduplication also occurred more actively. Thus, the increase in the degree of polyteny in males after a dose of 8 Gy and even more after 25 Gy is a consequence of an increase in the level of endoreduplication and is not associated with an elongation of the developmental period. In females, development was also significantly accelerated after irradiation at a dose of 25 Gy. The median decreased by 4.1 h (p < 0.001). At lower doses, radiation did not affect the rate of development of females.
Table 2 shows the impact of power of irradiation on the development rate of offspring. The exposure had a statistically significant effect (p < 0.001): in males it was 4.4%, in females − 7.5%.
Thus, data on the rate of development of flies in combination with data on the chromosome polyteny degree indicate an increase in endoreduplication in the offspring of irradiated parents.
To assess the possible selective effect of radiation, we examined embryonic mortality under different experimental conditions. The results are presented in Fig. 5. The total level of embryonic mortality in the offspring of irradiated parents was 2.1–4.3 times higher than the control values (p < 0.001). A clear dose–response relationship is shown.
The obtained data quite clearly differentiate the effect of the applied doses in relation to the survival/mortality of individuals. Obviously, the survival of individuals under irradiation conditions depends on their radioresistance: the resistant individuals survive, the sensitive individuals die. It can be concluded from this that the enhancement of endoreduplication in the offspring of irradiated Drosophila parents is due to selection for radioresistance. However, we cannot exclude from discussion the effect of other mechanisms, for example, of an epigenetic nature.
Discussion
Genome amplification by endoreduplication is a characteristic phenomenon for cells of many differentiating tissues of eukaryotes. Endoreduplication is an effective mechanism for enhancing gene expression and increasing the metabolic potential of cells. Endocycles also promote accelerated growth (Zhimulev and Koryakov 2009; Marguerat and Bähler 2012), response to physiological stress (Zhuravleva et al. 2004; Fox and Duronio 2013) and adaptation to environmental conditions (Strashnyuk et al. 1997; Zhuravleva et al. 2004). According to experts (Sugimoto-Shirasu and Roberts 2003; Zielke et al. 2011), about half the world’s biomass is produced with the participation of endoreduplication.
At the cellular level, the endocycles are controlled by key regulators of the cell cycle, such as cyclins, cyclin-dependent kinases and their inhibitors. In Drosophila, switching from the mitotic cycle to endocycling is associated with the loss of mitosis-activating cyclins A and B and the subsequent periodic expression of cyclin E, activating the S-phase (Fox and Duronio 2013; Shakina and Strashnyuk 2011; Zielke et al. 2011). Endocycles are initiated as part of a developmental program, which involve signaling and epigenetic reprogramming. Cell growth in Drosophila is regulated by multiple pathways. In Drosophila larval salivary glands, endocycle rates appear to be controlled, downstream of the TOR pathway, by the expression of the single Drosophila activator E2F: E2F1 (Zielke et al. 2011; Øvrebø and Edgar 2018).
Humoral factors also play an important role. Data on the dynamics of polytenization in ontogenesis (Rodman 1967) and an experimental study of the hormonal effects (Sihna and Lakhotia 1983) indicate that the key role in the implementation of the genetic program responsible for genome amplification is played by juvenile hormone. Regarding the role of ecdysterone in these processes, the available data are highly contradictory (Shakina and Strashnyuk 2011).
Hereditary factors make a significant contribution to the variability of chromosome polyteny (Strashnyuk et al. 1995; Larkins et al., 2001). In addition, the modifying effect on the ploidy of cells is exerted by external conditions, such as temperature (Strashnyuk et al. 1997), culture density (Rarog et al. 1999; Zhuravleva et al. 2004), and food composition (Britton and Edgar 1998).
As for the present study, we believe that the observed increase in endoreduplication in the progeny of irradiated parents was due to the selection factor. This is evidenced by the presented data on a dose-dependent increase in the level of embryonic mortality in the Oregon-R strain after irradiation. An additional factor is probably gametic selection, which can also contribute to the variability of the trait after ionizing radiation (Hourcade et al. 2010).
Earlier we showed changes in the offspring fitness after irradiation of the parents in Drosophila. The lifespan of adults in F1 increased or did not change (Skorobagatko et al. 2016). Under conditions analogous to our experiment, at a dose of 25 Gy, the average lifespan increased in males, in females it did not change. Izmaylov et al. (1993) also observed increased longevity of flies in the first generation after irradiation of parents.
The frequency of dominant lethal mutations increased in F1 progeny after irradiation, but returned to the control values or (at 25 Gy) decreased in the progeny of F2 (Skorobagatko et al. 2015b). This indicates the appearance of genetic changes in the strain, at least at a dose of 25 Gy. Thus, selection did occur, and the offspring after that became more viable.
We can also assume the effect of hormesis, that is, the action of epigenetic mechanisms. Epigenetic mechanisms begin to act already when the egg is formed, when gradients of concentrations of biologically active substances are formed (Korochkin 2006). We applied the exposure to radiation at this stage. However, the hormesis effect requires justification (Mushak 2007). In our case, this is difficult to do, since selection takes place. If we are talking about epigenetic phenomena, then we must take into account that they do not concern the changes in the genotype. At the same time, the epigenetic mechanisms of the action of radiation are discussed in the literature (Vaiserman et al. 2004; Sarup and Loeschcke 2011). Perhaps the different mechanisms operate at different doses, or epigenetic mechanisms function along with selection.
The differences between males and females in response to the action of radiation may be explained by different viability of the sexes. It is known that the homogametic sex in this respect is superior to the heterogametic sex. This follows from the well-known Haldane rule (Haldane 1922), as well as the hypothesis of sex-linked lethal and semi-lethal genes (Huxley 1924).
Geodakian (1998) considers the phenomenon of sexual differentiation from the standpoint of their specialization at the population–species level. According to his view, evolutionary innovations in the male genome occur before they are transferred to the female genome. This can be explained from the positions of dichronic evolution, when the evolutionary changes in the males are faster than in the females.
In our study, the changes in polyteny are detected only in males: in females they are absent. The polyteny in males increased. This suggests that selection for an increase in radioresistance implies an increase in the metabolic potential of cells.
In our opinion, an accelerated development with an unchanged degree of polyteny should also be considered as an increase in endoreduplication: the same result was achieved in a shorter time. We mentioned above that endoreduplication promotes accelerated growth (Zhimulev and Koryakov 2009; Marguerat and Bähler 2012). The growth of larval tissues in Drosophila occurs due to endocycles (Britton and Edgar 1998). In terms of causality, acceleration of endocycles is the reason for the increase in the rate of development. A dose-dependent acceleration of development was observed both in males (at 16 Gy and 25 Gy) and in females (at 25 Gy).
The situation with the polyteny is somewhat more complicated. Effects were found only in males when irradiated at doses of 8 and 25 Gy. At 16 Gy, the average level of polyteny did not change, however, a statistically significant acceleration of development was observed, which was not the case with irradiation dose of 8 Gy. Thus, increased endoreduplication at 8 Gy and 16 Gy occurred in different forms. At dose of 25 Gy, both an increase in polyteny and accelerated development were observed, which was not observed at lower doses. This can be seen as a manifestation of the dose response. Thus, the dose–response relationship becomes visible if we analyze polyteny along with the rate of development.
Several arguments indicate an increase in endoreduplication in the offspring of irradiated flies:
- (1)
Analysis of variance with a high level of significance showed the effect of radiation on polyteny (Table 1).
- (2)
Statistical analysis also showed the effect of radiation on the rate of development (Table 2).
- (3)
In males, stimulation of endoreduplication in various forms (increased polyteny degree or process rate) is shown for all doses studied. In females, accelerated development was observed with unchanged average polyteny at a maximum dose of 25 Gy.
- (4)
In none of the experimental variants, inhibition of endoreduplication in the offspring of irradiated flies was found.
Two circumstances must also be taken into account: (1) as already mentioned, we do not consider the enhancement of endoreduplication as a result of the direct action of radiation. In our opinion, this is a consequence of increased selection pressure after exposure. (2) In contrast to embryonic mortality, effects at the level of polyteny are distant: the development time from an egg to the end of the larval stage takes 5 days. During this time, many compensatory reactions at the cellular level could occur: repair of DNA damage, detoxification of free radicals, apoptosis and other protective mechanisms (Wichmann et al. 2010; Moskalev et al. 2011). These two circumstances can significantly modify the dose–response relationship.
As a possible function of polyteny, some authors suggest the modulation of stress response (Cookson et al. 2006) or buffering of the genome (Edgar and Orr-weaver 2001). In our previous work, we found that the differences in polyteny degree of chromosomes in Drosophila positively correlated with heat resistance, body weight of adults, and general fitness (Strashnyuk et al. 1995, 1997; Zhuravleva et al. 2004). According to Hassel et al. (2014), cells in which the endocycle occurs are less likely to respond to DNA damage, for example, in the case of radiation-induced instability of the genome. Endocycles also contribute to the repair of damaged tissues, which is an alternative or complement to the function of stem cells (Losick et al. 2013; Xiang et al. 2017; Øvrebø and Edgar 2018). The above facts can be useful for understanding the possible connection between an increase in selection pressure and the degree of genome amplification in Drosophila polytene chromosomes after exposure to γ-irradiation.
In response to exposure to ionizing radiation, the E2F1 transcription factor is overexpressed (Wichmann et al. 2010). E2F1 is the central component of the endocyclic molecular oscillator which regulates the periodic expression of Cyclin E. In turn, Cyclin E catalyzes kinase CDK2 in the G–S transition (Zielke et al. 2011; Edgar et al. 2014; Hua and Orr-Weaver 2017).
According to the literature, the E2F family of proteins plays a dual role. The transcription factor E2F1 induces both cell cycle progression and, in certain settings, apoptosis. In proliferating Drosophila cells, E2F1 is necessary for the transcriptional induction of pro-apoptotic hid gene after ionizing irradiation (Wichmann et al. 2010). Overexpression of E2F1 can also transcriptionally induce pro-apoptotic genes in mammalian cells (Irwin et al. 2000; Nahle et al. 2002).
A special feature of cells undergoing endocycles is their ability to prevent apoptosis and tolerate genotoxic stress (Mehrotra et al. 2008; Ullah et al. 2009). In proliferating Drosophila cells, DNA damage or incomplete DNA replication results the arrest of CDK-dependent cell cycle events and then apoptosis. DNA damage is also induced in endocycling cells, but not apoptosis. In Drosophila, this is apparently due to the absence of a checkpoint that insures completion of S-phase (Lilly and Spradling 1996). Downregulation of several pro-apoptotic genes in these cells is also discussed (Ullah et al. 2009). Suppression of apoptosis is also characteristic of mammalian endocyclic cells exposed to radiation or other DNA-damaging agents (Ullah et al. 2008). Given this, there remains only one role for the transcription factor E2F1 in endocyclic cells—the induction of cell cycle progression.
The data presented indicate the possible involvement of epigenetic component in the mechanism of action of radiation on the endoreduplication. Other authors (Can and Hicks 2006; Deckbar et al. 2011; Moskalev et al. 2011) also point to the cell cycle control as one of the adaptive responses to radiation exposure.
References
Alexander M, Bergendahl J (1964) Dose rate effects in the developing germ cells of male Drosophila. Genetics 49:1–16
Bandura JL, Zielke N (2017) Polyploidy in animal development and desease. In: Li XQ (ed) Somatic genome variation: in animals, plants, and microorganisms, 1st edn. Wiley-Blackwell, New York, pp 3–44
Britton JS, Edgar BA (1998) Environmental control of the cell cycle in Drosophila: nutrition activated mitotic and endoreduplicative cells by distinct mechanisms. Development 125:2149–2158
Can KL, Hicks GG (2006) Adsence of an immediate G1/S checkpoint in primary MEFs following gamma-radiation identified a novel checkpoint switch. Cell Cycle 5:1823–1830
Cookson SJ, Radziejwoski A, Granier C (2006) Cell and leaf size plasticity in Arabidopsis: what is the role of endoreduplication. Plant Cell Environ 29:1273–1283
Deckbar D, Jeggo PA, Löbrich M (2011) Understanding the limitation of radiation-induced cell cycle checkpoints. Crit Rev Biochem Mol Biol 46:271–283
Dubrova YuE (2006) Genomic instability in the offspring of irradiated parents: facts and interpretations. Russ J Genet 42:1116–1126
Dyka LD, Shakina LA, Strashnyuk VYu, Shckorbatov YuG (2016) Effects of 36,6 GHz and static magnetic field on degree of endoreduplication in Drosophila melanogaster polytene chromosomes. Int J Radiat Biol 92:222–227
Edgar BA, Orr-Weaver TI (2001) Endoreduplication cell cycle: more or less. Cell 105:297–306
Edgar BA, Zielke N, Gutierrez C (2014) Endocycles: a recurrent evolutionary innovation for post-mitotic cell growth. Nat Rev Mol Cell Biol 15:197–210
Einor D, Bonisoli-Alquati A, Costantini D, Mousseau TA, Møller AP (2016) Ionizing radiation, antioxidant response and oxidative damage: a meta-analysis. Sci Total Environ 548–549:463–471
Fox DT, Duronio RJ (2013) Endoreplication and polyploidy: insights into development and disease. Development 140:3–12
Geodakian VA (1998) Evolutionary role of sex chromosomes (a new concept). Russ J Genet 34:1171–1184 (In Russian)
Golub NI, Chernyk II (2008) Mutations induced by X-ray irradiation and certain chemical reagents that alter the life span of Drosophila melanogaster. Cytol Genet 42:30–36
Haldane JBS (1922) Sex ratio and unisexual sterility in hybrid animals. J Genet 12:101–110
Hassel C, Zhang B, Dixon M, Calvi BR (2014) Induction of endocycles represses apoptosis independently of differentiation and predisposes cells to genome instability. Development 141:112–123
Hourcade JD, Pérez-Crespo M, Fernández-González R, Pintado B, Gutiérrez-Adán A (2010) Selection against spermatozoa with fragmented DNA after postovulatory mating depends on the type of damage. Reprod Biol Endocrinol 8:9. https://doi.org/10.1186/1477-7827-8-9
Hua BL, Orr-Weaver TL (2017) DNA replication control during Drosophila development: insights into the onset of S phase, replication initiation, and fork progression. Genetics 207:29–47
Hudson AM, Cooley L (2014) Methods for studying oogenesis. Methods 68:207–217
Huxley JS (1924) Sex determination and related problems. Med Sci Abstr Rev 10:91–124
Irwin M, Marin MC, Phillips AC, Seelan RS, Smith DI, Liu W, Flores ER, Tsai KY, Jacks T, Vousden KH, Kaelin WG Jr (2000) Role for the p53 homologue p73 in E2F-1-induced apoptosis. Nature 407:645–648
Izmaylov DM, Obukhova LK, Okladnova OV, Akifyev AP (1993) Phenomenon of life span instability in Drosophila melanogaster: II. Change in rhythm of natural variations of life span after single exposure to γ-irradiation. Exp Gerontol 28:181–194
Kiknadze II, Gruzdev AD (1970) Change in chromosome length related to polyteny in the chironomid salivary gland. Genetica 12:953–960 (In Russian)
Korochkin LI (2006) What is epigenetics. Russ J Genet 42:1156–1164 (In Russian)
Larkins BA, Dilkes BP, Dante RA, Coelho CM, Woo Y, Liu Y (2001) Investigating hows and why of DNA endoreduplication. J Exp Bot 52:183–194
Lee HO, Davidson JM, Duronio RJ (2009) Endoreduplication: polyploidy with a purpose. Genes Dev 23:2461–2477
Lilly MA, Spradling AC (1996) The Drosophila endocycle is controlled by Cyclin E and lacks a checkpoint ensuring S-phase completion. Genes Dev 10:2514–2526
Lindsley DL, Tokuyasu KT (1980) Spermatogenesis. In: Ashburner M, Wright TRF (eds) The genetics and biology of Drosophila. Acad Press, London, pp 225–294
Losick VP, Fox DT, Spradling AC (2013) Polyploidization and cell fusion contribute to wound healing in the adult Drosophila epithelium. Curr Biol 23:2224–2232
Marguerat S, Bähler J (2012) Coordinating genome expression with cell size. Trends Genet 28:560–565
McKee BD, Tsai JH, Yan R (2012) Meiosis in male Drosophila. Spermatogenesis 2(3):167–184
McLaughlin JM, Bratu DP (2015) Drosophila melanogaster oogenesis: an overview. In: Walker JM (ed) Methods in molecular biology. Humana Press, Clifton, pp 1–20
Mehrotra S, Maqbool SB, Kolpakas A, Murnen K, Calvi BR (2008) Endocycling cells do not apoptose in response to DNA rereplication genotoxic stress. Genes Dev 22:3158–3171
Moskalev AA, Plyusnina EN, Shaposhnikov MV (2011) Radiation hormesis and radioadaptive response in Drosophila melanogaster flies with different genetic backgrounds: the role of cellular stress-resistance mechanisms. Biogerontology 12:253–263
Mushak P (2007) Hormesis and its place in nonmonotonic dose–response relationships: some scientific reality checks. Environ Health Perspect 115:201–211
Nagl W (1976) DNA endoreduplication and polyteny understood as evolutionary strategies. Nature 261:614–615
Nahle Z, Polakoff J, Davuluri RV, McCurrach ME, Jacobson MD, Narita M, Zhang MQ, Lazebnik Y, Bar-Sagi D, Lowe SW (2002) Direct coupling of the cell cycle and cell death machinery by E2F. Nat Cell Biol 4:859–864
Øvrebø JI, Edgar BA (2018) Polyploidy in tissue homeostasis and regeneration. Development. https://doi.org/10.1242/dev.156034
Rarog MA, Strashnyuk VYu, Kondrat’eva AO, Dmitruk TV, Vorob’eva LI, Shakhbazov VG (1999) Effect of culture density on expressivity of character eyeless and polyteny of giant chromosomes in Drosophila melanogaster. Russ J Genet 35:766–769
Rodman TC (1967) DNA replication in salivary gland nuclei of Drosophila melanogaster at successive larval and prepupal stages. Genetics 55:375–386
Sarup P, Loeschcke V (2011) Life extension and the position of the hormetic zone depends on sex and genetic background in Drosophila melanogaster. Biogerontology 12:109–117
Shakina LA, Strashnyuk VYu (2011) Genetic, molecular, and humoral endocycle-regulating mechanisms. Russ J Genet 47:1151–1160
Sihna P, Lakhotia SC (1983) Replication in Drosophila chromosomes XI. Stimulation of initiation of polytene replication cycles in vitro by juvenile hormone. Cell Differ 12:11–17
Skorobagatko DA, Shakina LA, Strashnyuk VYu, Mazilov AA (2015a) Lethal and recombinative action of γ-radiation in genetically unstable Drosophila melanogaster Bar strain. Radiatsionnaya Biologiya Radioekologiya 55:145–154
Skorobagatko DA, Strashnyuk VYu, Mazilov AA (2015b) Fitness components in the offsprings of Drosophila melanogaster Meig. after acute γ-irradiation. Factors Exp Evol Org 16:78–82. http://utgis.org.ua/faktory(In Russian)
Skorobagatko DA, Strashnyuk VY, Mazilov AA (2016) Lifespan in the progeny of Drosophila melanogaster after acute γ-irradiation. J VN Karazin Kharkiv Natl Univ Ser Biol 26:74–84. http://periodicals.karazin.ua/biology(In Russian)
Strashnyuk VYu, Nepeivoda SN, Shakhbazov VG (1995) Cytomorphometric analysis of Drosophila melanogaster Meig. polytene chromosomes in relation to heterosis, selection for adaptively valuable traits, and sex. Russ J Genet 31:17–21
Strashnyuk VYu, Al-Hamed S, Nepeivoda SN, Shakhbazov VG (1997) Cetogenetic and cytobiophysical investigation of mechanisms of temperature adaptation and heterosis in Drosophila melanogaster Meig. Russ J Genet 33:793–799
Sugimoto-Shirasu K, Roberts K (2003) “Big it up”: endoreduplication and cell-size control in plants. Curr Opin Plant Biol 6:544–553
Tikhomirova MM (1990) Genetic analysis (In Russian). LSU Publition, Leningrad
Ullah Z, Kohn MJ, Yagi R, Vassilev LT, DePamphilis ML (2008) Differentiation of trophoblast stem cells into giant cells is triggered by p57/Kip2 inhibition of CDK1 activity. Genes Dev 22:3024–3036
Ullah Z, Lee CY, Lilly MA, DePamphilis ML (2009) Developmentally programmed endoreduplication in animals. Cell Cycle 8(10):1501–1509
Vaiserman AM, Koshel NM, Mechova LV, Voitenko VP (2004) Cross-life stage and crossgenerational effects of gamma irradiations at the egg stage on Drosophila melanogaster life histories. Biogerontology 5:327–337
Vasil’eva LA, Antonenko OV, Zakharov IK (2011) Role of transposable elements in the genome of Drosophila melanogaster. Russ J Genet 47:463–488 (In Russian)
Wichmann A, Uyetake L, Tin Tin Su (2010) E2F1 and E2F2 have opposite effects on radiation-induced p53-independent apoptosis in Drosophila. Dev Biol 346:80–89
Xiang J, Bandura J, Zhang P, Jin Y, Reuter H, Edgar BA (2017) EGFR-dependent TOR-independent endocycles support Drosophila gut epithelial regeneration. Nat Commun 8:1–13. https://doi.org/10.1038/ncomms15125
Zhimulev IF, Koryakov DE (2009) Polytene chromosomes. In: Encyclopedia of life sciences (ELS). JohnWiley & Sons, Ltd: Chichester. https://doi.org/10.1002/9780470015902.a0001183.pub2
Zhuravleva LA, Strashnyuk VYu, Shakhbazov VG (2004) Influence of culture density on the polyteny degree of giant chromosomes in inbred lines and hybrids of Drosophila melanogaster. Cytol Genet 38:46–51 (In Russian)
Zielke N, Kim KJ, Tran V, Shibutani ST, Bravo MJ, Nagarajan S, van Straaten M, Woods B, von Dassow G, Rottig C, Lehner CF, Grewal SS, Duronio RJ, Edgar BA (2011) Control of Drosophila endocycles by E2F and CRL4CDT2. Nature 480:123–127
Acknowledgements
This work was supported by the Ministry of Education and Science of Ukraine (Project State registration number: 0117U004836).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Skorobagatko, D.A., Mazilov, A.A. & Strashnyuk, V.Y. Endoreduplication in Drosophila melanogaster progeny after exposure to acute γ-irradiation. Radiat Environ Biophys 59, 211–220 (2020). https://doi.org/10.1007/s00411-019-00828-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00411-019-00828-8