Abstract
The relative biological effectiveness (RBE) based on the induction of dicentrics in any cell type is principally an important information for the increasing application of high-LET radiation in cancer therapy. Since the standard system of human lymphocytes for measuring dicentrics are not compatible with our microbeam irradiation setup where attaching cells are essential, we used human–hamster hybrid AL cells which do attach on foils and fulfil the special experimental requirement for microbeam irradiations. In this work, the dose–response of AL cells to photons of different energy, 70 and 200 kV X-rays and 60Co γ-rays, is characterized and compared to human lymphocytes. The total number of induced dicentrics in AL cells is approximately one order of magnitude smaller. Despite the smaller α and β parameters of the measured linear–quadratic dose–response relationship, the α/β-ratio versus photon energy dependence is identical within the accuracy of measurement for AL cells and human lymphocytes. Thus, the influence of the reference radiation used for RBE determination is the same. For therapy relevant doses of 2 Gy (60Co equivalent), the difference in RBE is around 20% only. These findings indicate that the biological effectiveness in AL cells can give important information for human cells, especially for studies where attaching cells are essential.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The use of ion beams for radiation therapy is currently undergoing investigation at different institutions in Germany (IAEA 2008). A few years ago, projects were started at our ion microbeam laboratory to investigate and understand the relative biological effectiveness (RBE) of special irradiation situations which may gain clinical relevance in future. In the context of possible use of laser-accelerated proton or particle beams for tumour treatment (Dollinger et al. 2009; Martin 2009) we showed that the RBE of nanosecond proton pulses does not change compared to dose deposition within milliseconds, analysing both the induction of micronuclei (MN) in HeLa cells (Schmid et al. 2009) and chromosome aberrations in human–hamster hybrid (AL) cells (Schmid et al. 2011). In addition, the RBE dependence on the spatial dose distribution, especially on submicron dose clustering, was investigated with the aim to elucidate the effects underlying heavy ion RBE (Schmid et al. 2012). In fact, it could be demonstrated that low--LET radiation focused to submicrometer diameters but depositing the same average dose as quasi homogeneous low-LET radiation results in an increase in RBE for the induction of MN and dicentrics in AL cells similar to the increase observed with high-LET radiations.
The best characterized cell line for such RBE studies would certainly be human lymphocytes, because the ‘International Commission on Radiological Protection’ (ICRP 2003) and the ‘Committee to Assess Health Risks from Exposure to low Levels of Ionizing Radiation’ (BEIR VII Report 2006) stated that the most reliable and detailed data on photon RBE exist for chromosome aberrations in human lymphocytes. Predominately, their statement was based on analogous dicentric data sets over a broad range of photon energies reported either by Sasaki (1991) and coworkers (Sasaki et al. 1989) or by our cytogenetic group (Schmid et al. 2002b). However, human lymphocytes are not compatible with our microbeam irradiation setup as, in general, they do not attach on foils. Thus, we used AL cells which do attach on foils and fulfil the special experimental requirement for microbeam irradiations. Two decades ago, this cell line was established in our laboratory to analyse either spindle disturbances after exposure to non-ionizing radiation (Schrader et al. 2008; Hintzsche et al. 2011) or structural chromosome aberrations after exposure to ionizing radiation (Schmid et al. 2011, 2012, 2015).
The aim of the present work is to characterize the AL cells with respect to the RBE of photons with various energies to be able to determine the influence of reference radiation to measure high-LET RBE and to compare photon response of AL cells to human lymphocytes. Using 70 and 200 kV X-rays as well as 60Co γ-rays as reference radiations, the RBE of high-LET radiation qualities, e.g. heavy ions, should be estimated in the present investigation. The factor of the RBE of a certain radiation quality, T, which changes by switching from reference radiation A to B is given by the RBE of radiation B with respect to A (compare “Appendix”):
Here RBEref Y(X) stands for the RBE of radiation quality X with respect to reference radiation Y. Thus, discussing the influence of reference radiation on RBE is equivalent to discuss their RBE.
Materials and methods
Cell line
The present investigations were carried out in monolayers of AL cells, which were irradiated when their chromosomes were under unduplicated conditions (pre-DNA synthesis, G1-phase of the cell cycle). This cell line was derived from the CHO-K1 wild-type cell line, a subclone of the parental CHO cell line, which was derived from the ovary of an adult Chinese hamster. The cell line has a modal number of 21 chromosomes (85% of analysed cells), whereas 15% of the cells has a loss or gain of chromosomes (Schmid et al. 2011). Chromosome analysis was only focused on cells containing 21 chromosomes.
Cell culture
For each experiment of exposure of cells to 70 kV X-rays, 200 kV X-rays or 60Co γ-rays, a frozen aliquot from the same stock culture of AL cells was thawed and seeded as cultures with exponentially growing unsynchronized cells into irradiation containers. A detailed description of the construction of these containers for cell irradiation was given previously (Schmid et al. 2009). About 30,000 AL cells were seeded in RPMI-1640 medium, supplemented with 16% foetal calf serum, 100 U/ml penicillin and 50 µg/ml streptomycin to obtain sub-confluent cultures. The AL cell monolayers were maintained in a humidified atmosphere containing 5% CO2 and 95% air at 37 °C. Under these growth conditions, the cells had a doubling time of 18 h. The cells were allowed to recover from the freeze/thaw procedure for more than two passages.
Irradiation conditions
The exposure of AL cells to X-rays generated at a tube voltage of 200 kV (mean energy 88 keV) was performed at the new X-ray calibration facility (RS225, Gulmay Medical, Surrey, UK) of our Department of Radiation Oncology. AL cells were seeded as cultures with exponentially growing unsynchronized cells into special containers which were also applied for our irradiation experiments with 20 MeV protons (Schmid et al. 2011, 2012). Using a dose rate of 0.88 Gy min−1, AL cells were exposed to 200 kV X-rays (15 mA, 0.8 mm Be and 0.5 mm Cu filter) at a source–cell distance of 50 cm. Dosimetry was performed using standard dosimetry protocols as the IAEA-TRS 398 Code of Practice and, additionally, radiochrome film-based dosimetry. Gafchromic EBT2-film, a self-developing radiochromic film, was selected for dose verification after irradiation. Films were digitized with an EPSON Perfection V700 Photo scanner using the manufacturer’s scan software in professional mode with all image correction features turned off. Forty-eight-bit RCB tiff images with resolution of 1200 dpi were acquired from films oriented in landscape format at the earlier 40 h after irradiation to minimize uncertainty in optical density due to post-irradiation growth. The applied doses to AL cells ranged from 0.5 to 8 Gy.
For comparison, the results of our earlier exposure of AL cells to X-rays generated at a tube voltage of 70 kV (mean energy 39 keV) have been used in the present investigation. Cell exposure was performed in two independently performed experiments at the former X-ray calibration facility (Philips RT100, Philips Medical Systems, Eindhoven, The Netherlands) of the Department of Radiation Oncology. To avoid any saturation effect at high doses, the dose points for estimating the dose–response relationship were restricted to those below 4 Gy in this earlier study (Schmid et al. 2011). Presently, however, the complete results for exposure of AL cells with doses up to 8 Gy of 70 kV X-rays summarized from both experiments have been used for estimating the dose–response relationship.
The 60Co γ-ray irradiation of AL cells was performed in the Secondary Standard Dosimetry Laboratory of the Research Unit Medical Radiation Physics and Diagnostics (Helmholtz Centre Munich). Radiation doses were based on secondary standard ionization chamber dosimetry with the calibration traceable to the primary standards of the German national laboratory PTB (Physikalisch-Technische Bundesanstalt, Braunschweig). Monolayer of AL cells placed in cylindrical polypropylene chambers were tightly embedded at a depth of 1 cm in a polymethyl methacrylate (PMMA) phantom (7 cm × 11.5 cm × 2.3 cm). Applying a dose rate of 0.5 Gy min−1, the cells were also exposed to doses up to 8 Gy. The exposures of AL cells to all three reference radiation qualities were performed at a temperature of 20–22 °C (room temperature). This temperature had to be applied because experiments using ion beams at the ion microprobe SNAKE [Superconducting Nanoprobe for Applied nuclear (Kern) physics Experiments] of the 14 MV Munich tandem accelerator could only be carried out at this condition.
Post-irradiation culture and chromosome analysis
Immediately after irradiation, AL cells were trypsinized and reseeded in 4-ml RPMI-1640 medium supplemented with 20% foetal calf serum and antibiotics (penicillin/streptomycin) and incubated at 37 °C in a humidified atmosphere of 5% CO2 in air. Colcemid at a final concentration of 0.03 µg ml−1 was added to the cultures 6 h after incubation and incubated for a further 18 h. By the presence of colcemid during this incubation period, a synchronization of the cycling AL cells that were at the G1-phase of the cell cycle during irradiation could be achieved, whereas AL cells irradiated at the S- and G2-phases could not be arrested by colcemid at the metaphase because these cells had already finished their first cell cycle after irradiation. Subsequently, the cultures were centrifuged, the supernatant removed and replaced by a hypotonic potassium chloride solution (0.075 M) at 37 °C for 10 min. All subsequent steps of the chromosome preparation were carried out according to a standardized laboratory procedure originally described for human lymphocytes (IAEA 2001), which was also used in our former experiments for exposure of human lymphocytes to X-rays and γ-rays (Table 1). The cells were fixed, i.e. the culture medium was removed and the cell pellet resuspended in a mixture of three parts of methanol and one part of glacial acetic acid. Aliquots of fixative were added drop by drop on individual slides. After air drying, the cells were stained with 2% acetic Orcein for 10 min. Using this technique, the cytoplasm of the cells remained well preserved and chromosome loss due to preparation procedure was avoided. All object slides were coded. Only complete cell nuclei containing the modal number of 21 chromosomes were analysed for dicentrics.
Statistics
A null hypothesis that the observed differences between either coefficients of the dose–response curves determined in two independently performed experiments or RBE values are not different was tested according to the z test statistics. Since the conditions for the application of the normal probability distribution hold for these data (i.e. both sample sizes are much larger than 100 cells and the products of sample sizes and proportions are greater than five), this statistical procedure could be applied. A difference at the two-sided p value <0.05 was considered statistically significant.
Determination of RBE and α/β
The RBE is defined as the ratio of the necessary doses of the reference radiation and the test radiation to induce a certain effect RBE = D ref/D test. The dose of reference radiation is calculated by inverting the dose–response curve which is parameterized with the linear quadratic model: y(D) = c + αD + βD 2. The parameter α and β are determined by a weighted least square fit with the inverse variance of individual data points used as weighting factors. The constant c is fixed to the background level during the fit. The uncertainties for α and β follow from the fit by propagation of uncertainties of each data point. The uncertainties of derived values, RBE and α/β, are calculated by propagation of the uncertainties of the parameters α and β, the background level c, the effect y and the relative uncertainty of the dosimetry between reference and test radiation, which is assumed to be 5%. For propagation of uncertainties the propagation function is linearized around the point of interest, the mean values of the parameters. As the fit parameters α and β are highly anticorrelated, the covariance or correlation coefficient, respectively, of α and β is considered, too (compare, e.g. Ku 1966). Inclusion of correlation is crucial for proper estimation of uncertainty as neglecting correlation would lead to overestimation of RBE uncertainty and underestimation of α/β uncertainty.
Results
Tables 2, 3 and 4 present measured data for induction of dicentrics in AL cell induced by 200 kV X-rays, 70 kV X-rays and 60Co γ-rays at doses between 0 and 8 Gy. The intercellular distributions of dicentrics are given together with the results of the test for Poisson distribution. The relative variance, σ 2 divided by the mean effect y, approximates unity if the intercellular distribution of dicentrics follows a Poisson distribution. Values of the test quantity u in excess of 1.96 indicate overdispersion at the 5% level of significance (Schmid et al. 1995). This test quantity is standard normal distributed under the null-hypothesis. Therefore, only the distribution after 2 Gy of 70 kV X-rays shows a significant overdispersion. All other intercellular distributions of dicentrics are not significantly different from Poisson distribution.
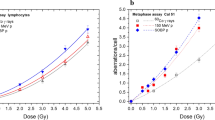
In Fig. 2, the yield for induction of dicentrics in AL cells is plotted for these three radiation qualities at doses between 0 and 8 Gy. The yield is fitted with a linear quadratic function, y(D) = c + αD + βD 2. The constant term, c, is fixed in the fit to the background level of the three experiments, c = 0.00067. A weighted least squares approximation with the reciprocal of the variances as weighting factors results in the α- and β-parameters, which are demonstrated in Table 5, together with the standard errors and the α/β-ratios. In addition, the correlations of both parameters, cor (α, β), are given. These are necessary for estimations of uncertainties of values derived from α and β. In Fig. 3, the linear quadratic parameters α and β as well as the α/β-ratio are plotted versus the mean photon energy of the used spectrum together with corresponding data for human lymphocytes. In Fig. 4, the RBE for 70 and 200 kV X-rays with 60Co γ-rays as reference is calculated from the fit parameters and plotted versus the effect, yield of dicentrics per metaphase, (bottom axis) and the necessary dose of 60Co γ-rays to induce the given yield of dicentrics (top axis). The error bars indicate the 95% confidence level.
Discussion
The use of ion beams for radiation therapy is currently undergoing investigation at different institutions. Before such a new non-conventional beam becomes available for therapy, it is practice to carry out a variety of laboratory experiments to determine RBE values and RBE–dose relationships for a variety of experimental systems and a variety of endpoints. Thus, for example, a “Meeting on RBE in Ion Beam Therapy” (IAEA 2008) dealt primarily with a review of experimental measuring of RBE and approaches to the clinical use of the concept of RBE based on experimental findings, theoretical models and previous clinical experience with fast neutrons and ions. For example, based on the induction of dicentrics in human lymphocytes, a comparable modification had initiated our former studies on analysing both the RBE values of the mixed fission-neutron and γ-ray beam of the RENT neutron therapy facility (Schmid et al. 1998) at the former FRM I research reactor and the MEDAPP therapy facility (Schmid et al. 2009a, b) at the new FRM II research reactor (both reactors in Garching, Germany). In the present study, however, we used the induction of dicentrics in AL cells for RBE evaluation because these cells do attach on foils and fulfil the special experimental requirement for microbeam irradiations.
In general, the linear–quadratic model (y = αD + βD 2) is applied to describe the dose–effect curves for dicentrics in human lymphocytes. For example, Sasaki et al. (1989) and Sasaki (1991) as well as Schmid et al. (1984, 1995, 2002b, 2008) reported on consistent results on the energy dependence. The α-coefficient is small for ultra-soft X-rays, increases with photon energies, reaches a maximum at around 10 keV and then decreases with increasing photon energy up to 60Co γ-rays. The β-coefficient, however, decreases with increasing photon energy and reaches a plateau level at moderate to high energy beyond about 100 keV. In Table 1, a selection of our coefficients α and β for induction of dicentrics in human lymphocytes is given for photon energies between 17.4 keV and 1.25 MeV (Schmid et al. 1984, 1995, 2002b, 2008). To elucidate the impact of this energy dependence of photon response on the effect of different reference radiations, we calculated the RBE of the radiation qualities in Table 1 with 60Co γ-rays as reference. In Fig. 1, this RBE is plotted versus induction of dicentrics (bottom axis) or the corresponding 60Co γ-ray dose (top axis). For small doses/effects, α dominates the dose–response, y = αD. In the limit of zero doses, the RBE is given by the ratio of the α-coefficients. The change of α with X-ray energy leads to large RBE of low-energy X-rays. For example, a RBE up to six was found for 29 kV X-rays (Schmid et al. 2002b). At high doses, the dose–response is dominated by the β coefficient, y = βD 2, and the RBE in the limit of high doses is given by the square root of the β ratio. This ratio is close to one due to the small dependence of β on photon energy.
RBE for induction of dicentrics in human lymphocytes for mean photon energies of 17.4, 96, 135 and 662 keV versus the effect (bottom axis) and the corresponding 60Co dose (top axis). As reference radiation, 60Co γ-rays are used. For the RBE calculation the dose–response curves are parameterized with the α and β values of Table 1
While such differences in RBE values at low doses have found particular attention with regard to radiation protection purposes, RBE values at higher doses around one gray, based on the induction of dicentrics in any cell type are important, when discussing RBE values with regard to tumour therapy. Especially, when using high-LET radiation qualities for cancer treatment, its RBE values base on a well-defined RBE of the applied reference radiation (Eq. 1). In the present investigation, we measured dose–effect curves for induction of dicentrics in AL cells applying doses up to 8 Gy. Notably, we observed no indication of a saturation effect. The linear quadratic model is suitable to describe the dose–effect curve within the accuracy of measurement (Fig. 2) and no significant deviation of the intercellular distribution of dicentrics from Poisson statistics could be detected for the high doses of 4 and 8 Gy. A significant deviation on a 5% level would result in a u value above +1.96 or below −1.96 in Tables 2, 3 and 4 (Schmid et al. 1995). The negligible saturation even at therapy relevant doses makes the AL cell line well suited for such studies.
Dose–effect curve for induction of dicentric chromosomes in AL cell after irradiation with 70, 200 kV X-rays and 60Co γ-rays together with the linear–quadratic fit of the data: y = c + αD + βD 2. The fit parameters are given in Table 5
Within a similar mean photon energy range, both coefficients α and β of the linear–quadratic dose–response curves for dicentrics in AL cells are lower by about one order of magnitude than the corresponding coefficients in human lymphocytes (Tables 1, 5; Fig. 3). Beside the cell line the irradiation conditions are different. Whereas human lymphocytes were irradiated at 37 °C, AL cell irradiation was performed at room temperature. However, it should be noted that Gumrich et al. (1986) reported no change in the induction of dicentric yields in human lymphocytes irradiated by 150 kV X-rays between 20 and 37 °C. In our cytogenetic laboratory, a 40% decrease of α together with an anticorrelated 40% increase of β was observed when changing irradiation temperature from 21 to 37 °C (Schmid et al. 2002a). Both works suggest that at least only a small fraction of the change in α and β values can be caused by different irradiation temperature. A further part of variation in RBE values may likely to be due to different exposure conditions of AL cells and human lymphocytes to ionizing radiation qualities. Whereas the linear–quadratic coefficients α and β were determined for induction of dicentrics in lymphocytes of irradiated whole blood by different photon energies (Table 1), the corresponding coefficients α and β obtained for induction of dicentrics in AL cells by the three photon energies have been analysed in cell monolayer (Tables 2, 3, 4). However, in special former experiments of our cytogenetic laboratory, separated lymphocytes from human peripheral blood were also exposed as attached monolayer on Mylar foils either to 137Cs γ-rays (Schmid et al. 1996) or to monochromatized synchrotron radiation (monoenergetic photon energy of 17.4 keV) at the electron storage ring BESSY II in Berlin, Germany (Krumrey et al. 2004). As demonstrated in Table 6, the estimated coefficients α and β obtained for the dose–response of dicentrics in human lymphocytes after irradiation of samples as monolayer or as whole blood (Schmid et al. 1995, 2003) are consistent. Another explanation might be the lower number of chromosomes of AL cells of only 23, which is half the number of chromosomes in human cells. Thus, the probability for joining double-strand breaks from different chromosomes can be reduced.
Apart from the different absolute numbers, the dependency of α and β values on the mean photon energy shows the same characteristic for human lymphocytes and AL cells. The β value shows nearly no dependence on photon energy whereas α decreases with increasing photon energy by about a factor of 6–7. For human lymphocytes, the α value drops from α = (0.0655 ± 0.0097) Gy−1 for 29 kV X-rays (17.4 keV mean photon energy) to α = (0.0107 ± 0.0041) Gy−1 for 1250 keV photons (60Co γ-rays), whereas for AL cells we found α = (0.0077 ± 0.0025) Gy−1 for 70 kV X-rays (39 keV mean photon energy) and α = (0.0011 ± 0.0020) Gy−1 for 60Co γ-rays (1250 keV mean photon energy). Astonishingly, the α/β-ratios for both cell types show exactly the same behaviour with changing photon energy (see Fig. 3c). In the double logarithmic plot of Fig. 3c, the data points follow a linear behaviour indicating a power function: α/β = m × E p. Here, E denotes the mean photon energy. The parameter m and p can be determined by a least square fit to: m = (8.1 ± 3.0) Gy (keV) and p = −0.542 ± 0.084. In particular, p is not significantly different to 0.5, thus the data are compatible with the hypothesis that α/β-ratio is proportional to the square root of the mean photon energy.
To elucidate the second aim of the present work, i.e. to investigate the effect of different reference radiations on RBE determination for induction of dicentrics in AL cells, the photon RBE was calculated and shown in Fig. 4. The RBE for 70 and 200 kV X-rays with 60Co γ-rays as reference are plotted against the induction of dicentrics (bottom axis) and the corresponding 60Co γ-ray dose (top axis). The error bars are calculated by propagation of uncertainties of α and β and its correlation for the X-rays as well as the 60Co γ-rays and represent the 95% confidence level. For large effects or high-dose photon, RBE approaches approximately unity. It exactly approaches the square root of the β value of the X-rays and 60Co γ-rays, which is 0.96 for 70 kV and 0.94 for 200 kV. For small effects or doses, the RBE approaches the ratio of the α values, 6.5 for 70 kV and 3.0 for 200 kV X-rays. Nevertheless, the differences at small doses are not significant. This is mostly caused by the much lower numbers of dicentrics induced in AL cells compared to human lymphocytes. Thus, especially at small doses a higher number of cells must be analysed, which makes AL cells a less suited model for radiation protection studies in the low-dose regime. Neither 70 kV nor 200 kV X-ray RBE is, at any dose, significantly different from unity on a 5% level. For therapy relevant doses of 2 Gy (60Co γ-ray equivalent dose) a RBE of 1.23 ± 0.13 is determined for 70 kV and 1.03 ± 0.11 for 200 kV X-rays. Therefore, by changing reference radiation from 200 to 70 kV X-rays, a measured high-LET RBE will change by about 20%.
RBE of 70 and 200 kV X-rays with respect to 60Co γ-rays as reference radiation plotted against the yield of dicentric chromosomes per metaphase (bottom, axis) and the equivalent 60Co dose (top axis). The error bars indicate the 95% confidence interval. A relative error in photon dosimetry of 5% is assumed. Error bars are tilted for clarifications to avoid overlap
The same behaviour of the α/β-ratio as well as the photon RBE indicates that AL cells are a useful model to study RBE and allows transferring the results to human cells. The influence of photon RBE on measured RBE values is small at therapy relevant doses in the range of a few gray. This is also demonstrated in Table 7, where data of two studies in AL cells at our microbeam laboratory are displayed together with the RBE with respect to the different reference radiations, 70 and 200 kV X-rays as well as 60Co γ-rays. In a previous study (Schmid et al. 2011), we showed that the effectiveness of nanosecond pulse proton application is comparable to 100 millisecond proton application at a dose of 3.6 Gy. In a further project it was demonstrated that submicron dose clustering, applied with a low-LET proton microbeam, increases the induction of dicentrics in AL cells at 1.7 Gy (Schmid et al. 2012). Understanding dose clustering may be a key to understand high-LET RBE quantitatively. In Table 7, it is demonstrated that at any single experiment the estimated RBE does not change significantly (z ≥ 0.86; p ≥ 0.20) when changing photon radiation, 70 kV X-rays, 200 kV X-rays and 60Co γ-rays, used as reference. This result holds true even at different exposure conditions or differences in the magnitude of estimated RBE of proton or carbon radiations.
Conclusion
In the present work, we characterized induction of dicentrics in AL cells after irradiation with photons of different energy. The total yield of dicentrics in AL cells is nearly one order of magnitude lower than in human lymphocytes, which forbids a direct comparison of α and β values. But the identical dependence of the α/β ratio on photon energy and a comparable photon RBE of AL cells and human lymphocytes shows that AL cells are a suitable model for RBE studies, especially for microbeam studies, where attaching cells are essential.
References
BEIR VII Report (2006) Health risks from exposure to low levels of ionizing radiation. National Academies Press, Washington, DC
Dollinger G, Bergmaier A, Hable V, Hertenberger R, Greubel C, Hauptner A, Reichart P (2009) Nanosecond proton microbeam. Nucl Instrum Methods Phys Res B 267:2008–2012
Gumrich K, Virsik-Peukert RP, Harder D (1986) Temperature and the formation of radiation-induced chromosome aberrations. I. The effect of radiation temperature. Int J Radiat Biol 49:665–672
Hintzsche H, Jastrow C, Kleine-Ostmann T, Stopper H, Schmid E, Schrader T (2011) Terahertz radiation induces spindle disturbances in human-hamster hybrid cells. Radiat Res 175:569–574
IAEA (2001) Cytogenetic analysis for radiation dose assessment. IAEA technical report series 405, Vienna
IAEA (2008) Relative biological effectiveness in ion beam therapy. IAEA technical report series 461, Vienna
ICRP (2003) Relative biological effectiveness (RBE), quality factor (Q), and radiation weighting factor (wR). Annals of the ICRP; Publication 92, Pergamon Press, Oxford
Krumrey M, Ulm G, Schmid E (2004) Dicentric chromosomes in monolayer of human lymphocytes produced by monochromatized synchrotron radiation with photon energies from 1.83 keV to 17.4 keV. Radiat Environ Biophys 43:1–6
Ku H (1966) Notes on the use of propagation of error formulas. J Res Nat Bur Stand 70:263–273
Martin M (2009) Laser accelerated radiotherapy: is it on its way to the clinic? J Natl Cancer Inst 101:450–451
Sasaki MS (1991) Primary damage and fixation of chromosomal DNA as probed by monochromatic soft X-rays and low-energy neutrons. In: Fielden EM, O’Neil P (Eds.) The early effects of radiation on DNA. NATO ASI Series, vol H54. Springer, Berlin (Germany) pp 369–384
Sasaki MS, Kobayashi K, Hieda K, Yamada T, Ejima Y, Maezawa H, Furusawa Y, Ito T, Okada S (1989) Induction of chromosome aberrations in human lymphocytes by monochromatic X-rays of quantum energy between 4.8 and 14.6 keV. Int J Radiat Biol 56:975–988
Schmid E, Bauchinger M, Streng S, Nahrstedt U (1984) The effect of 220 kVp X-rays with different spectra on the dose response of chromosome aberrations in human lymphocytes. Radiat Environ Biophys 23:305–309
Schmid E, Braselmann H, Nahrstedt U (1995) Comparison of gamma-ray induced dicentric yields in human lymphocytes measured by conventional analysis and FISH. Mutat Res 348:125–130
Schmid E, Hieber L, Heinzmann U, Roos H, Kellerer AM (1996) Analysis of chromosome aberrations in human peripheral lymphocytes induced by in vitro a- particle irradiation. Radiat Environ Biophys 35:179–184
Schmid E, Schraube H, Bauchinger M (1998) Chromosome aberration frequencies in human lymphocytes irradiated in a phantom by a mixed beam of fission neutrons and gamma rays (1998). Int J Radiat Biol 73:263–267
Schmid E, Regulla D, Guldbakke S, Schlegel D, Roos M (2002a) Relative biological effectiveness of 144 keV neutrons in producing dicentric chromosomes in human lymphocytes compared with 60Co gamma rays under head-to-head conditions. Radiat Res 157:453–460
Schmid E, Regulla D, Kramer HM, Harder D (2002b) The effect of 29 kV X-rays on the dose response of chromosome aberrations in human lymphocytes. Radiat Res 158:771–777
Schmid E, Krumrey M, Ulm G, Roos H, Regulla D (2003) The maximum low-dose RBE of 17.4 and 40 keV monochromatic X-rays for the induction of dicentric chromosomes in human peripheral lymphocytes. Radiat Res 160:499–504
Schmid E, Roos H, Kramer HM (2008) The dependence of the biological effectiveness of 60Co gamma rays in a large absorber determined by dicentric chromosomes in human lymphocytes. Radiat Prot Dosim 130:442–444
Schmid E, Wagner FM, Romm H, Walsh L, Roos H (2009a) Dose-response relationship of dicentric chromosomes in human lymphocytes obtained for the fission neutron therapy facility MEDAPP at the research reactor FRM II (2009). Radiat Environ Biophys 48:67–75
Schmid TE, Dollinger G, Hauptner A, Hable V, Greubel C, Auer S, Friedl AA, Molls M, Röper B (2009b) No evidence for a different RBE between pulsed and continuous 20 MeV protons. Radiat Res 172:567–574
Schmid TE, Dollinger G, Hable V, Greubel C, Zlobinskaya O, Michalski D, Auer S, Friedl AA, Schmid E, Molls M, Röper B (2011) The effectiveness of 20 MeV protons at nanosecond pulse lengths in producing chromosome aberrations in human-hamster hybrid cells. Radiat Res 175:719–727
Schmid TE, Greubel C, Hable V, Zlobinskaya O, Michalski D, Girst S, Siebenwirth C, Schmid E, Molls M, Multhoff G, Dollinger G (2012) Low LET protons focused to submicrometer shows enhanced radiobiological effectiveness. Phys Med Biol 57:5889–5907
Schmid TE, Friedland W, Greubel C, Girst S, Reindl J, Siebenwirth C, Ilicic K, Schmid E, Multhoff G, Schmitt E, Kundrat P, Dollinger G (2015) Sub-micrometer 20 MeV protons and 45 MeV lithium spot irradiation enhances yields of dicentric chromosomes due to clustering of DNA double-strand breaks. Mutat Res 793:30–40
Schrader T, Münter K, Kleine-Ostmann T, Schmid E (2008) Spindle disturbances in human-hamster hybrid (AL) cells induced by mobile communication frequency range signals. Bioelectromagnetics 29:626–639
Acknowledgements
This work was supported by the DFG Cluster of Excellence: Munich-Centre for Advanced Photonics and by the project ‘LET-Verbund’ (Funding Nos. 02NUK031A, 02NUK031B) of the German Federal Ministry of Education and Research.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
The RBE of a certain radiation quality, here called test radiation T, with respect to a reference radiation is defined as the ratio of the doses of the reference radiation, D ref, and the test radiation, D T, necessary to induce the same effect: RBE = D ref/D T. Changing the reference radiation the RBE will change, too. The RBE depends on the reference radiation. The RBE of a test radiation with respect to reference radiation B, RBErefB (T), can be expressed by the RBE with respect to reference radiation A, RBEref A (T), and the RBE of reference radiation B with respect to reference radiation A, RBEref A (B), in the following way:
Rights and permissions
About this article
Cite this article
Schmid, T.E., Greubel, C., Dollinger, G. et al. The influence of reference radiation photon energy on high-LET RBE: comparison of human peripheral lymphocytes and human–hamster hybrid AL cells. Radiat Environ Biophys 56, 79–87 (2017). https://doi.org/10.1007/s00411-016-0680-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00411-016-0680-3