Abstract
The use of phosphate mineral products in animal nutrition, as a major source of phosphor and calcium, can lead to uranium entering the food chain. The aim of the present study was to determine the protective effect of natural sepiolite and sepiolite treated with acid for broilers after oral intake of uranium. The broilers were contaminated for 7 days with 25 mg/uranyl nitrate per day. Two different adsorbents (natural sepiolite and sepiolite treated with acid) were given via gastric tube immediately after the oral administration of uranium. Natural sepiolite reduced uranium distribution by 57 % in kidney, 80 % in liver, 42 % in brain, and 56 % in muscle. A lower protective effect was observed after the administration of sepiolite treated with acid, resulting in significant damage of intestinal villi in the form of shortening, fragmentation, and necrosis, and histopathological lesions on kidney in the form of edema and abruption of epithelial cells in tubules. When broilers received only sepiolite treated with acid (no uranyl nitrate), shortening of intestinal villi occurred. Kidney injuries were evident when uranium concentrations in kidney were 0.88 and 1.25 µg/g dry weight. It is concluded that adding of natural sepiolite to the diets of broilers can reduce uranium distribution in organs by significant amount without adverse side effects.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Intensive animal husbandry involves the use of phosphate mineral supplements as the major source of phosphorus and calcium, which are considered as essential metabolic elements. For farm animals, a key source of uranium is phosphate mineral supplementation, mostly which is dicalcium phosphate, in which the specific activity of uranium can reach 3,000 Bq/kg dry weight (Arruda-Neto et al. 1997; Casacuberta et al. 2009; Mitrović et al. 2014). Dicalcium phosphate can be also used as a mineral fertilizer. According to the regulations of the Republic of Serbia, the import and sale of mineral fertilizers is forbidden when the activity concentration of 238U and 226Ra is higher than 1,600 and 1,000 Bq/kg fresh weight, respectively, but the regulations do not include phosphate mineral supplements for animal nutrition (Official Gazette of RS 2011, 2013). This mineral supplement is often given to animals from an early period of life to slaughtering and can to lead uranium entering the food chain. Ingestion of feed, water, and soil is a major pathway for animal contamination with radionuclides (IAEA 2010). Water contaminated with uranium would be the most influential parameter contributing to uranium activity concentration in chicken meat (Jeambrun et al. 2012).
Although uranium is radioactive, its adverse health effects are primarily a result of its chemical rather than radiological toxicity (Keith et al. 2013). Very young animals can absorb greater quantities of uranium than older animals, as shown by Sullivan and Gorham (1982). Previous investigations performed on broilers show that after uranium contamination, the most uranium was accumulated in kidney and liver, while muscles were not a major target for uranium deposition (Mitrović et al. 2014). In rats, the transcellular pathway is dominant for gastrointestinal absorption of dissolved uranium compounds in the small intestine (Dublineau et al. 2006).
To protect animals from different toxins, such as mycotoxins, heavy metals, and radionuclides, the use of adsorbents applied as a feed additive has been recommended (Karovic et al. 2013; Mitrović et al. 2012; Oguz 2011; Papaioannou et al. 2005; Rizzi et al. 2003). Sepiolite (Si12O30 Mg8 (OH)4(H2O)4·8H2O) is a naturally occurring fibrous clay mineral of sedimentary origin. It is porous clay with a large specific surface area and with a high ability to absorb inorganic as well as organic compounds and heavy metals (Lazarević et al. 2007, 2009). It can be used as a pellet binder for improving pellet quality (Angulo et al. 1996). Adding 2 % of sepiolite in starter and growth periods improved growth performances and feed efficiency (Ayed et al. 2008). When added as a feed supplement, it significantly increased organic matter digestibility, decreased the water-relative viscosity of jejunal digesta in broilers, and improved the activity of digestive enzymes (Ouhida et al. 2000).
The adsorption capacity of sepiolites suggests that this mineral is effective for the removal of Pb2+, Cd2+, and Sr2+ from polluted waters (Lazarević et al. 2007). Donat (2009) founded that sepiolite is suitable as sorbent material for recovery and adsorption of uranium (VI) ions from aqueous solutions. However, there is insufficient data about using this mineral adsorbent to protect animals from the alimentary contamination with uranium.
Consequently, the aim of the present study was to determine the ability of sepiolite to adsorb uranium (VI) at different solution pH values (in vitro conditions), and to explore the possibility of its use to adsorb uranium in broilers digestive tract to reduce gut transfer. Since the acid treatment changed the sorption capacity of uranium (Kilislioglu and Aras 2010), sorption of uranium on natural sepiolite (S) and sepiolite treated with acid (TS) was investigated. The experiments were performed with broilers receiving uranyl nitrate and natural sepiolite (S) or sepiolite treated with acid (TS), via gastric tube, during a 7-day period. In order to determine the toxicity of uranium, histopathological examinations of kidney, liver, brain, and small intestine were performed.
Materials and methods
Adsorbents
Sepiolite from deposits near Čačak, Republic of Serbia, was used as initial raw material. The samples were dried for 2 h in an oven at 150 °C, then pulverized in a porcelain mortar, and passed through a sieve with varying mesh size. Fractions containing particles of 250–800 µm diameter were used. The sepiolite activated by acid was prepared with 4 mol/dm3 HCl. Activation was performed at room temperature, at a ratio of 10 g of sepiolite and 100 cm3 acid solution, for 10 h. Following activation, the solid phase was separated by filtration and washed with hot distilled water until there was a negative reaction to Cl ions. The solid was dried for 2 h at 150 °C.
Uranium sorption (in vitro conditions)
In order to investigate the uranium (VI) adsorption on natural sepiolite (NS) and sepiolite treated with acid (AS), different amounts (0.01, 0.025, 0.05, 0.1, 0.25, 0.5, 0.7, 0.9, and 1 g) of adsorbent were added to 50 ml of uranium (VI) solution. The adsorption of uranium (VI) ions on natural sepiolite (NS) and sepiolite treated with acid (AS) was determined at two pH values, pH values 3 and 6. These pH values were selected because in the digestive tract of broilers, the pH values are in the range from 2.5 to 8 (crop: 5.5, proventriculus/gizzard: 2.5–3.5, duodenum: 5–6, jejunum: 6.5–7.0, ileum: 7.0–7.5, colon: 8) (Denbow 2000; Klasing 1999). The pH value of the solution was adjusted with HNO3. The uranium (VI) solutions were prepared using uranyl nitrate hexahydrate [UO2(NO3)2·6H2O] (Sigma-Aldrich Co.). The initial concentration of uranium (VI) ions was constant at each probe, i.e., 10 mg U/ml (500 mg U/50 ml) in pure distilled water. The samples were shaken for 5, 10, 15, 30, 60, 120, 240, 360, and 1,440 min in a mechanical shaker (150 rpm).
After the reaction time, solids were separated by filtration and the concentration of the uranium (VI) remaining in the solution was determined using a fluorometric method (see below). The percentage of uranium adsorbed (%) was calculated by Eq. 1:
where C i is the initial uranium (VI) concentration and C f is the uranium (VI) concentration in the final solution. All experiments were performed in duplicate.
Uranium sorption (in vivo conditions)
Experiments were performed with 35-day-old broilers of linear hybrid Hybro, weighing around 1,000 g. Thirty-six broilers were randomly selected for the experiment. Food and water intake were ad libitum. They were kept under constant temperature (21 ± 2 °C) with a 12:12-h (light–dark) cycle. The birds were divided into six experimental groups, with six birds per group. The zero group (0) was administered neither the uranyl nitrate solution nor adsorbents.
During the seven days, the birds in groups 1, 2, and 3 were contaminated via gastric tube, with a uranyl nitrate water solution (UO2(NO3)2·6H2O) (Sigma-Aldrich Co.), in a quantity of 25 mg uranyl nitrate/per day. The control group (1) received only uranyl nitrate and no adsorbents. Immediately after the uranyl nitrate contamination, the broilers in the group 2 received a dose of 2 g of natural sepiolite (S) daily, while the broilers in group 3 received 2 g of sepiolite treated with acid (TS). Broilers in group 4 received only the adsorbent natural sepiolite (2 g/day), whereas the broilers in group 5 received only sepiolite treated with acid (2 g/day), also via gastric tube.
All the birds from each group were stunned and then killed by cervical dislocation, on the 8th day of the experimental period. Uranium concentration was determined in muscle, kidney, liver, and brain. Histopathological analyses were performed on samples of the small intestine, kidney, liver, and brain.
Sample preparation
The organs (kidney, liver, brain, muscle) were weighed to measure the fresh weight and then dried at 105 °C. After drying, all samples were dissolved by microwave digestion using “Milestone Ethos 1.”
Quantification of uranium
The uranium content in the initial solution, filtrate, muscle, and organs of broilers (kidney, liver, brain) was determined by a fluorometric method based on the fluorescence of uranium in a fused mixture of NaF, Na2CO3, and K2CO3, using a “Jarrell Ash 26-000 Division.” Dried samples (20 g) were ashed at 450 °C in a muffle furnace for 2 h, after which the ash was dissolved in 5.0 ml 10.3 M HNO3 + 5.0 ml of 24 M HF and then dried on a hot plate. The residue was redissolved in 5.0 ml 10.3 M HNO3 and dried again followed by another dissolution in 5.0 ml 10.3 M HNO3, and again dried (to remove free fluoride). The final ash was dissolved in 25.0 ml 12.7 M HNO3 for the determination of uranium. Aliquot samples (5.0–10.0 ml) of the dissolved ash were transferred to 125-ml separatory funnels containing 10.0 ml saturated Al(NO3)3 and 10.0 ml 0.1 M TOPO (trioctylphosphinoxide, [CH3(CH2)7]3PO) in ethyl acetate. Funnels were vigorously shaken for 5 min, and the organic (upper) and aqueous (lower) phases were allowed to separate. The uranium complex was separated into the organic phase. Small volumes (0.1 ml) of the organic phase were transferred to platinum fusion dishes (10 mm in diameter) containing 0.75 mg 9 % NaF + 91 % NaKCO3 pellets, dried under high-intensity lamps, fused at 700 °C for 5 min, and then cooled. Finally, the intensity of fluorescence was determined in a fluorometer (Thermo-Jarrell Ash Corp., Franklin, MA, USA). The concentration of uranium was determined from standard uranium calibration curves (lower detection limit of 0.01 mg/g fused pellet, correlation coefficient R > 0.997) (Stojanovic et al. 2010).
Histopathological analyses
After the birds were killed, samples of the small intestine, kidney, liver, and brain were rinsed with NaCl 0.9 % and fixed in 10 % formaldehyde solution, dehydrated, embedded in paraffin, and cut into 5-µm-thick sections. Histopathological slides of 5 µm were stained with hematoxylin and eosin (H&E).
Results
In vitro experiments
The kinetics of uranium (VI) ions adsorption at different pH values was studied. The pH values of the initial solution are an important variable for the adsorption of uranium (VI) ions on the adsorbents (Donat and Aytas 2005). The uptake of uranium (VI) ions at pH 6 by natural sepiolite and sepiolite treated with acid was rapid reaching 100 % after 5 min, for 0.5 g adsorbents, remaining constant during 24 h. Similar adsorption was observed for same amounts of natural sepiolite at pH 3 (Fig. 1). However, uranium (VI) adsorption by 0.5 g sepiolite treated with acid at pH 3 was lower and significantly slower, but increased over time. Desorption processes were not observed during the time of investigation.
The influence of the mass of adsorbents to remove uranium is shown in Table 1. Binding capacity increases with mass for both scenarios. Natural sepiolite, at pH 3, showed higher adsorption index of uranium (VI) ions than sepiolite treated with acid. Lazarevic et al. (2007) reported that acid activation of sepiolite does not lead to an increase in the adsorption capacity of heavy metal, which is confirmed by the present study. Results shown in Fig. 1 and Table 1 were used for setting up the parameters for the in vivo studies described below, where a mass ratio adsorbent/uranium of 80:1 (2 g of each adsorbent and 25 mg uranium) was used.
In vivo experiments
Uranium was detected in kidney, liver, and brain of broilers in the control contaminated group (group 1). The same results were obtained for groups (2 and 3) where the broilers received adsorbents after the contamination (Table 2). In the second group (uranyl nitrate + S), uranium concentration in liver was lower by a factor of 5 than in liver from the control group, while the concentration of uranium in kidneys and brain was reduced by 2.3 times and 1.7 times. Administration of sepiolite treated with acid (group 3) reduced uranium concentration in liver by a factor of 2 in comparison with the control group (group 1), while in kidney and brain uranium concentration decreased by a factor of 1.4 and 1.2, respectively. In muscle and selected organs of broilers in groups 0, 4, and 5, uranium could not be detected (0.001 µg/g). No effects of broilers’ food and water intake and behavior on uranium concentrations were observed.
Histopathological changes in broilers contaminated with a dose of 25 mg 238U daily were found in the kidney, liver, and small intestine, in the form of dystrophic changes in the kidney tubules epithelium, edema and vacuolization of the cytoplasm of hepatocytes, and necrosis of intestinal villi (Mitrović et al. 2014). In the examined tissues of broilers that received adsorbent sepiolite after their contamination with uranyl nitrate (group 2), and of broilers that received only adsorbents sepiolite (group 4), no difference from the normal histological structure could be observed.
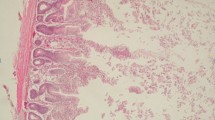
In broilers that received sepiolite treated with acid immediately after the contamination of uranium (group 3), histopathological changes were manifested in the form of shortening, fragmentation and necrosis of intestinal villi (Fig. 2), and edema, and abruption of epithelial cells in renal tubules (Fig. 3). Histopathological changes in the form of shortening of intestinal villi (Fig. 4) were also detected in broilers that received only sepiolite treated with acid (group 4), while changes in the liver, kidneys, and brain were not observed.
Intestine from broilers a in zero group (group 0): normal structure of intestinal villi; HE (hematoxylin and eosin) × 10 (microscope magnification); b in the experimental group where the broilers received sepiolite treated with acid and uranyl nitrate (group 3): shortening, fragmentation, and necrosis of intestinal villi (arrow); HE (hematoxylin and eosin) × 10 (microscope magnification)
Renal cortex from broilers a in zero group (group 0): glomerular and the tubular basement membrane are normal; HE (hematoxylin and eosin) × 40 (microscope magnification); b Renal cortex from broilers in the experimental group where the broilers received sepiolite treated with acid and uranyl nitrate (group 3): edema and abruption of epithelial cells in the tubules (arrow); HE (hematoxylin and eosin) × 40 (microscope magnification)
Discussion
Different adsorbents are often used in poultry nutrition, in order to prevent the absorption of various toxins from the digestive tract. These additives should be safe for animal health and should have the ability to bind harmful substances in the digestive tract. Phosphate mineral products such as dicalcium phosphate and monocalcium phosphate are important in animal nutrition, as a source of phosphorus and calcium (Roessler 1990, Arruda-Neto et al. 2005), but they may be enriched in significant quantities of naturally occurring radionuclides (Casacuberta et al. 2009), such as uranium.
In intensive poultry production, there is the possibility of animal contamination with uranium, using phosphate mineral products, but the discussion about human health risk is controversial. Arruda-Neto et al. (1997) reported that the uranium transfer coefficients from feed supplemented with dicalcium phosphate to animal meat were 1.2 day/kg in chicken and 0.2 day/kg for bovines. These authors calculated that poultry and bovine meat may accumulate 9 Bq/kg and 25 Bq/kg of 238U and, consequently, the dose for Brazilian consumers could reach 10 mSv per year if the contributions of all 238U decay series radionuclides were considered (Arruda-Neto et al. 1997). Opposite to this, Casacuberta et al. (2009) calculated the dose humans would receive due to the ingestion of poultry meat based on 210Pb and 210Po, because these two radionuclides of the 238U decay series mostly contribute to radioactive exposure after ingestion. These authors estimated the dose to humans via consumption of chicken meat and found values between 2 and 11 µSv per year. This does not suggest any radiological risk to man associated with the consumption of meat contaminated by radioactivity in dicalcium phosphate fed to the animals.
The pH value in digestive tract of broilers ranges from 2.5 to 8 (Denbow 2000; Klasing 1999). Therefore, an in vitro experiment was performed on two initial pH values, 6 and 3. The results obtained showed that adsorption of uranium ions (VI) on natural sepiolite was high and fast, both for initial pH values 6 and 3. Donat (2009) reported that uptake of uranium (VI) ions on natural sepiolite reached the maximum at pH 3 and then decreased, which is not in accordance with the present results, probably due to the different structure and surface properties of these sepiolites. The uptake of uranium (VI) ions on sepiolite treated with acid was rapid at pH 6. In contrast, uranium adsorption at pH 3 was lower, but after four hours of contact, the adsorption had reached more than 90 % (Fig. 1). The present results also show that the administration of natural sepiolite and sepiolite treated with acid to broilers, immediately after uranium administration, will reduce the uranium transfer to tissues and organs significantly.
After absorption via the digestive tract, uranium is mostly deposited in bones (ICRP 1996) in humans. Uranium and calcium qualitatively follow the same metabolic pathway and adult ducks incorporate on average 10 times more uranium than broilers (Arruda-Neto et al. 2014). After 7 days of uranium administration, broilers had accumulated uranium in kidney and liver. The uranium concentration in brain was three times less than that in kidney, and 2.5 times less than that in liver. In muscle, the uranium concentration was 14 times lower than in the kidney, 11 times lower than in the liver, and 4.5 times lower than in the brain, indicating that muscle is not a major target for uranium deposition (Mitrović et al. 2014). No histopathological changes in muscle were observed after the administration of uranyl nitrate in drinking water in Sprague–Dawley rats (Gilman et al. 1998a), or in New Zealand rabbits after 91 days (up to 43 mg U/kg/day) (Gilman et al. 1998b, c).
After uranium administration to broilers and immediate protection with natural sepiolite (group 2), uranium concentration in selected organs and muscle was less than those of the control group (group 1). Application of natural sepiolite reduced uranium concentration by 57 % in kidney, 80 % in liver, 42 % in brain, and 56 % in muscle (Table 2). Lower protective effects were observed after the administration of sepiolite treated with acid (group 3): Uranium concentration was lower by 30 % in kidney, 52 % in liver, 15 % in brain, and 22 % in muscle (Table 2). The efficiency of protection in kidney, liver, and brain was on average 60 % for sepiolite and 32 % for sepiolite treated with acid. In our previous study, we reported that the efficiency of protection in kidney, liver, and brain was on average 60 % for organobentonite and 61 % for organozeolite (Mitrović et al. 2014). Comparing these results with those of the present study, it is observed that the highest protective effect of adsorbents (organobentonite, organozeolite, natural sepiolite, and sepiolite treated with acid) was in liver (52–80 %), while the lowest protective effect was in brain (15–49 %). The use of these adsorbents reduced uranium distribution in kidney by 30–67 and 22–67 % in muscle. In addition to this high efficiency of uranium adsorption, there are various possibilities of sepiolite use in poultry production. For example, it can be used as a pellet binder for improving pellet quality (Angulo et al. 1996), growth performances, and feed efficiency (Ayed et al. 2008). Adding 1.5 % sepiolite in chicken diet showed a reduction in the intestinal transit time, which might correspond to a better nutrient utilization (Tortuero et al. 1992).
After uranium administration to the broilers in the control group (group 1), histopathological changes were observed in the small intestine, liver, and kidney (Mitrović et al. 2014). In contrast, in group 2, where the broilers received natural sepiolite immediately after uranyl nitrate contamination, no histopathological lesions were observed in the investigated organs and tissues. Accordingly, injuries were also not observed in group 4, where the broilers received only natural sepiolite. Lesions were observed in broilers that received sepiolite treated with acid (groups 3 and 5). In group 5, where broilers received only sepiolite treated with acid without contamination with uranyl nitrate administration, shortening of intestinal villi was observed. Significant damage of intestinal villi in the form of necrosis, fragmentation and shortening, and histopathological lesions on kidney in the form of edema and abruption of epithelial cells in tubules was observed in group 3 (uranyl nitrate + TS). The uranium concentration in kidneys of broilers in group 3 (uranyl nitrate + TS), where histopathological changes were detected, was greater (0.88 μg/g) than that in kidneys from group 2 (uranyl nitrate + S). In rats that received small doses of uranyl fluoride, changes in kidneys such as necrosis of the proximal tubules, as well as proteinuria and enzimuria, were found at low concentrations of uranium from 0.7 to 1.4 μg/g fresh weight of kidney (Diamond et al. 1989), while a much more serious renal impairment was observed when concentrations in kidneys were 3.4 and 5.6 μg/g of fresh weight. The changes observed in the small intestine in group 3 are not associated with the toxic effects of uranium, because in broilers that received only sepiolite treated with acid, the shortening of the small intestine was also observed (group 5). These changes are probably caused by the HCl, which was used for sepiolite activation. Histopathological changes observed in kidneys are the result of their physiological ability to reabsorb and accumulate divalent metals (Vicente–Vicente et al. 2010; Kurttio et al. 2002). In rats, uranium specifically accumulates in the proximal tubules in the inner cortex and the outer stripe of the outer medulla, where it causes apoptosis and renal lesions (Homma-Takeda et al. 2009). Studies of overexposure in experimental animals (rats and rabbits) showed a chronic nephrotoxicity after a short-term high dose of uranium administered via drinking water (Gilman et al. 1998a, b).
Conclusion
With the actual increase in uranium accumulation in the environment due to huge uranium production for nuclear and nonnuclear purposes, it is useful to understand its distribution and toxicity for humans and animals, and its potential to enter the human food chain. The results obtained in the present study show that uranium has enterotoxic effects on the intestinal villi. Target organs for uranium accumulation are kidneys and liver, where histopathological changes were also observed. Uranium transfer in muscle was low, indicating that muscles are not a target tissue for uranium. The efficiency of protection in kidney, liver, and brain for sepiolite and organobentonite was on average 60, and 61 % for organozeolite. In all situations where there is a risk of contamination by uranium either through phosphate mineral additives or water, adding sepiolite, organobentonite, and organozeolite as mineral adsorbents to animal feed prevents enterotoxic effects on intestine and reduces uranium transfer in organs and tissue.
References
Angulo E, Brufau J, Esteve-Garcia E (1996) Effect of sepiolite product on pellet durability in pig diets in particle size and in broiler starter and finisher diets. Anim Feed Sci Technol 63:25–34
Arruda-Neto JDT, Tavares MV, Filadelfo M (1997) Concentrations of uranium in animal feed supplements: measurements and dose estimates. J Radioanal Nucl Chem 221:97–104
Arruda-Neto JDT, Cestari AC, Nogueira GP, Fonseca LEC, Saiki M, Oliveira E, Manso-Guevara MV, Vanin VR, Deppman A, Mesa GF (2005) Metabolical aspects associated with incorporation and clearance of uranium by broilers—case study and a biophysical approach. Int J Poult Sci 4:511–517
Arruda-Neto JDT, Cavalcante GT, Nogueira GP, Rodrigues TE, Fonseca LEC, Saiki M, Genofre GC (2014) Uranium metabolism associated with ontogenetic growth of birds—case studies with broilers and ducks. Adv Biosci Biotechnol 5:768–776
Ayed MH, Zghal I, Rekik B (2008) Effect of sepiolite supplementation on broiler growth performances and carcass yield. In: Proceedings, Western Section, American Society of Animal Science, vol 59. pp 169–172
Casacuberta N, Masquéa P, Garcia-Orellana J, Bruach JM, Anguita M, Gasa J, Villa M, Hurtado S, Garcia-Tenorio R (2009) Radioactivity contents in dicalcium phosphate and the potential radiological risk to human populations. J Hazard Mat 170:814–823
Denbow D (2000) Gastrointestinal anatomy and physiology. In: Whittow G (ed) Sturkie’s avian physiology. Academic PressUniversity of Hawaii at Manoa, Honolulu, pp 299–325
Diamond GL, Morrow PE, Panner BJ, Gelein RM, Baggs RB (1989) Reversible uranyl fluoride nephrotoxicity in the Long Evans rat. Fundam Appl Toxicol 13:65–78
Donat R (2009) The removal of uranium (VI) from aqueous solutions onto natural sepiolite. J Chem Thermodyn 41(7):829–835
Donat R, Aytas S (2005) Adsorption and thermodynamic behavior of uranium (VI) on Ulva sp.-Na bentonite composite adsorbent. J Radioanal Nucl Chem 1:107–114
Dublineau I, Grison S, Grandcolas L, Baudelin C, Tessier C, Suhard D, Frelon S, Cossonnet C, Claraz M, Ritt J, Paquet P, Voisin P, Gourmelon P (2006) Absorption, accumulation and biological effects of depleted uranium in Peyer’s patches of rats. Toxicology 227(3):227–239
Gilman AP, Moss MA, Villeneuve DC, Secours VE, Yagminas AP, Tracy BL, Quinn JM, Long G, Vallit VE (1998a) Uranyl nitrate: 91-day exposure and recovery studies in the male New Zealand white rabbit. Toxicol Sci 41:138–151
Gilman AP, Villeneuve DC, Secours VE, Yagminas AP, Tracy BL, Quinn JM, Valli VE, Moss MA (1998b) Uranyl nitrate: 91-day toxicity studies in the New Zealand white rabbits. Toxicol Sci 41:129–137
Gilman AP, Villeneuve DC, Secours VE, Yagminas AP, Tracy BL, Quinn JM, Valli VE, Willes RJ, Moss MA (1998c) Uranyl nitrate: 28-day and 91-day toxicity studies in the Sprague–Dawley rat. Toxicol Sci 41:117–128
Homma-Takeda S, Terada Y, Nakata A, Sahoo SK, Yoshida S, Ueno S, Inoue M, Iso H, Ishikawa T, Konishi T, Imaseki H, Shimada Y (2009) Elemental imaging in kidney of adult rats exposed to uranium acetate. Nucl Inst Methods Phys Res B 267:2167–2170
International Atomic Energy Agency (IAEA) (2010) Handbook of Parameter Values for the Prediction of Radionuclide Transfer in Terrestrial and Freshwater Environments, Technical Reports Series No. 472, p 194
International Commission for Radiation Protection (ICRP) (1996) Age-dependent doses to members of the public from intake of radionuclides: Part 4 inhalation dose coefficients. Publication 72 Annals of the ICRP Pergamon Press, Oxford, UK
Jeambrun M, Pourcelot L, Mercat C, Boulet B, Loyen J, Cagnat X, Gauthier-Lafaye F (2012) Study on transfers of uranium, thorium and decay products from grain, water and soil to chicken meat and egg contents. J Environ Monit 14:2170–2180
Karovic D, Djermanovic V, Mitrovic S, Radovic V, Okanovic D, Filipovic S, Djekic V (2013) The effect of mineral adsorbents in poultry production. Worlds Poult Sci J 69:335–342
Keith S, Faroon O, Roney N, Scinicariello F, Wilbur Sh, Ingerman L, Llados F, Plewak D, Wohlers D, Diamond G (2013) Toxicological profile for uranium. Agency for Toxic Substances and Disease Registry (ATSDR) (US), Atlanta
Kilislioglu A, Aras G (2010) Adsorption of uranium from aqueous solution on heat and acid treated sepiolites. Appl Radiat Isot 68:2016–2019
Klasing KC (1999) Avian gastrointestinal anatomy and physiology. Semin Avian Exot Pet Med 8(2):42–50
Kurttio P, Auvinen A, Salonen L, Saha H, Pekkanen J, Mäkeläinen I, Väisänen SB, Penttilä IM, Komulainen H (2002) Renal effects of uranium in drinking water. Environ Health Perspect 110:337–342
Lazarević S, Janković-Častvan I, Jovanović D, Milonjić S, Janaćković Đ, Petrović R (2007) Sorption of Pb2+, Cd2+ and Sr2+ ions on the natural and acid-activated sepiolites. Appl Clay Sci 37(1–2):47–57
Lazarević S, Radovanović Ž, Veljović DJ, Onjia A, Janaćković DJ, Petrović R (2009) Characterization of sepiolite by inverse gas chromatography at infinite and finite surface coverage. App Clay Sci 43:41–48
Mitrović B, Vitorović G, Vićentijević M, Vitorović D, Pantelić G, Lazarević Macanović M (2012) Comparative study of 137Cs distribution in broilers and pheasants and possibilities for protection. Radiat Environ Biophys 51:79–84
Mitrović B, Vitorović G, Jovanović M, Lazarević-Macanović M, Andrić V, Stojanović M, Daković A, Vitorović D (2014) Uranium distribution in broiler organs and possibilities for protection. Radiat Environ Biophys 53:151–157
Official Gazette of Republic of Serbia (2011, 2013) Rulebook on limits of radionuclides content in drinking water, foodstuffs, feeding stuffs, medicines, general use products, construction materials and other goods that are put on market. Publication No. 86/11 from 18.11.2011. and 97/13 from 6.11.2013. Available on: http://www.srbatom.gov.rs/srbatom/zakonska-regulativa.htm
Oguz H (2011) A review from experimental trials on detoxification of aflatoxin in poultry feed. Eurasian J Vet Sci 27(1):1–12
Ouhida I, Pérez JF, Piedrafita J, Gasa J (2000) The effects of sepiolite in broiler chicken diets of high, medium and low viscosity. Productive performance and nutritive value. Anim Feed Sci Technol 85:183–194
Papaioannou D, Katsoulos PD, Panousis N, Karatzias H (2005) The role of natural and synthetic zeolites as feed additives on the prevention and/or the treatment of certain farm animal diseases: a review. Microporous Mesoporous Mater 84:161–170
Rizzi L, Simioli M, Roncada P, Zaghini A (2003) Aflatoxin B1 and clinoptilolite in feed for laying hens: effects on egg quality mycotoxin residues in livers and hepatic mixed-function oxygenase activities. J Food Prot 66:860–865
Roessler CE (1990) Control of radium in phosphate mining, beneficiation and chemical processing. The Environmental Behavior of Radium, vol. 2, Technical Report Series No. 310, IAEA Vienna, pp 269–279
Stojanović M, Stevanović D, Milojković J, Grubišić M, Ileš D (2010) Phytotoxic effect of the uranium on the growing up and development the plant of corn. Water Air Soil Pollut 209:401–410
Sullivan MF, Gorham LS (1982) Further studies on the absorption of actinide elements from the gastrointestinal tract of neonatal animals. Health Phys 43:509–519
Tortuero F, Fernandez Gonzalez E, Martin ML (1992) Effects of dietary sepiolite on the growth, visceral measurements and food passage in chickens. Arch Zootec 41:209–217
Vicente-Vicente L, Quiros Y, Pérez-Barriocanal F, López-Novoa JM, López Hernández FJ, Morales AI (2010) Nephrotoxicity of uranium: pathophysiological diagnostic and therapeutic perspectives. Toxicol Sci 118(2):324–347
Acknowledgments
This work was supported by the Ministry of Education, Science and Technological Development of Serbia, in the frame of Innovation Project No. 451-03-2802/2013-16/160 and Projects No. TR 31003 and TR 34013.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mitrović, B.M., Jovanović, M., Lazarević-Macanović, M. et al. Efficiency of sepiolite in broilers diet as uranium adsorbent. Radiat Environ Biophys 54, 217–224 (2015). https://doi.org/10.1007/s00411-015-0589-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00411-015-0589-2