Abstract
Titanite textures and chemistry have been investigated from the Roxby Downs Granite, host to the Olympic Dam Cu–U–Au–Ag deposit, South Australia. Three textural subtypes of titanite are documented: primary magmatic (cores and rims); deuteric; and hydrothermal (low T recrystallisation). Magmatic cores are defined by enrichment in LREE (~ 3 wt%), Nb (up to 1 wt%) and Zr relative to rims, which typically contain < 1 wt% LREE and Nb, as well as greater concentrations of Al, Ca, Fe and F. Deuteric titanite occurs as overgrowths on pre-existing titanite and other magmatic accessory minerals (magnetite and ilmenite), and is depleted in HFSE compared to magmatic rims, showing geochemical trends consistent with substitution of Ca2+ + Ti4+ ↔ REE3+ + (Al, Fe)3+. Hydrothermal titanite forms as a low-temperature hydrothermal overprint on primary titanite as well as an alteration product of chloritised phlogopite. Applying Zr-in-titanite geothermometry, three temperature ranges are obtained for titanite crystallisation: magmatic cores ~ 765 to 780 °C; rims ~ 705 to 740 °C; and deuteric ~ 680 to 690 °C. Titanite breakdown is a ubiquitous feature of the Roxby Downs Granite, and occurs through interaction with CO2- and F-rich fluids, forming pseudomorphs characterised by the presence of REE-fluorocarbonates, which are subsequently overprinted by REE-phosphates with increased proximity to the Olympic Dam Breccia Complex. This change is related to interaction with fluids containing appreciable PO42− liberated from local dissolution of fluorapatite. Such observations are consistent with and linked to later/retrograde stages in the formation of the Olympic Dam deposit.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Titanite is a common accessory mineral in intermediate-silicic igneous rocks and acts as a major repository for rare-earth elements (REE) in the continental crust (Gromet and Silver 1983; Bea 1996). The ability for titanite to sequester appreciable REE and other transition metals and high-field strength elements, coupled with its high closure temperature and sluggish diffusion rate, make it an applicable tool in the study of petrogenetic processes and geochronology (Dodson 1973; Cherniak 1993, 1995, 2006; Scott and St-Onge 1995; Corfu 1996; Corfu and Stone 1998; Della Ventura et al. 1999; Piccoli et al. 2000; Tiepolo et al. 2002; Prowatke and Klemme 2005; Hayden et al. 2008; McLeod et al. 2010; Kohn 2017). Many studies have demonstrated the potential for titanite geochemistry to reflect bulk compositions of source rocks and track the physicochemical conditions (e.g., temperature, pressure, fO2) of evolving magmatic systems (Gromet and Silver 1983; Wones 1989; Nakada 1991; Piccoli et al. 2000; Frost et al. 2001; McLeod et al. 2010).
The applicability of titanite as an indicator mineral in magmatic-hydrothermal ore deposits and igneous-metamorphic terranes has also been documented (Buick et al. 2007; Storey et al. 2007; Smith et al. 2009; Ismail et al. 2014; Storey and Smith 2017). The mineral has proven particularly useful in porphyry and skarn systems whereby Mo, Sn and W concentrations in titanite have been used to distinguish between barren and mineralised porphyry stocks, with implications for development of a vector approach in exploration (Che et al. 2013; Cao et al. 2015; Xu et al. 2015). Here, we document the textures and geochemistry of titanite in the Roxby Downs Granite, host to the Olympic Dam Cu–U–Au–Ag deposit, South Australia, in an attempt to further constrain the magmatic, subsolidus and hydrothermal stages preserved in the host rock, as well as to highlight the utility of titanite as a petrogenetic tool in intrusion-hosted magmatic-hydrothermal systems.
Background
Titanite chemistry
Structurally, titanite, CaTi(SiO4)(O,OH,F), comprises chains of Ti-octahedra bonded by isolated Si-tetrahedra, which surround and enclose sevenfold Ca-polyhedra. There are three sites at which element substitutions typically occur: the octahedral Ti site, sevenfold Ca site and underbonded O1 site (Ribbe 1980). Oberti et al. (1991) deemed substitution in the tetrahedral Si site to be inconsequential in natural systems. Due to a net imbalance in the electrostatic charge of pure titanite, the O1 oxygen, which connects the Ti octahedral together, becomes underbonded (Ribbe 1980), and is consequently involved in monovalent substitutions with OH− and F−, compensating for charge through coupled substitution of trivalent cations (predominantly Al3+ or Fe3+) for Ti4+. Other element substitutions include: at the Ca site—Na, Mn, REE, Pb, Th; Ti site—Al, Fe2+, Fe3+, Mg, Zr, Nb, Ta, Sb, V, Cr, Mo, Sn, W; and O1 site—OH, F, Cl (Deer et al. 1982; Franz and Spear 1985; Bernau and Franz 1987; Enami et al. 1993; Russell et al. 1994; Della Ventura et al. 1999; Frost et al. 2001; Tiepolo et al. 2002; Liferovich and Mitchell 2005; Vuorinen and Hålenius 2005).
The most common substitutions suggested for REE in titanite are: Ca2+ + Th4+ ↔ 2REE3+ (Gromet and Silver 1983), Ca2+ + Ti4+ ↔ REE3+ + (Al, Fe)3+ (Green and Pearson 1986; Enami et al. 1993), 2Ca2+ ↔ 2REE3+ + Na+ (Tiepolo et al. 2002) and Ti4+ + Ca2+ + Th4+ ↔ REE3+ + Zr4+ + Al3+ (Seifert 2005).
Geological setting and sample suite
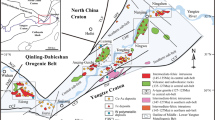
The ~ 1595 Ma Roxby Downs Granite (RDG) hosts the world-class Olympic Dam hematite-dominated Cu–U–Au–Ag deposit, which is located in the north of the Olympic Cu–Au Province on the eastern margin of the Gawler Craton (Fig. 1). Rocks of the Hutchison Group (~ 2000 to 1860 Ma), Donington Suite (~ 1845 to 1810 Ma) and Wallaroo Group equivalents (~ 1750 Ma) form the Paleoproterozoic basement of the Olympic Dam region (Creaser 1989; Ferris et al. 2002; Jagodzinski 2005). These units are unconformably overlain and intruded by felsic and mafic–ultramafic lithologies of the Hiltaba Suite (HS) and Gawler Range Volcanics (GRV). The HS intrusives are grouped together within the larger Burgoyne batholith, which makes up the basement of the northern Stuart Shelf (Creaser 1989). The batholith is separated into two granitoid suites: the Wirrda Suite and the White Dam Suite, both of which comprise intrusive rocks varying from quartz monzodiorite to granite (Creaser 1989). The RDG is affiliated with the Wirrda Suite, which is situated on the western side of the Burgoyne batholith (Creaser 1996).

(modified from Kontonikas-Charos et al. 2018a)
Geological map of bedrock in the Olympic Dam region displaying sampled drillholes and other prospects within the area. ‘Biotite out’ represents the boundary between weakly sericite–hematite-altered and least-altered Roxby Downs Granite. The Olympic Dam Breccia Complex (grey dashed line) signifies the outer limits of the ore-body at Olympic Dam which is defined by whole-rock Fe > 5% (Ehrig et al. 2012). See text for detail. Inset: Location of Olympic Dam within South Australia
Deposition of Cu–U–Au–Ag mineralisation at Olympic Dam is considered to be contemporaneous with regional magmatism at ~ 1590 Ma (e.g., Johnson and Cross 1995; Jagodzinski 2005; Skirrow et al. 2007; Ciobanu et al. 2013; Courtney-Davies et al. 2016, 2019). Hydrothermal breccias of the Olympic Dam Breccia Complex host the Cu–U–Au–Ag mineralisation and are contained within the RDG. Compositions and textures within the Olympic Dam Breccia Complex are gradational and comprise a continuum from sericite–hematite-altered granite through mineralised, hematite-rich breccias to barren hematite–quartz breccias in the deposit centre (Ehrig et al. 2012).
The RDG is equigranular to porphyritic, medium- to coarse-grained and peraluminous to weakly metaluminous (Creaser 1989; Kontonikas-Charos et al. 2017). Dominant minerals are alkali feldspar (40–50%), plagioclase (20–25%), quartz (20–25%), with minor amphibole and biotite (5–10%). Magmatic accessory minerals (~ 5%) include magnetite, titanite, ilmenite, fluorapatite, zircon, and allanite. Other minor minerals include: fluorite, tourmaline, uranothorite, REE-fluorocarbonates (parisite, bastnäsite and the unnamed polytype B2S) and sulphides (pyrite, chalcopyrite, galena, sphalerite and molybdenite) (Creaser 1989; Ehrig et al. 2012; Ciobanu et al. 2017; Kontonikas-Charos et al. 2017, 2018a, b).
Approach and methodology
Samples of RDG were obtained from various drillholes surrounding the Olympic Dam Breccia Complex (Fig. 1). Drillholes RD2488, RD2494 and RD2495, ~ 5 km SW of the deposit, were chosen as representative samples of least-altered RDG, whereas drillholes RD2280 and RD2531, < 2 km SE of the deposit, contain variably sericite–hematite-altered RDG and were selected for comparison; see Kontonikas-Charos et al. (2017) for detailed mineralogy and petrography.
Textural characterisation of titanite was achieved using a combination of optical microscopy and scanning electron microscopy (SEM), performed on a FEI Quanta 450 field emission gun (FEG) scanning electron microscope with energy-dispersive X-ray spectrometry (EDS) and back-scatter electron (BSE) imaging capabilities. Operating conditions were at a constant accelerating voltage of 20 kV, beam current of 10 nA and 130 Pa chamber pressure. Sample characterisation and imaging was carried out in BSE mode, supported by semi-quantitative EDS analyses.
Quantitative concentrations of major and minor elements for titanite were determined using a Cameca SX-Five Electron Probe Microanalyzer fitted with five wavelength-dispersive spectrometers for electron probe microanalysis (EPMA), as well as an EDS, SEM and optical microscope. EPMA was also used for mineral identification where necessary. All analyses were carried out under constant beam operating conditions of 15 keV, 20 nA (100 nA was used for REE), a take-off angle of 40° and beam size of 1 µm. Titanite was analysed for Si, Al, Na, Ca, Fe, Mg, Mn, P, Ti, Cr, V, Zr, Nb, Sn, La, Ce, Nd, Y, Cl and F. Certified natural and synthetic standards for probe analysis were supplied by Astimex Ltd. and P&H Associates. Calibration and reduction of data were completed using Probe for EPMA software. For detail on standards, detection limits, spectrometers and count times, see Electronic Supplementary Material.
Laser-ablation inductively-coupled mass spectrometry (LA-ICP-MS) was used to provide quantitative trace element data for titanite in selected samples. The following basic set of isotopes were monitored: 23Na, 24Mg, 27Al, 29Si, 31P, 39K, 43Ca, 45Sc, 47Ti, 51V, 53Cr, 55Mn, 57Fe, 59Co, 60Ni, 65Cu, 66Zn, 69Ga, 75As, 85Rb, 88Sr, 89Y, 90Zr, 93Nb, 95Mo, 118Sn, 133Cs, 137Ba, 139La, 140Ce, 141Pr, 146Nd, 147Sm, 153Eu, 157Gd, 159Tb, 163Dy, 165Ho, 166Er, 169Tm, 172Yb, 175Lu, 178Hf, 181Ta, 182W, 206Pb, 207Pb, 208Pb, 232Th and 238U. Spot analysis and element mapping was performed on an ASI M-50-LR 193-nm Excimer laser equipped to an Agilent 7700cx Quadrupole ICP-MS. The laser system was operated at pulse rates of 10 Hz and power levels of 50% power level; laser energy was typically 6–9 J/cm2, giving an ablation rate of approximately 1.5 μm/s. A relatively large beam size of 29 µm was used for titanite spot analyses to generate increased counts during ablation, whereas for element mapping a beam size of 12–18 µm was used at a scan speed of 1.5 × beam size (µm/s). In addition, dwell times on heavier elements, including REE, U and Th, were increased to reduce signal disturbance. The standard reference material used was the NIST-610, and data were processed through Iolite software. LA-ICP-MS methodology is detailed in Electronic Supplementary Material. All microanalytical instruments used are hosted at Adelaide Microscopy, The University of Adelaide.
Results
Petrography
Titanite in the RDG exhibits a range of textural morphologies, zonation patterns and mineralogical associations. Primary titanite occurs as orange–brown, euhedral grains ranging from 300 µm to ~ 1 mm in size, often developed along {111} (elongated or wedge-shaped) (Fig. 2a). Most grains display sectorial zonation and many preserve overgrowth rims (with minor oscillatory zonation), as well as evidence of dissolution and reprecipitation (Fig. 2b). Irregular and rounded inclusions of ilmenite, magnetite, fluorapatite and zircon may be observed within titanite, generally towards core interiors. Larger (mm-sized) titanite phenocrysts are observed in contact with alkali feldspar and quartz, whereas < 500-µm-sized grains are typically associated with edenite, phlogopite and accessory phases (magnetite, ilmenite, fluorapatite and zircon). Deuteric titanite occurs as overgrowths on primary titanite and interstitial to other magmatic accessory minerals (magnetite and ilmenite) (Fig. 2c, d).
Photomicrographs in plane-polarised light (a) and back-scatter electron (BSE) images (b–d) of titanite morphologies and textures in the Roxby Downs Granite. a Euhedral {111} titanite with anhedral rim and partial dissolution (black areas). b BSE image of partially altered primary titanite (Ttn I), with recrystallised areas representing hydrothermal titanite (Ttn III), adjacent deuteric titanite (Ttn II). c BSE image of primary titanite (Ttn I) with deuteric rims (Ttn II) and partial dissolution reprecipitation zones (Ttn III). d Typical appearance of deuteric titanite occurring interstitial between magnetite, hosting apatite and zircon. Mineral abbreviations: Ab albite, Afs alkali feldspar, Ap apatite, Mag magnetite, Qz quartz, Ttn titanite, Zrc zircon
Hydrothermal titanite appears green in plane-polarised light and is distinguished from primary and deuteric titanite by its irregular or lack of zonation. It also overprints and replaces primary titanite (Fig. 3a). Extensive replacement is more commonplace in smaller grains and often produces pseudomorphs displaying patchy zonation and harbouring primary titanite relicts as well as other magmatic accessory inclusions (Fig. 3b, c). In this case, inclusions have typically undergone subsolidus re-equilibration, e.g., magnetite with ilmenite trellis exsolutions and ilmenite breakdown to symplectites composed of hematite + Ti-oxide. Hydrothermal titanite is also observed to propagate along and replace ilmenite trellis exsolutions in magnetite. It also occurs as an alteration product of phlogopite, forming fine-grained, cleavage-oriented lenticular inclusions enclosed within clinochlore (Fe# ~ 0.35 to 0.45), typically along rims and parallel to the basal plane (Fig. 3d).
BSE images of hydrothermal titanite. a Recrystallised titanite with patchy zonation and inclusions of ilmenite (now hematite + Ti-oxide symplectites) and magnetite. b Detail of hydrothermal titanite and primary relict. c Hydrothermal titanite inclusions oriented along chloritised basal plane of phlogopite. d Detail of porous hydrothermal titanite in c. Mineral abbreviations: Chl chlorite, Ilm ilmenite, Mag magnetite, Phl phlogopite, Qz quartz, Ttn titanite
Partial to complete breakdown of titanite is observed throughout the RDG. Initial dissolution occurs along the crystal face and produces a microporous phase at the reaction interface which propagates towards grain interiors (Fig. 4a). The chemistry of this phase at ~ 1 µm resolution (obtained via EPMA) implies a mixed composition likely representative of initial titanite and an Fe-, Mg- and Al-bearing phase (chlorite). Further destabilisation of titanite produces coarser symplectites of a clinochlore (Fe# ~ 0.3) + calcite matrix intergrown with hetero-oriented Ti-oxide, Ce-dominant REE-fluorocarbonates (parisite and bastnäsite), and quartz. The predominance of calcite over chlorite in the alteration matrix increases with sample depth. Most primary titanite shows evidence of such breakdown, and in many cases, large polymineralic pseudomorphs are all that remain. In some grains, dissolution of primary titanite appears to be overprinted by the formation of hydrothermal titanite.
BSE images detailing dissolution of titanite. a Partial breakdown of titanite (Ttn) to symplectites of Ti-oxide (TiO2), REE-fluorocarbonates (REE-F-carbonates), calcite (Cal) and chlorite (Chl) in the RDG. b Typical appearance of euhedral pseudomorphs after titanite in sericite–hematite-altered RDG. Mineral assemblage consists of Ti-oxide + REE-fluorocarbonates + REE-phosphates + quartz in chamosite + dolomite matrix. c Detail of Ti-oxide + monazite (Mnz) + quartz (Qz) + sericite (Ser) + hematite (Hm) after titanite in intensely sericite–hematite-altered RDG. d Xenotime (Xtm) inclusion in Ti-oxide within quartz (Qz) + K-feldspar (Kfs) matrix after titanite
In granite samples affected by sericite–hematite alteration, titanite is no longer preserved. Rather, complete breakdown of titanite has produced euhedral pseudomorphs consisting of Ti-oxide, REE-fluorocarbonates + REE-phosphates (monazite, xenotime) + quartz within a chamosite (Fe# ~ 0.65) + dolomite matrix (Fig. 4b). With increased proximity to the Olympic Dam Breccia Complex, quartz + K-feldspar + sericite (Fe, Mg-rich) ± hematite predominate over chamosite and dolomite in the matrix, whereas REE-phosphates are the sole REE-minerals within the pseudomorphs (Fig. 4c, d).
Crystal chemistry
Electron microprobe analyses reveal intracrystalline variation in major and minor elements of titanite cores and rims, as well as between deuteric and hydrothermal titanite (Table 1). Cores are markedly enriched in LREE (~ 3 wt%), Nb (up to 1 wt%) and Zr relative to rims. In contrast, rims typically contain < 1 wt% LREE (La, Ce, Nd) and Nb, however, they are richer in Al, Ca, F and calculated OH than cores (Fig. 5). Intracrystalline variation in primary titanite cores is particularly evident for Nb and LREE (Fig. 5c, d). Deuteric and hydrothermal titanite are also distinguished by major and minor element variation, defined by progressive increases in F and Al/Fe ratio accompanied by depletion in LREE (La, Ce, Nd), Nb and Y. In many instances, these textural subtypes overlap and cluster with primary titanite rims.
Most titanite analysed is characterised by LREE abundance such that Ce ≫ Nd > La; however, this varies for deuteric and hydrothermal titanite, in which La may predominate over Nd. Linear traverses across oscillatory zoned rims illustrate variation between LREE, Y and Nb at 10-µm intervals (Fig. 6). In general, overall concentrations of these elements decreases with distance from cores; however, several zones indicate a pairing of Y and Nb coupled with a slight dissociation from La, Ce and Nd. Deuteric and hydrothermal titanite are geochemically comparable to outer zones of primary rims.
Laser-Ablation ICP-MS analysis was used to further discriminate titanite zonation and textural subtypes based on trace elements (Table 2, Fig. 7). As determined from EPMA data, primary titanite cores are particularly well distinguished from rims by their higher concentrations of REE and HFSE. Total REE can exceed 5 wt% in titanite cores, in contrast with rims which typically contain 0.5–1 wt%. Zirconium (300–1400 ppm), Hf, Nb, Ta (10–300 ppm) and Th (100–500 ppm) show similar preference for titanite cores, and all appear to reliably differentiate between titanite textural subtypes. Arsenic (10–50 ppm), P and Na positively correlate and are also concentrated in cores. Conversely, rims and deuteric titanite have greater abundances of Sc (up to 100 ppm), V (200–400 ppm), Cr, Sn (up to 400 ppm), 206Pb and U (80–150 ppm) relative to primary cores. Data for Cu, Co, Zn, Ga and Mo are generally < 10 ppm, and do not exhibit any systematic distribution patterns at the grain-scale. Potassium, Rb, Ba, Cs and Ni are typically below minimum detection limits.
Laser-Ablation ICP-MS maps are shown for primary titanite (Fig. 8) as well as deuteric and hydrothermal titanite (Fig. 9). Zirconium, REE (Ce, Eu, Yb), Y and Th show a very strong correlation and preference for primary titanite cores. All these elements are significantly depleted in deuteric and hydrothermal titanite. Minor dissolution of the primary titanite has resulted in the redistribution of REE in irregular inclusions, identified as REE-fluorocarbonates within the REE-poor calcite ± clinochlore and Ti-oxide matrix. Interestingly, some zones within deuteric and hydrothermal titanite show decoupling of Eu from other REE. Uranium and 206Pb are negatively correlated with Th, and generally concentrate along rims and within deuteric titanite. As deduced from spot analyses, Nb and Ta correlate and show sectorial zonation in primary titanite. Hydrothermal titanite is almost devoid of these elements; however, they appear to preferentially partition into newly formed Ti-oxide. Tin and Sc show a consistently low abundance within titanite cores, which steadily increases towards grain rims. They also strongly correlate with areas of hydrothermal titanite. Manganese, P, As and Na (not shown in Figs. 8 and 9) display comparable spatial distributions, and are typically hosted in primary titanite. Vanadium shows a preference for certain zones towards grain boundaries and, like Sc, is concentrated in hydrothermal titanite.
Chondrite-normalised REE patterns for titanite vary with respect to anomalies, slope and ∑REE (Fig. 10). Primary titanite cores are LREE-enriched with a pronounced negative Eu-anomaly (Eu/Eu* = EuCN/[(SmCN + GdCN)/2]), weak negative Y-anomaly (Y/Y* = YCN/[(DyCN + HoCN)/2]) and slightly negative slope. There exists intracrystalline variation to this pattern, whereby some zones show a minor positive Y-anomaly coupled with a vertical displacement of MREE due to lower ∑REE. Rims and deuteric titanite either mimic this primary trend with lower ∑REE or show a change in Eu-anomaly to slightly positive or negative with minor HREE enrichment relative to MREE. Average ∑REE values vary from 4.5 wt% (cores), 2 wt% (rims) and 1.2 wt% (deuteric).
Discussion
Minor and trace element crystal chemistry
Variation diagrams (Fig. 5) show strong correlations consistent with a coupled substitution at the octahedral Ti site: (Al, Fe)3+ ↔ Ti4+ + (OH, F)−. The low Al/Fe ratios in magmatic titanite compared with deuteric and hydrothermal are largely temperature dependent. This is based on the increased stability of the CaAlFSiO4 component of titanite at lower temperatures (e.g., Enami et al. 1993). High Al/Fe ratios and F coupled with low LREE and Th have recently been reported for metamorphic titanite elsewhere (Garber et al. 2017; Olierook et al. 2019). In addition, variable Nb/Ta ratios in titanite have been used to discern metamorphic titanite (Chen and Zheng 2015). Our data indicate a change in Nb/Ta ratios between magmatic and deuteric titanite, from 8–9.5 to 12–50, respectively. Based on their comparable ionic charge and radii, both Nb and Ta are generally considered to display similar geochemical behaviours in magmatic systems. However, experimental studies on partition coefficients between titanite and melts have shown strong Nb/Ta fractionation, with DNb/DTa in titanite ranging from 0.07 to 0.55 (Tiepolo et al. 2002; Prowatke and Klemme 2005). The elevated Nb/Ta ratios in deuteric titanite would likely be a result of variable concentrations within deuteric fluids.
Substitution of REE at the Ca site is influenced by ionic radii, such that LREE would be preferred over HREE due to more compatible ionic radii (e.g., La3+ = 1.10 Å, Ca2+ = 1.06 Å). However, experimental studies indicate higher partition coefficients for MREE in titanite (e.g., Tiepolo et al. 2002; Prowatke and Klemme 2005). This is not observed in titanite from the RDG, which instead shows pronounced LREE enrichment relative to MREE and HREE. Similar findings have been reported from numerous other intermediate-silicic igneous rocks (Frost et al. 2001; Smith et al. 2009; Ismail et al. 2014; Kontonikas-Charos et al. 2014), and can be attributed to the relative timing of titanite crystallisation. As it forms at a late magmatic stage in this case (see next section), the crystallisation of earlier accessory minerals such as zircon (HREE-rich) produce a relatively LREE-enriched residual melt thus explaining the observed REE fractionation in titanite. This may also explain the observed high Th/U ratios in magmatic titanite. In addition, it has been shown that an increase in melt polymerisation, P, fO2 and decrease in temperature can drastically increase REE partition coefficients for titanite (Mahood and Hildreth 1983; Green and Pearson 1986; Wones 1989).
Chondrite-normalised REE fractionation patterns for titanite show significant variation between textural subtypes. The typical LREE-enriched and strong negative Eu-anomaly, characteristic of primary titanite cores and some rims, likely reflect earlier crystallisation of MREE and HREE accessory phases, and the preferential partitioning of Eu in magmatic plagioclase. This primary trend is similar to that of magmatic apatite (Krneta et al. 2016) and whole-rock geochemistry of the RDG. The strength of Eu-anomaly increases with ∑REE, consistent with geochemical differences between titanite cores and rims. Due to the fine scale of oscillatory zonation in many primary titanite rims and the ablation spot size used (29 µm), differences form cores in REE fractionation trends are difficult to distinguish; however, elemental maps (Figs. 8, 9) reveal decoupling of Eu relative to other REE in certain zones. This, along with diminishing of the Eu-anomaly, may reflect increasing fO2 in the melt, which would inhibit Eu2+ speciation and explain observed geochemical trends. This is further substantiated by REE fractionation patterns for deuteric titanite, which exhibit weak negative to positive Eu-anomalies (Fig. 11). As Eu2+ is able to directly substitute for Ca2+ in titanite, Eu decoupling from other REE may also result from fluctuations in the activity of charge-compensating ions within the melt, which could potentially change partition coefficients for REE3+, but not Eu2+ (e.g., Brugger et al. 2000, Smith et al. 2004).
Previous authors have interpreted the positive Eu-anomaly in titanite as being of metamorphic origin (e.g., Buick et al. 2007), likely a result of deuteric fluid-mediated alteration following crystallisation. Alternatively, in this case, deuteric and/or hydrothermal titanite may derive Eu2+ liberated through albitisation of local plagioclase, although determination of REE concentrations in albite (Kontonikas-Charos et al. 2018a) indicates that much of the Eu is retained during this reaction.
Titanite thermometry
Hayden et al. (2008) successfully developed the Zr-in-titanite thermometer based on the substitution of Zr4+ for Ti4+ in titanite within assemblages of coexisting zircon, rutile and quartz. Applications of the thermometer are, however, only calibrated for {111} sectors and at P–T conditions ranging from 0.1 to 2.5 GPa and ~ 600 to 1000 °C. All these criteria are met for titanite in the RDG. Ti-oxide, however, occurs as a hydrothermal phase, thus \(a_{{{\text{TiO}}_{ 2} }}\) is primarily dependent on the presence of ilmenite and magnetite. Activity levels used for calculations here are \(a_{{{\text{SiO}}_{ 2} }}\) = 1 and \(a_{{{\text{TiO}}_{ 2} }}\) = 0.7. Given reasonable lower limits of \(a_{{{\text{TiO}}_{ 2} }}\) = 0.5 for typical crustal rocks (Hayden and Watson 2007), the maximum overestimate would be ~ 25 °C. Pressure estimates used here are based on plagioclase–amphibole geobarometry (Kontonikas-Charos et al. 2017), who obtained values of ~ 2.2 kbar.
Three temperature ranges are obtained for titanite crystallisation in the RDG. Primary titanite cores typically contain Zr > 1000 ppm, corresponding to temperature estimates ranging from 765 to 780 °C. Rims yield temperatures around 705–740 °C (~ 500 ppm Zr), whereas deuteric titanite is relatively Zr-poor (< 400 ppm) and corresponds to crystallisation temperatures of 680–690 °C. Note, however, that different P estimates were used for deuteric titanite (see below).
Implications for petrogenetic and subsolidus evolution of the Roxby Downs Granite
Textural observations and geochemistry are indicative of multiple generations of titanite within the RDG. Primary magmatic titanite, characterised by variable sectorial and oscillatory zonation as well as high REE, Ti and Nb concentrations, yields crystallisation temperature estimates indicative of formation at a late magmatic stage within the RDG (~ 750 to 780 °C). This is consistent with observed mineralogical relationships between other accessory minerals zircon, fluorapatite, ilmenite and magnetite, and also in agreement with apatite saturation temperatures for the RDG (~ 850 °C; Krneta et al. 2016). As crystallisation proceeds, rims on primary titanite form, and become progressively depleted in HFSE and enriched in Ca, Al and F, relative to cores.
The presence of ilmenite and magnetite inclusions within many primary titanite cores has implications for melt evolution. Textural relationships indicate a progression from ilmenite stable (low fO2, high temperature) to titanite stable (high fO2, low temperature). Xirouchakis and Lindsley (1998) performed phase equilibrium experiments under controlled fO2 conditions and at 600–1100 °C and < 1 bar–3.8 kbar, showing that stabilisation of titanite at the expense of ilmenite can occur via the following reaction: clinopyroxene + ilmenite + O2 = titanite + magnetite + quartz. Such a reaction is particularly appropriate in the case of the RDG, and many other felsic igneous rocks, and represents the boundary between reduced and oxidised conditions in granitic rocks (e.g., Wones 1989).
Deuteric titanite exclusively forms in association with primary titanite or interstitial to other magmatic accessory minerals. Based on geochemistry and textures, this generation of titanite is interpreted to form at the subsolidus stage during cooling. Geothermometry indicates crystallisation temperatures around 680–695 °C (calibrated for P = 1 kbar, corresponding to an assumed depth of 2–3 km), which may correlate with a high-temperature onset of deuteric alteration. Harlov et al. (2006) attributed the formation of titanite rims on ilmenite as a result of hydration and oxidation reactions such as amphibole + ilmenite + O2 = titanite + magnetite + quartz + H2O, generated during high-temperature amphibolite facies metamorphism. Based on mineral assemblages and the close spatial association between deuteric titanite and edenite, such a reaction may be appropriate for the RDG.
In contrast, hydrothermal titanite forms through recrystallisation of pre-existing titanite and concomitantly as a product of low-temperature chloritisation of phlogopite. This either represents a later retrogressive metamorphic stage or a continuum down temperature during subsolidus re-equilibration. High concentrations of U within deuteric titanite as well as hydrothermal titanite (e.g., Figs. 8, 9) indicate that either the residual melt or hydrothermal fluids were enriched with U, or that U was mobilised from earlier crystallised phases such as thorite and zircon. Regardless, this observation is important considering evidence for multiple uranium mineralisation and dissolution/reprecipitation events recorded from Olympic Dam (e.g., Macmillan et al. 2016, 2017).
Destabilisation of titanite
Titanite breakdown is a ubiquitous feature of the RDG. Textural observations support initial fluid permeation along grain boundaries and microfractures at the titanite crystal interface. The microporous matrix represents the first stage in the dissolution process. As the reaction progresses, the chemical components of titanite mix with percolating fluids (likely introducing additional Fe, Al and Mg) and eventually precipitate as pseudomorphs comprising a fine-grained calcite + clinochlore matrix with coarser Ti-oxide + REE-fluorocarbonates ± quartz. Although the overall precursor grain shape is retained (e.g., pseudomorphic), neither calcite nor clinochlore (the dominant matrix minerals) share any structural similarity with titanite. This explains the extremely fine crystalline nature of the initial matrix, which would be a consequence of complete breakdown of titanite structural bonds. That titanite rims and deuteric titanite may be relatively unaffected by dissolution in comparison to primary cores indicates that hydrothermal fluids were in disequilibrium with the F-poor, REE-Ti–rich cores, and that F and REE contents may influence titanite stability under certain physicochemical conditions.
Fluids are inferred to have been rich in CO2 and F, based on the presence of fluorocarbonates and calcite as alteration products. Similar alteration assemblages in corroded titanite grains have been reported from numerous granitic rocks (Pan et al. 1993; Morad et al. 2009; Middleton et al. 2013), with inferences for interaction with hydrothermal fluids characterised by high CO2 and HF activity levels. This is supported by experimental studies, which indicate that both CO2 and F promote titanite destabilisation (Hunt and Kerrick 1977; Troitzsch and Ellis 2002).
Suitable element sources in the RDG include the chloritisation of phlogopite (~ 3 wt% F) which likely produced FCO3− fluids and released Fe, Al and/or Mg, which may account for the presence of chlorite in the resultant pseudomorph assemblage. A similar source of F has been suggested in other altered granitic rocks (e.g., Förster 2000; Middleton et al. 2013). In addition to phlogopite, albitisation of adjacent and nearby plagioclase could be a suitable source of Al. The initial destabilisation of titanite in the RDG can be represented by the following equation:
It should be noted that this equation does not consider minor Fe (0.29–0.36 apfu) and Al (0.29–0.49 apfu) contained in the Ti site of titanite, which were likely incorporated into clinochlore during alteration.
The prevalence of REE-phosphates over REE-fluorocarbonates in pseudomorphs, observed in sericite–hematite-altered RDG, would require a change in physicochemical conditions that would promote the availability of free phosphate anions. In the case of the RDG, fluorapatite is the main carrier of PO42− and would thus be the most likely source. Previous mineralogical and chemical studies on these samples (Krneta et al. 2016, 2017) provide substantial evidence of multistage dissolution of fluorapatite and eventual replacement by quartz through interaction with highly acidic, CO2-rich fluids. The initial dissolution of fluorapatite would thus release PO42−, as well as Ca2+ and F−, to the fluid, allowing for precipitation of monazite and xenotime. At this later stage, low temperature and high acidity (low pH) buffer F− in the fluids as HF (e.g. Migdisov et al. 2016). Florencite may also form on behalf of fluorapatite during alteration (Krneta et al. 2016, 2017). The observation of early REE-fluorocarbonate and later REE-phosphate crystallisation is consistent with REE-mineral speciation and paragenesis in the Olympic Dam deposit (Schmandt et al. 2017, 2019).
Titanite in the RDG not only provides insight into granite petrogenesis through the preservation of intrinsic zonation, but also records post-magmatic subsolidus re-equilibration and multiple fluid-rock interaction events (producing early REE-fluorocarbonates and later REE-phosphates) which mimic the alteration and mineralisation sequences at Olympic Dam. As a significant repository for REE (> 5 wt%) in the RDG, the destabilisation of titanite and subsequent element recycling likely contributed to the anomalous REE concentrations reported in the Olympic Dam deposit. The work presented here further substantiates the utility of titanite and its alteration products to reflect physicochemical changes in large intrusion-hosted magmatic-hydrothermal deposits and their enclosing alteration zones.
Conclusions
-
Textural subtypes and geochemistry of titanite track the evolution of the RDG from magmatic to subsolidus and hydrothermal stages. Geochemical indices to distinguish between different titanite subtypes are LREE, Zr, Nb, Th concentrations, Al/Fe ratios and Eu-anomalies.
-
The presence of ilmenite and magnetite inclusions within titanite cores, along with diminishing of Eu-anomaly in magmatic rims, is consistent with a progressive increase in fO2 of the melt.
-
Breakdown of titanite is selective and typically affects cores more than rims. The resultant alteration assemblage is indicative of interaction with dominantly F- and CO2-bearing fluids, with additional elements likely scavenged from concomitant alteration of adjacent phlogopite and plagioclase.
-
Dissolution of fluorapatite in sericite–hematite-altered RDG samples is favoured as the source of PO42− for crystallisation of REE-phosphates in polymineralic pseudomorphs (after titanite).
References
Bea F (1996) Residence of REE, Y, Th and U in granites and crustal protoliths; implications for the chemistry of crustal melts. J Petrol 37:521–552
Bernau R, Franz G (1987) Crystal chemistry and genesis of Nb-, V-, and Al-rich metamorphic titanite from Egypt and Greece. Can Mineral 25:695–705
Brugger J, Lahaye Y, Costa S, Lambert D, Bateman R (2000) Inhomogeneous distribution of REE in scheelite and dynamics of Archaean hydrothermal systems (Mt. Charlotte and Drysdale gold deposits, Western Australia). Contrib Mineral Petrol 139:251–264
Buick IS, Hermann J, Maas R, Gibson RL (2007) The timing of sub-solidus hydrothermal alteration in the Central Zone, Limpopo Belt (South Africa): constraints from titanite U–Pb geochronology and REE partitioning. Lithos 98:97–117
Cao M, Qin K, Li G, Evans NJ, Jin L (2015) In situ LA-(MC)-ICP-MS trace element and Nd isotopic compositions and genesis of polygenetic titanite from the Baogutu reduced porphyry Cu deposit, Western Junggar, NW China. Ore Geol Rev 65:940–954
Che XD, Linnen RL, Wang RC, Groat LA, Brand AA (2013) Distribution of trace and rare earth elements in titanite from tungsten and molybdenum deposits in Yukon and British Columbia, Canada. Can Mineral 51:415–438
Chen Y-X, Zheng Y-F (2015) Extreme Nb/Ta fractionation in metamorphic titanite from ultrahigh-pressure metagranite. Geochim Cosmochim Acta 150:53–73
Cherniak D (1993) Lead diffusion in titanite and preliminary results on the effects of radiation damage on Pb transport. Chem Geol 110:177–194
Cherniak D (1995) Sr and Nd diffusion in titanite. Chem Geol 125:219–232
Cherniak D (2006) Zr diffusion in titanite. Contrib Mineral Petrol 152:639–647
Ciobanu CL, Wade BP, Cook NJ, Mumm AS, Giles D (2013) Uranium-bearing hematite from the Olympic Dam Cu–U–Au deposit, South Australia: a geochemical tracer and reconnaissance Pb–Pb geochronometer. Precambrian Res 238:129–147
Ciobanu CL, Kontonikas-Charos A, Slattery A, Cook NJ, Wade BP, Ehrig K (2017) Short-range stacking disorder in mixed-layer compounds: a HAADF STEM study of bastnäsite-parisite intergrowths. Minerals 7:227
Corfu F (1996) Multistage zircon and titanite growth and inheritance in an Archean gneiss complex, Winnipeg River Subprovince, Ontario. Earth Planet Sci Lett 141:175–186
Corfu F, Stone D (1998) The significance of titanite and apatite U–Pb ages: constraints for the post-magmatic thermal-hydrothermal evolution of a batholithic complex, Berens River area, northwestern Superior Province, Canada. Geochim Cosmochim Acta 62:2979–2995
Courtney-Davies L, Zhu Z, Ciobanu CL, Wade BP, Cook NJ, Ehrig K, Cabral AR, Kennedy A (2016) Matrix-matched iron-oxide laser ablation ICP-MS U–Pb geochronology using mixed solution standards. Minerals 6:85
Courtney-Davies L, Tapster SR, Ciobanu CL, Cook NJ, Verdugo-Ihl MR, Ehrig KJ, Kennedy AK, Gilbert SE, Condon DJ, Wade BP (2019) A multi-technique evaluation of hydrothermal hematite U–Pb isotope systematics: implications for ore deposit geochronology. Chem Geol 513:54–72
Creaser RA (1989) The geology and petrology of Middle Proterozoic felsic magmatism of the Stuart Shelf, South Australia. La Trobe University, Melbourne
Creaser RA (1996) Petrogenesis of a Mesoproterozoic quartz latite-granitoid suite from the Roxby Downs area, South Australia. Precambrian Res 79:371–394
Deer WA, Howie RA, Zussman J (1982) Rock-forming minerals. Orthosilicates Longman, London
Della Ventura G, Bellatreccia F, Williams C (1999) Zr- and LREE-rich titanite from Tre Croci, Vico volcanic complex (Latium, Italy). Mineral Mag 63:123–130
Dodson MH (1973) Closure temperature in cooling geochronological and petrological systems. Contrib Mineral Petrol 40:259–274
Ehrig K, McPhie J, Kamenetsky V (2012) Geology and mineralogical zonation of the Olympic Dam Iron Oxide Cu–U–Au–Ag deposit, South Australia. In: Hedenquist JW, Harris M, Camus F (eds) Geology and genesis of major copper deposits and districts of the world: a tribute to Richard H. Sillitoe, vol 16. SEG Special Publication, Tulsa, pp 237–267
Enami M, Suzuki K, Liou J, Bird DK (1993) Al–Fe3+ and F–OH substitutions in titanite and constraints on their PT dependence. Eur J Mineral 5:219–232
Ferris GM, Schwarz MP, Heithersay P (2002) The geological framework, distribution, and controls of Fe-oxide Cu–Au mineralisation in the Gawler Craton, South Australia: Part 1—geological and tectonic framework. In: Porter TM (ed) Hydrothermal iron oxide copper–gold & related deposits: a global perspective, vol 2. PGC Publishing, Adelaide, pp 9–32
Förster H-J (2000) Cerite-(Ce) and thorian synchysite-(Ce) from the Niederbobritzsch granite, Erzgebirge, Germany: implications for the differential mobility of the LREE and Th during alteration. Can Mineral 38:67–79
Franz G, Spear FS (1985) Aluminous titanite (sphene) from the eclogite zone, south-central Tauern Window, Austria. Chem Geol 50:33–46
Frost BR, Chamberlain KR, Schumacher JC (2001) Sphene (titanite): phase relations and role as a geochronometer. Chem Geol 172:131–148
Garber J, Hacker B, Kylander-Clark A, Stearns M, Seward G (2017) Controls on trace element uptake in metamorphic titanite: implications for petrochronology. J Petrol 58:1031–1057
Green TH, Pearson NJ (1986) Rare-earth element partitioning between sphene and coexisting silicate liquid at high pressure and temperature. Chem Geol 55:105–119
Gromet LP, Silver LT (1983) Rare earth element distributions among minerals in a granodiorite and their petrogenetic implications. Geochim Cosmochim Acta 47:925–939
Harlov D, Tropper P, Seifert W, Nijland T, Förster H-J (2006) Formation of Al-rich titanite (CaTiSiO4O–CaAlSiO4OH) reaction rims on ilmenite in metamorphic rocks as a function of fH2O and fO2. Lithos 88:72–84
Hayden LA, Watson EB (2007) Ti-oxide saturation in hydrous siliceous melts and its bearing on Ti-thermometry of quartz and zircon. Earth Planet Sci Lett 258:561–568
Hayden LA, Watson EB, Wark DA (2008) A thermobarometer for sphene (titanite). Contrib Mineral Petrol 155:529–540
Hunt J, Kerrick D (1977) The stability of sphene; experimental redetermination and geologic implications. Geochim Cosmochim Acta 41:279–288
Ismail R, Ciobanu CL, Cook NJ, Teale GS, Giles D, Mumm AS, Wade B (2014) Rare earths and other trace elements in minerals from skarn assemblages, Hillside iron oxide–copper–gold deposit, Yorke Peninsula, South Australia. Lithos 184–187:456–477
Jagodzinski E (2005) Compilation of SHRIMP U–Pb geochronological data, Olympic Domain, Gawler Craton. South Australia, 2001–2003: Geoscience Australia Record 2005/020
Johnson JP, Cross KC (1995) U–Pb geochronological constraints on the genesis of the Olympic Dam Cu–U–Au–Ag deposit, South Australia. Econ Geol 90:1046–1063
Kohn MJ (2017) Titanite petrochronology. Rev Mineral Geochem 83:419–441
Kontonikas-Charos A, Ciobanu CL, Cook NJ (2014) Albitization and redistribution of REE and Y in IOCG systems: insights from Moonta-Wallaroo, Yorke Peninsula, South Australia. Lithos 208–209:178–201
Kontonikas-Charos A, Ciobanu CL, Cook NJ, Ehrig K, Krneta S, Kamenetsky VS (2017) Feldspar evolution in the Roxby Downs Granite, host to Fe-oxide Cu–Au–(U) mineralisation at Olympic Dam, South Australia. Ore Geol Rev 80:838–859
Kontonikas-Charos A, Ciobanu CL, Cook NJ, Ehrig K, Krneta S, Kamenetsky VS (2018a) Rare earth element geochemistry of feldspars: examples from Fe-oxide Cu–Au systems in the Olympic Cu–Au Province, South Australia. Mineral Petrol 112:145–172
Kontonikas-Charos A, Ciobanu CL, Cook NJ, Ehrig K, Ismail R, Krneta S, Basak A (2018b) Feldspar mineralogy and rare-earth element (re) mobilization in iron-oxide copper gold systems from South Australia: a nanoscale study. Mineral Mag 82:S173–S197
Krneta S, Ciobanu CL, Cook NJ, Ehrig K, Kontonikas-Charos A (2016) Apatite at Olympic Dam, South Australia: a petrogenetic tool. Lithos 262:470–485
Krneta S, Ciobanu CL, Cook NJ, Ehrig K, Kontonikas-Charos A (2017) Rare earth element behaviour in apatite from the Olympic Dam Cu–U–Au–Ag deposit, South Australia. Minerals 7:135
Liferovich RP, Mitchell RH (2005) Composition and paragenesis of Na-, Nb- and Zr-bearing titanite from Khibina, Russia, and crystal-structure data for synthetic analogues. Can Mineral 43:795–812
Macmillan E, Cook NJ, Ehrig K, Ciobanu CL, Pring A (2016) Uraninite from the Olympic Dam IOCG-U–Ag deposit: linking textural and compositional variation to temporal evolution. Am Mineral 101:1295–1320
Macmillan E, Cook NJ, Ehrig K, Pring A (2017) Chemical and textural interpretation of late-stage coffinite and brannerite from the Olympic Dam IOCG-Ag–U deposit. Mineral Mag 81:1323–1366
Mahood G, Hildreth W (1983) Large partition coefficients for trace elements in high-silica rhyolites. Geochim Cosmochim Acta 47:11–30
McLeod GW, Dempster TJ, Faithfull JW (2010) Deciphering magma-mixing processes using zoned titanite from the Ross of Mull Granite, Scotland. J Petrol 52:55–82
Middleton AW, Förster H-J, Uysal IT, Golding SD, Rhede D (2013) Accessory phases from the Soultz monzogranite, Soultz-sous-Forêts, France: implications for titanite destabilisation and differential REE, Y and Th mobility in hydrothermal systems. Chem Geol 335:105–117
Migdisov A, Williams-Jones A, Brugger J, Caporuscio F (2016) Hydrothermal transport, deposition, and fractionation of the REE: experimental data and thermodynamic calculations. Chem Geol 439:13–42
Morad S, El-Ghali MA, Caja MA, Al-Ramadan K, Mansurbeg H (2009) Hydrothermal alteration of magmatic titanite: evidence from Proterozoic granitic rocks, southeastern Sweden. Can Mineral 47:801–811
Nakada S (1991) Magmatic processes in titanite-bearing dacites, central Andes of Chile and Bolivia. Am Mineral 76:548–560
Oberti R, Smith DC, Rossi G, Caucia F (1991) The crystal-chemistry of high-aluminium titanites. Eur J Mineral 3:777–792
Olierook HK, Taylor RJ, Erickson TM, Clark C, Reddy SM, Kirkland CL, Jahn I, Barham M (2019) Unravelling complex geologic histories using U–Pb and trace element systematics of titanite. Chem Geol 504:105–122
Pan Y, Fleet ME, MacRae ND (1993) Late alteration in titanite (CaTiSiO5): redistribution and remobilization of rare earth elements and implications for U/Pb and Th/Pb geochronology and nuclear waste disposal. Geochim Cosmochim Acta 57:355–367
Piccoli P, Candela P, Rivers M (2000) Interpreting magmatic processes from accessory phases: titanite—a small-scale recorder of large-scale processes. Earth Environ Sci Trans R Soc Edinb 91:257–267
Prowatke S, Klemme S (2005) Effect of melt composition on the partitioning of trace elements between titanite and silicate melt. Geochim Cosmochim Acta 69:695–709
Ribbe PH (1980) Titanite. Rev Mineral Geochem 5:137–154
Russell JK, Groat L, Halleran AA (1994) LREE-rich niobian titanite from Mount Bisson, British Columbia; chemistry and exchange mechanisms. Can Mineral 32:575–587
Schmandt DS, Cook NJ, Ciobanu CL, Ehrig K, Wade BP, Gilbert S, Kamenetsky VS (2017) Rare earth element fluorocarbonate minerals from the Olympic Dam Cu–U–Au–Ag deposit, South Australia. Minerals 7:202
Schmandt DS, Cook NJ, Ciobanu CL, Ehrig K, Wade BP, Gilbert S, Kamenetsky VS (2019) Rare earth element phosphates from the Olympic Dam Cu–U–Au–Ag deposit, South Australia: recognizing temporal-spatial controls on REE mineralogy in an evolved IOCG system. Can Mineral 57:1–22
Scott DJ, St-Onge MR (1995) Constraints on Pb closure temperature in titanite based on rocks from the Ungava orogen, Canada: implications for U–Pb geochronology and PTt path determinations. Geology 23:1123–1126
Seifert W (2005) REE-, Zr-, and Th-rich titanite and associated accessory minerals from a kersantite in the Frankenwald, Germany. Mineral Petrol 84:129–146
Skirrow RG, Bastrakov EN, Barovich K, Fraser GL, Creaser RA, Fanning CM, Raymond OL, Davidson GJ (2007) Timing of iron oxide Cu–Au–(U) hydrothermal activity and Nd isotope constraints on metal sources in the Gawler craton, South Australia. Econ Geol 102:1441–1470
Smith M, Henderson P, Jeffries T, Long J, Williams C (2004) The rare earth elements and uranium in garnets from the Beinn an Dubhaich Aureole, Skye, Scotland, UK: constraints on processes in a dynamic hydrothermal system. J Petrol 45:457–484
Smith M, Storey C, Jeffries T, Ryan C (2009) In situ U–Pb and trace element analysis of accessory minerals in the Kiruna district, Norrbotten, Sweden: new constraints on the timing and origin of mineralization. J Petrol 50:2063–2094
Storey CD, Smith M (2017) Metal source and tectonic setting of iron oxide–copper–gold (IOCG) deposits: evidence from an in situ Nd isotope study of titanite from Norrbotten, Sweden. Ore Geo Rev 81:1287–1302
Storey C, Smith M, Jeffries T (2007) In situ LA-ICP-MS U–Pb dating of metavolcanics of Norrbotten, Sweden: records of extended geological histories in complex titanite grains. Chem Geol 240:163–181
Tiepolo M, Oberti R, Vannucci R (2002) Trace-element incorporation in titanite: constraints from experimentally determined solid/liquid partition coefficients. Chem Geol 191:105–119
Troitzsch U, Ellis DJ (2002) Thermodynamic properties and stability of AlF-bearing titanite CaTiOSiO4–CaAlFSiO4. Contrib Mineral Petrol 142:543–563
Vuorinen JH, Hålenius U (2005) Nb-, Zr- and LREE-rich titanite from the Alnö alkaline complex: crystal chemistry and its importance as a petrogenetic indicator. Lithos 83:128–142
Wones DR (1989) Significance of the assemblage titanite + magnetite + quartz in granitic-rocks. Am Mineral 74:744–749
Xirouchakis D, Lindsley DH (1998) Equilibria among titanite, hedenbergite, fayalite, quartz, ilmenite, and magnetite: experiments and internally consistent thermodynamic data for titanite. Am Mineral 83:712–725
Xu L, Bi X, Hu R, Tang Y, Wang X, Xu Y (2015) LA-ICP-MS mineral chemistry of titanite and the geological implications for exploration of porphyry Cu deposits in the Jinshajiang-Red River alkaline igneous belt, SW China. Mineral Petrol 109:181–200
Acknowledgements
BHP Olympic Dam is kindly thanked for providing financial support and access to Olympic Dam samples and facilities. Staff at Adelaide Microscopy are also acknowledged for assistance with microanalysis. This work was supported by the ARC Research Hub for Australian Copper–Uranium (Grant IH130200033), co-supported by BHP Olympic Dam and the South Australian Mining and Petroleum Services Centre of Excellence. We gratefully acknowledge the comments from two anonymous reviewers and Associate Editor Chris Ballhaus who assisted us to clarify ideas expressed in this contribution.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Chris Ballhaus.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kontonikas-Charos, A., Ehrig, K., Cook, N.J. et al. Crystal chemistry of titanite from the Roxby Downs Granite, South Australia: insights into petrogenesis, subsolidus evolution and hydrothermal alteration. Contrib Mineral Petrol 174, 59 (2019). https://doi.org/10.1007/s00410-019-1594-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00410-019-1594-2