Abstract
In principle, compositional profiling of the near-rim region of minerals can provide insight into cooling rates, but presumes that loss or gain of material from the crystal rim is not kinetically restricted. Trace element depth profiles collected for Zr, Hf, Ta, Nb, and U in amphibolite-facies rutile grains of the Catalina Schist, southern California, show significant variability within a single rock: Profiles of the same element among different grains can have significantly different slopes, grains with indistinguishable Zr profiles show vastly different Nb profiles, and grains with indistinguishable Nb profiles show different Zr profiles. Textural and kinetic idiosyncrasies within the matrix apparently affect the ability of specific crystals to accept or release trace elements, and impugn the common assumption that mineral surfaces maintain equilibrium at amphibolite-facies conditions. A new model that limits the flux of Zr from rutile grains helps explain commonly observed compositional profiles, and implies that inversion of compositional profiles assuming equilibrium among grain surfaces will invariably overestimate cooling rates. Few grains may record the low closure temperatures that experimentally determined diffusivities imply. Rather, higher temperatures will be retained, depending on the proximity of reactants and products in the matrix. Silicon diffusion does not control Zr reequilibration in rutile, and relative diffusion coefficients (D’s) of trace elements in rutile are D Zr ~ D Hf ~ 10D Nb ~ 20D Ta ~ 40D U.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rutile, the most common polymorph of TiO2, enjoys an ascending role in the investigation of petrologic processes. Straightforward analysis of Zr contents and, in some cases, U–Pb ratios via in situ methods constrain temperatures and ages of metamorphism. Indeed the Zr-in-rutile thermometer (Zack et al. 2004; Watson et al. 2006; Ferry and Watson 2007; Tomkins et al. 2007; Thomas et al. 2010) is now one of the most commonly applied thermometers in rutile-bearing rocks. Simultaneously, experimental constraints on diffusion rates of the trace elements Pb, Zr, Hf, Nb, and Ta suggest that diffusive reequilibration should reset rutile compositions during cooling on scales of microns to tens of microns over a key range of temperatures between ~800 and ~600 °C (Cherniak et al. 2007; Marschall et al. 2013). Thus, diffusion profiles potentially constrain cooling rates during initial stages of cooling for amphibolite- and granulite-facies rocks (e.g., Smye and Stockli 2014).
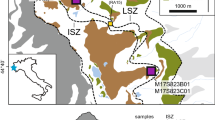
In this study, we exploited the depth profiling capabilities of laser ablation inductively coupled plasma mass spectrometry (LA-ICP-MS) to characterize trace element zoning in the outer ~15 µm of rutile grains from the Catalina Schist, southern California (Fig. 1a). Samples were collected from a subduction mélange unit that reached relatively uniform peak metamorphic conditions approaching 700 °C, 1 GPa, before being exhumed and cooled (Platt 1975, 1976; Sorensen and Barton 1987). The duration at the peak of metamorphism was <5 Ma, and initial cooling rates were between ~25 and ~100 °C/Ma (Fig. 1b; Mattinson 1986; Grove et al. 2008). Our original hope was to use diffusion profiles in rutile to better constrain rates of initial cooling that might be linked to tectonic processes. In that respect, our intentions almost completely failed. Depth profiles show such profound differences among grains within individual samples that a key assumption of modeling cooling rates—that crystal surfaces maintain rock-wide equilibrium with surrounding matrix minerals—was certainly false. Yet this failure (of tectonic interests) provides startling new insight into the behavior of trace elements during metamorphism.
a Combined metamorphic zone—geological map of Santa Catalina Island, California (Platt 1975, 1976; Grove et al. 2008). b Temperature–time constraints for amphibolite-facies rocks; likely rates of initial cooling range from 25 to 100 °C (Mattinson 1986; Grove et al. 2008; data for rutile from Kohn and Penniston-Dorland, unpubl). Grt garnet, Ttn titanite, Rt rutile, Hbl hornblende, Ms muscovite
Here, we present near-rim zoning data from rutile grains separated from several rocks of the Catalina Schist. We demonstrate that equilibrium could not have been maintained among all rutile grains in each rock during cooling and propose a new model to explain data trends. Finally, we explore the implications of our data for several petrogenetic applications including geospeedometry, geothermometry, and closure temperatures. While less useful for inferring tectonic processes, our data open a new avenue for understanding element mobility during metamorphism.
Samples
Samples were collected from the amphibolite-facies mélange unit of the Catalina Schist on Santa Catalina Island, California, USA. This unit has been subject to numerous studies ranging from structural/metamorphic characterizations, investigations of fluid flow, and integration of thermochronologic and petrologic data into tectonic models (e.g., Platt 1975; Sorensen 1988; Bebout and Barton 1993, 2002; Grove et al. 2008; Penniston-Dorland et al. 2012). Three of the samples we analyzed (A14-29, A14-59c, and A15-01b) are from mafic amphibolite blocks, 1–10 m in exposed diameter. The other three samples are from garnet quartzite blocks (A14-59a and A15-10) and a felsic gneiss block (A14-57b), all <1 m in exposed diameter. All blocks are surrounded by finer-grained amphibolite-facies sheet-silicate and amphibole-rich matrix, and samples were collected as close to block cores as possible to minimize potential effects of fluid flow that preferentially affected block margins. Typically, the amphibolites contain 40–80 % hornblende, 5–20 % garnet, 0–5 % epidote, trace to 40 % plagioclase that is partly replaced with an epidote + albite symplectite, trace to 10 % quartz, 1–2 % rutile, and trace to 2 % apatite (Fig. S1). Quartz is not always present, but compositions of amphiboles in quartz-absent rocks indicate near-saturation (a Qtz ≥ ~0.95; Penniston-Dorland and Kohn, unpubl. data). The origin of the epidote symplectites is equivocal and could represent a near-peak-metamorphic epidote-forming reaction. The garnet quartzites contain up to 98 % quartz, with 2–5 % garnet, trace muscovite or talc, rutile, and apatite (Fig. S1). The felsic gneiss contains ~30 % plagioclase that is partly replaced with an epidote–plagioclase symplectite, ~50 % quartz, ~5 % garnet that is altered along fractures to muscovite and chlorite, ~15 % fine- and coarse-grained muscovite, and trace matrix epidote, rutile, magnetite, graphite, and titanite (Fig. S1). Zircon crystals are ubiquitous, ranging in size up to ~5 µm in diameter in the amphibolites and quartzites, and up to 50 µm in diameter in the felsic gneiss. The felsic gneiss does contain evidence for retrograde fluid infiltration (fine-grained muscovite, altered garnet, possibly graphite), but clear evidence for retrograde fluid infiltration in the other rocks is not apparent.
Chemical substitutions and diffusion in rutile
Van Orman and Crispin (2010) and Marschall et al. (2013) provide helpful overviews of geochemical substitutions and diffusion mechanisms in rutile, which we summarize here. All trace elements of interest to this study substitute for Ti in the octahedral site of rutile, but smaller cations (e.g., Si4+, Ti4+, Cr3+, Fe3+) can also occupy interstitial positions, whereas larger cations (Nb5+, Ta5+, Zr4+, Hf4+, Pb2+, U4+) generally do not. Smaller cations diffuse faster than larger cations, probably because diffusion of interstitial cations is considerably faster than across octahedra. Chemically, Zr4+, Hf4+, and U4+ can substitute directly for Ti4+, but Nb5+ and Ta5+ must undergo coupled substitution, likely either (Nb5+, Ta5+) + (Cr3+, Fe3+) = 2 Ti4+, or 2(Nb5+, Ta5+) + O2− = 2 Ti4+ + an oxygen vacancy (rutile can have abundant Ti3+ and oxygen vacancies at low \(f_{{{\text{O}}_{2} }}\)). Diffusion of trivalent cations and oxygen in rutile is sufficiently fast that they should not rate limit Nb or Ta diffusion.
Several experimental studies have investigated diffusion of high field strength elements in rutile, but results for the same element from different studies are difficult to reconcile. Sasaki et al. (1985) used a radiotracer technique to investigate Zr diffusion in synthetic rutile between 1100 and 1500 °C at high \(f_{{{\text{O}}_{2} }}\). Diffusivities increased with decreasing \(f_{{{\text{O}}_{2} }}\), and diffusion parallel to c was about five times slower than diffusion perpendicular to c, the opposite of the behavior of other cations. In contrast, Cherniak et al. (2007) employed diffusion couples (Zr-bearing HfSiO4 powder or ZrSiO4 with single synthetic and natural rutile crystals) between 750 and 1100 °C, and found diffusion rates c. 3 orders of magnitude lower than Sasaki et al. (1985) and no dependence on \(f_{{{\text{O}}_{2} }}\) or purity of rutile. Possibly, low concentrations of Zr diffuse rapidly via interstitials, whereas higher concentrations of Zr diffuse slowly via octahedral vacancies (Cherniak et al. 2007). Extrapolating the results of Sasaki et al. (1985) to natural rocks requires calculating the concentration of interstitial Ti4+ cations, which depends on both \(f_{{{\text{O}}_{2} }}\) and the concentration of impurities. Because no method is proposed for calculating how numerous variable valence impurities in natural rutile affect Ti4+ interstitials, we cannot apply the results of Sasaki et al. (1985) to geological materials. Consequently, we use the results of Cherniak et al. (2007) for diffusion of Zr (and Hf) in our discussion and models.
For Nb, Sheppard et al. (2007, 2009) measured diffusion rates between 800 and 1300 °C, but complex composition profiles are difficult to interpret. Van Orman and Crispin (2010) express skepticism that these results have value. Marschall et al. (2013) investigated Nb and Ta diffusion between 850 and 1250 °C using a Nb- and Ta-enriched thin film plated to rutile surfaces and a diffusion couple between rutile and Nb- and Ta-enriched silicate melt. We use the results of Marschall et al. (2013) in our discussion and models, which imply diffusivities at 500–750 °C c. 3 orders of magnitude lower than Sheppard et al. (2007, 2009).
Methods
Compositional measurements
Samples were crushed and rutile grains with flat faces were handpicked for analysis and mounted by pressing into a flattened putty layer with dimensions of a standard petrographic thin section (Fig. S2). This mounting approach allows adjacent mounting of removable samples with permanent standards and minimizes surface topography that might affect gas flow through the laser ablation cell. Laser ablation ICP-MS analysis generally followed techniques described in Kohn and Corrie (2011) with a spot size of 65–80 µm, a repetition rate of 5 Hz, and a fluence of 5–6 J/cm2. A single preablation pulse to clear any surface contamination was succeeded by ablation times of 30 s, yielding depths of approximately 15 µm. Analytes included 29Si (50 ms), 49Ti (10 ms), 51V (10 ms), 52Cr (10 ms), 57Fe (10 ms), 91Zr (50 ms), 93Nb (10 ms), 95Mo (10 ms), 118Sn (10 ms), 177Hf (40 ms), 181Ta (40 ms), and 238U (40 ms), but detailed results are presented here for only Zr, Nb, and U. High background count rates for 29Si and susceptibility to even the slightest surface contamination by silicates precluded interpretation of Si profiles. Profiles for Hf and Ta parallel Zr and Nb and are used for inferring relative diffusivities. Count times yielded approximately 90 points per profile (0.15–0.2 µm/point). Raw counts were normalized to Ti and standardized against R10 rutile (Luvizotto et al. 2009) and a rutile megacryst from Rietfontein, whose composition was determined via electron probe microanalysis and comparison to the R10 standard. Data were processed using Iolite 2.5 (Paton et al. 2011).
A key concern is whether analytical artifacts bias apparent near-rim zoning. During the initial stages of ablation, count rates for all elements increase rapidly and in parallel. Analytical windows were chosen with a lower bound that initiated as early as possible in the data stream, but still returned constant concentrations on unzoned crystals. This window initiates ~1 s after the break in count rate from the preablation background signal, and with an ablation rate of ~0.5 µm/s, we assume that the first reliable data reflect a distance ~0.5 µm from the crystal surface. Including the first ~1 s data increases data scatter in the near-edge region, but does not change the principal conclusions of our study. The upper bound was taken where the count rate for Ti started to decrease (after the laser was turned off). Inadvertently, we analyzed a few rutile crystals either that were isolated from reequilibration or whose surfaces were fractures rather than external crystal faces. These crystals return constant concentration profiles (Fig. S3), so we do not believe our analytical windows introduce compositional artifacts. Profiles with constant concentrations for all elements were excluded from data compilations and interpretations.
Other analytical artifacts can include contamination (mixing) of deeper analysis with material ablated from the original surface or ablation pit walls, and washout. We discuss these issues in detail in the Supporting Data section, but contamination from the original surface and walls of the ablation pit are minor (<1 %) and can be neglected for Ti, Zr, Nb, Hf, Ta, and U. Washout serves to smear composition profiles, flattening them typically by ~10 %, but in some cases up to 25 %. However, two key results are consistently preserved: the shapes of continuous profiles and the apparent rim composition. Our interpretations emphasize rim compositions and profile shapes, so washout artifacts should not compromise our primary conclusions. Flattening artifacts will tend to lead to overestimated diffusion rates for steep profiles, and underestimated cooling rates, but tests suggest bias of less than a factor of 2, which is smaller than other sources of error.
Modeling
We modeled theoretical compositional profiles for Zr using an implicit finite difference scheme. Experimental evidence constrains diffusion rates (D) for Zr parallel to the c-axis and for Hf both parallel and perpendicular to the c-axis (Cherniak et al. 2007). Nearly all our profiles were collected perpendicular to the c-axis as determined from crystal elongation. Because D Zr is indistinguishable from D Hf parallel to c, we assumed that D Zr equaled D Hf for diffusion perpendicular to c. This assumption is supported by the similarity of diffusion profile shapes for Zr and Hf (Supplemental data). D Hf parallel to c (“D∥c ”) is 4–20 times faster than D Hf perpendicular to c (“D⊥c ”) over the temperature range of interest, so, taking into account the elongation of rutile crystals parallel to c, model crystals were assumed to have a spherical geometry. Because Zr diffusion is slow, affecting principally the outer 10–20 µm, the exact crystal geometry is unimportant—a cylindrical or slab geometry does not significantly change our interpretations. Grain radius was set at 100 µm, grid spacing (∆x) at 0.5 µm, maximum r-value at 0.1 (r = D∆t/∆x 2, where ∆t = time increment), and maximum temperature step at 1 °C.
Limitations to the maximum Zr flux play a key role in our interpretations of diffusion profiles (Fig. 2). Nearly all models of diffusive resetting assume that the boundary condition on the surface of the crystal reflects equilibrium with other minerals. For example, most modelers would assume that the Zr-in-rutile thermometer equilibrium:
defines Zr concentrations on the crystal surface and controls diffusional resetting of Zr in rutile with a strong temperature dependence (Watson et al. 2006):
In real rocks, however, ZrO2 and SiO2 must exit rutile and quartz, diffuse through the rock matrix to a zircon crystal, and attach as ZrSiO4 (Fig. 2a). Thus, the loss of Zr from a rutile crystal may be further limited by diffusion rates of ZrO2 or SiO2 along grain boundaries and/or through an intergranular medium, by the detachment or dissolution of SiO2 from quartz, or by attachment or precipitation of ZrSiO4 onto zircon. Grain size may also affect the efficacy of mass transport (Carlson and Gordon 2004), as will availability of fluids. For example, unless replenished from external sources, hydrous fluids along grain boundaries should be consumed during cooling to form hydrous minerals, and buffering reactions should further decrease dissolved and adsorbed H2O and OH contents in minerals and along grain boundaries (e.g., see Yardley 1981; Kohn 1999). Thus, intergranular transport may occur rapidly during prograde or near-peak metamorphism through a fluid medium along relatively open grain boundaries, whereas during cooling, transport may occur in the absence of a fluid along increasingly tighter grain boundaries. Ultimately, these processes may limit the maximum flux of ZrO2 out of the rutile crystal more than D Zr in rutile.
a Concept of flux limitations in the matrix. The rate of zircon formation and loss of Zr from rutile may be limited by slow diffusion of Zr or Si through the matrix, by detachment of SiO2 (dissolution of quartz), or by attachment of ZrSiO4 (precipitation of zircon). Rt rutile, Qtz quartz, Zrn zircon. b Schematic of temperature dependence of Zr flux. If rutile crystal surfaces maintain equilibrium with the matrix (“Equilibrium” models), flux rises to a maximum soon after cooling commences, then falls approximately exponentially. The High-T flux threshold model limits the maximum flux of Zr such that the total flux at the end of a simulation is a fixed proportion of the Equilibrium flux, e.g., 25 % of the total. Low-T zero flux cuts off all loss of Zr from rutile at a fixed temperature
To explore the compositional implications of these concepts, we first modeled Zr loss assuming equilibrium of the grain surface with zircon and quartz (Eqs. 1 and 2; “Equilibrium flux”), and monitored the Zr flux (red curve labeled “No flux threshold,” Fig. 2b), which we integrated to determine the total Zr flux. These models included different cooling rates from 10 to 100 °C/Ma, and are referred to as “Equilibrium models.” Next, we modeled Zr loss by imposing limitations on Zr fluxes. These flux limitation models assumed a cooling rate of 25 °C/Ma. For the simplest of these (“High-T flux threshold”), we imposed a maximum flux threshold (total ppm Zr/ °C) such that the total integrated flux at the end of a simulation was a fixed proportion (10–75 %) of the total integrated Zr flux for the 25 °C/Ma Equilibrium model. For example, if the total Zr flux in the Equilibrium model was 1 × 106 (arbitrary units), for the 10 % model, we iteratively adjusted the maximum flux threshold (purple line labeled “High-T flux threshold,” Fig. 2b) until the total Zr flux at the end of the model simulation was 1 × 105. We also explored the use of flux thresholds that assumed an exponential dependence of flux on temperature; these yield similar results to fixed thresholds and are not presented here.
During execution of the flux limitation models, if the rim composition was equal to the equilibrium Zr concentration and the equilibrium Zr flux over a temperature increment was below the maximum threshold, the composition on the rim remained at the equilibrium value. If the equilibrium Zr flux exceeded the maximum flux threshold, however, we adjusted the boundary concentration iteratively until the flux for that temperature increment equaled the maximum flux. That is, the boundary condition shifted from a fixed concentration (Fick’s second law) to a fixed flux (Fick’s first law).
Finally, we explored the possibility that at some low temperature, Zr loss might simply shut off (black dashed line labeled “Low-T zero flux,” Fig. 2b). Physically this might occur if detachment or attachment dramatically slows or if grain boundary diffusion decreases abruptly with the loss of fluid or available OH during cooling (i.e., decreasing \(a_{{{\text{H}}_{2} {\text{O}}}}\); Yardley 1981; Kohn 1999). For these models, the High-T flux was set at the High-T flux limit, but at 550 °C, the flux limit was set to zero. This allowed Zr to interdiffuse within the crystal as temperature decreased, but not out of the crystal.
Equilibrium models assume that the crystal surface of rutile maintains equilibrium with zircon and quartz throughout the cooling history, i.e., that the matrix of the rock does not limit the flux of Zr out of rutile. Flux limitation models implicitly assume that kinetic processes within the rock matrix somehow limit the rate of loss of Zr. We do not speculate on whether the High-T flux threshold reflects grain boundary diffusion, diffusion through an intergranular medium, or detachment/attachment limitations (and similarly for Low-T zero flux). The Low-T zero-flux models then assume that an additional process limits Zr flux through the matrix at a lower temperature.
Estimating relative diffusivities
The shapes of the composition profiles potentially permit estimates of relative diffusivities between element pairs. Although quantitatively incorrect, an error-function solution to a fixed-boundary one-dimensional halfspace provides a first-order approximation:
where the projected rim composition is C rim, interior composition is C interior, and composition at any point x is C x . The outermost composition data (C x ) are regressed versus distance (x) according to Eq. 3, adjusting assumed rim concentration to force a zero intercept and taking an innermost composition as C interior. The regression slope (=1/√4Dt) is inverted and squared to solve for relative D·t values (t = duration of diffusion). Profiles in grains from the same sample formed during exactly the same thermal history, so t is constant, and ratios of D·t values correspond with ratios of D.
Equation 3 is incorrect in detail because it assumes a fixed boundary composition, whereas compositions changed through time and in fact must have differed among grains in the same rock. Such calculations are also not fully quantitative because we cannot a priori constrain how boundary conditions changed with temperature. Nonetheless, if boundary conditions change systematically through time, the steepness versus flatness of profiles, interpreted using Eq. 3, should provide an approximation of relative D’s. To quantify accuracies, we calculated D-ratios from our forward models(whose boundary conditions also violate an error-function-type solution, but whose D values are known) and recovered D Zr,∥c > D Zr,⊥c ~ D Nb,∥c > D Ta,∥c , as expected from the experimental results of Cherniak et al. (2007) and Marschall et al. (2013). Calculated ratios, however, differed from ratios that were input into the models by factors of 3–5. To reduce the impact of single-profile idiosyncrasies, we averaged all profiles for a rock rather than modeling each individually. These averages even out idiosyncrasies so that the modeled profile more likely reflects a relatively systematic change in boundary conditions for each specific element.
Results
Compositional profiles
Most generally, compositional profiles show evidence for diffusive resetting of Zr, Nb, and U (i.e., decreases in Zr toward the rims of grains, typically with concomitant increases in Nb and U). For two reasons, we interpret these trends to reflect diffusion rather than growth during cooling. First, projection of diffusion experiments to metamorphic conditions implies that diffusive resetting over the outer several microns in rutile is inevitable. Decreasing Zr is expected through diffusive resetting of the Zr-in-rutile thermometer. Second, no source of Ti or Nb is available in the quartzites and felsic gneiss to drive new rutile growth. If retrograde fluid infiltration drove late-stage rutile growth, we might expect different blocks to show different types of zoning (depending on the trace element composition of the fluid), yet the broad similarities among profiles among grains and between blocks (e.g., all show decreasing Zr and most show increasing Nb and U) suggest that all profiles reflect diffusion. Significantly, although broadly similar profiles appear among different grains in the same rock, important differences also occur that are key to our interpretations (Figs. 3, 4, 5, S4).
Zoning in rutile grains from sample A15-10, illustrating the range of zoning patterns (lavender symbols) and emphasizing three specific profiles (different colored symbols labeled 1–3). a Zr. Profiles are relatively flat toward grain interiors, and all concentrations decrease toward the rim. Profiles 1–3 are virtually indistinguishable, suggesting they experienced nearly identical Zr boundary conditions during cooling. The average Zr-in-rutile temperature profile decreases toward rim (solid line), as expected for diffusive resetting during cooling. b Nb. The slopes of the Nb concentration profiles vary toward the rim (increase, decrease, or remain approximately flat). Profiles 1–3 must have experienced different Nb boundary conditions. c U. With the exception of one grain with a high U content, U profiles are either flat or increase toward the rim. Profiles 2 and 3 remain nearly flat (constant U boundary condition) while profile 1 increases (different U boundary conditions)
Zoning in rutile grains from sample A14-29, illustrating the range of zoning patterns (symbol colors as described for Fig. 3) and emphasizing three specific profiles (1–3). a Nb. Profiles are relatively flat toward grain interiors, and generally show concentration increases toward rim. Profiles 1–3 are virtually indistinguishable, suggesting they experienced nearly identical Nb boundary conditions during cooling. b Zr. Profiles of Zr concentration and the average Zr-in-rutile temperature (solid red line) are relatively flat toward grain interiors and generally decrease toward rim. Profile 2 has a different Zr concentration in interior, possibly a relict of rutile growth at a lower temperature, but projects toward a similar rim Zr content as profile 1, suggesting similar rim boundary conditions. Profile 3 projects to a higher Zr concentration on the rutile rim than profiles 1 and 2, implying a different Zr boundary condition
Zoning in rutile grains from sample A14-59a, illustrating the range of zoning patterns (symbol colors as described for Fig. 3) and emphasizing three specific profiles (1–3). a Zr. Profiles of concentration and the average Zr-in-rutile temperature (solid red line) are relatively flat toward grain interiors and generally decrease toward rim. Profiles 1–3 show different interior compositions, but project to similar rim compositions, suggesting they converged toward similar Zr boundary conditions during cooling. b Nb. Concentrations generally increase toward rim. Profiles 1 and 2 have similar interior concentrations (unlike Zr). Profiles 2 and 3 project toward similar rim concentrations (like Zr), but profile 1 projects to a much higher Nb concentration, implying a different Nb boundary condition. c U. Concentrations generally increase toward rim. Profiles 1 and 2 show similar trends, whereas profile 3 may project toward a different rim U concentration
Rutile grains in sample A15-10 (Fig. 3) show consistent decreases in Zr and increases in U toward their rims, but Nb profiles vary ranging from decreasing toward the rim to increasing sharply at the rim. Three essentially indistinguishable Zr profiles correspond with completely different Nb trends: Nb in profile 1 increases toward the rim, profile 2 remains flat, and profile 3 decreases. Profile 1 shows a different U rim concentration than profiles 2 and 3.
Rutile grains in sample A14-29 (Fig. 4) show broadly consistent increases in Nb and decreases in Zr toward the rutile rims. Three essentially indistinguishable Nb profiles yield differently sloped Zr profiles: The rim composition for profile 3 differs from profiles 1 and 2, whereas the interior composition for profile 2 differs from profiles 1 and 3.
Rutile grains in sample A14-59a (Fig. 5) consistently show decreasing Zr and increasing Nb and U toward crystal rims. In profiles 1–3, Zr rim compositions are indistinguishable, but interior compositions range widely (300–600 ppm). Profiles 2 and 3 also have indistinguishable Nb rim compositions and different Nb contents in the interior, but profile 1 has the same interior Nb concentration as profile 2 and trends toward a significantly different rim composition. Data scatter for U in the rutile interiors precludes definitive comparison, but profile 3 may trend toward a lower rim U content compared to profiles 1 and 2.
We calculated relative D Zr, D Hf, D Nb, and D Ta from sample A14-29 because individual profiles in this sample show the greatest profile-to-profile consistency. Regressed slopes (Fig. S5) including 2σ errors are 0.136 ± 0.005 (Zr), 0.14 ± 0.01 (Hf), 0.42 ± 0.04 (Nb), and 0.58 ± 0.06 (Ta). Average data for U from sample A14-59a, which are most systematic of the samples we analyzed, suggest a slope of 0.69 ± 0.14, compared to a slope of 0.11 ± 0.01 for Zr profiles from the same rutile grains (Fig. S5). Inverting these slopes and squaring (cf. Eq. 3) implies that D Zr ~ D Hf ~ 10D Nb ~ 20D Ta ~ 40D U (Figs. S5–6). Washout from the ablation cell tends to flatten profiles more for steep gradients than for shallow gradients, so diffusivity ratios are likely underestimated for Zr and Hf relative to Nb, and for Nb relative to U. Taking factor of ~4 uncertainties into account, these data imply that Zr and Hf diffuse faster than Nb, Ta, and U, and that Nb likely diffuses faster than U. Because data were derived from the same profiles, D-value differences reflect the same crystallographic orientation.
Discussion
Trace elements in rutile—where do they come from, where do they go?
The trace elements of interest to this study have different sources and sinks in the matrix, so the controls by which rutile takes up Nb, Ta, and U or loses Zr and Hf may differ. In the rocks we studied, nearly all Zr and Hf is hosted in zircon. Garnet and hornblende also contain Zr, but they should expel Zr during cooling (Kohn et al. 2015) so do not likely serve as a sink. Thus, loss of Zr and Hf from rutile during cooling must form new zircon. However, the amount of zircon that forms from this process is extremely small: The Zr loss we observe, if developed over a 50-µm-radius rutile crystal, would produce only a single zircon grain ~1.5 µm in radius, or a ~10-nm-thick overgrowth on a preexisting 10-µm-radius zircon crystal. Zircon also serves as the main host of U, but epidote may contain a substantial proportion (monazite is rare and could provide sufficient U to source the tiny U uptake profiles in rutile). Apatite might also provide some U.
In contrast to other trace elements, Nb and Ta are hosted almost wholly in rutile (e.g., Zack et al. 2002), but with a small proportion in amphibole. Our measurements of Nb concentrations in rutile and hornblende from Catalina Schist amphibolites suggest a ratio of ~1000:1; for example, rutile with 500–1000 ppm Nb coexists with hornblende with 0.5–1 ppm Nb (Kohn and Penniston-Dorland, unpublished data). For typical rutile and hornblende abundances of c. 1–2 % and c. 40–80 %, hornblende accounts for 3–5 % of the Nb budget of a rock. If rutile crystals are on average 50 µm in radius, the amount of excess Nb we observe on rutile rims is ~1 % of the total rock budget. Thus, hornblende might have sourced the excess Nb and Ta we see on rutile rims. In fact, if Nb and Ta partitioning between hornblende and rutile is temperature sensitive and Nb diffusion in hornblende is not too slow, hornblende must have contributed some fraction of the Nb uptake. Alternatively, dissolution of rutile could also provide Nb and Ta, and this is the likely source in the quartzites and felsic gneiss. Dissolution of c. 0.2 µm of the outer surface of rutile would also provide sufficient Nb and Ta to source the diffusion profiles. Although this process does imply a moving crystal surface, the distance over which the surface moves is many times smaller than the total length of the profile. Thus, our models and interpretations, which assume a fixed crystal boundary, should not be grossly in error.
The trace elements all substitute for Ti, so gain of Nb, Ta, and U implies loss of Ti, whereas loss of Zr and Hf implies gain of Ti. Besides the fact that gain of some elements tends to balance loss of others, hornblende from Catalina Schist amphibolites typically has 1–2 wt% Ti and could readily serve as a Ti source or sink. Trace titanite or magnetite are the likely sinks in the felsic gneiss. Exactly how quartzites conserve trace elements remains unexplained. Slight dissolution of rutile to source near-rim increases in Nb and Ta should deliver Ti to the matrix, but we can identify no other mineral that would likely accept it (Ti contents of muscovite and quartz should decrease during cooling; Wark and Watson 2006; Chambers and Kohn 2012).
Different crystals, different boundary conditions
We assume that if two or more rutile grains within a sample have the same concentration profile for a given element, they experienced the same series of boundary conditions during cooling for that element. The converse, however, is not necessarily true: The same boundary conditions might result in two rutile grains with different concentration profiles because (a) diffusive resetting is faster parallel to the c-axis than perpendicular, so the magnitude of resetting is different, and (b) interior compositions might differ if relict rutile grains are preserved that crystallized at lower T. Although we attempted to collect all analyses perpendicular to the c-axis (Fig. S2), the imperfect shape of some crystals did not always permit verification of crystal axes. Some profiles were collected closer to grain tips, and although their profiles do not differ systematically from others, tip proximity might also influence the degree of resetting because faster diffusion parallel to c would enhance loss or gain of trace elements. Although we recognize these complications, the simplest interpretation of the composition profiles is nonetheless that mineral rims do not all maintain rock-wide equilibrium with matrix minerals during cooling. For example, the close similarity of Zr profiles in sample A15-10 (Fig. 3) implies similar interior compositions (crystals equilibrated at the same temperature), boundary conditions, and crystal orientation, yet Nb profiles are vastly different. If Zr maintained equilibrium with the matrix on crystal surfaces, Nb did not. In sample A14-29 (Fig. 4), Zr profiles 1 and 2 might be reconciled with a similar boundary condition and crystal orientation, as implied by comparable Nb profiles, but with different interior compositions. Profile 3, however, implies a different Zr boundary condition from other grains, i.e., if profiles 1 and 2 reflect diffusive response to a consistent (possibly equilibrium) boundary condition (same Nb), profile 3 does not (different Zr). Analogously, profiles 1, 2, and 3 in sample A14-59a (Fig. 5) might reflect a consistent Zr boundary condition during cooling if crystal growth temperatures were different, but Nb profile 3 in A14-59a implies a different boundary condition from other grains.
These inconsistencies cannot reflect mineral growth or dissolution. If dissolution or growth removed or augmented the outer portions of some grains, they should show comparable profiles in all elements, yet they do not (Zr vs. Nb, Fig. 3; Nb vs. Zr, Fig. 4). For example, shifting profile 3 in sample A14-29 (Fig. 4) to the right by ~2 µm would cause nearly perfect overlap in Zr with profile 1, but also destroy the nearly perfect correspondence between their Nb profiles. Rather each grain must have experienced a different series of boundary conditions during cooling, possibly driven by variations in local reactions and scales of equilibrium.
Attempts to model consistent cooling histories for grains within individual samples further illuminate irreconcilable differences among Zr profiles. Disparities are most extreme for sample A14-57b (Figs. 6a, S4). Equilibrium models parallel Zr profiles 1 and 3 (presuming different crystal orientations; in fact, both profiles were collected ⊥c (spots 25 and 26, Fig. S2), but at different distances from crystal tips). These data imply initial cooling at rates of 25–50 °C/Ma, consistent with independent chronologic data (Grove et al. 2008). Factor of 2 differences in apparent cooling rate (~25 °C/Ma for profile 1 vs. 50 °C/Ma for profile 3) are not distinguishable within the experimental uncertainties of Cherniak et al. (2007), so in principle a single linear cooling path could explain both profiles. In contrast, relatively flat profiles 2 and 4 cut across model curves and imply accelerating or instantaneous cooling. Although each specific profile implies a cooling history consistent with chronologic constraints (Fig. 6a), the rock experienced only one cooling history, so all models cannot be simultaneously correct. Crystal orientation and experimental uncertainties can explain only some of these differences, implying differences in boundary conditions during cooling among different grains.
Model predictions (lines) compared to measured Zr profiles (dots) for sample A14-57b (Fig. S4), emphasizing four disparate profiles (1–4). a Equilibrium models. Lines are numbered according to cooling rate (°C/Ma). Profiles 1 and 3 are consistent with linear cooling but at different rates. Profile 2 implies either accelerated cooling or an unusually low (disequilibrium) interior composition. Profile 4 implies nearly instantaneous cooling. Whereas any one profile or a composite profile can be reconciled with independent chronologic constraints, they cannot all be correct. Inset shows complete dataset with numbered selected profiles. Thicker curves for 25 °C/Ma allow comparison to b–c. b Flux-limited models (High-T flux threshold) at fixed 25 °C/Ma cooling rate. Lines are numbered with assigned % of the equilibrium flux. 100 % models (thicker curves) are identical to Equilibrium models with 25 °C/Ma cooling (a). Profiles 1, 3, and 4 can be fit with different flux limits and constant cooling rate. Profile 2 still implies accelerated cooling rate. c Flux-limited models (Low-T zero flux) at fixed 25 °C/Ma cooling rate with zero-flux cutoff at 550 °C. Profiles 2–4 can be fit
Compositional effects of limited fluxes
High-T flux threshold models (Fig. 6b) help explain disparities among zoning profiles. In sample A14-57b, profiles 1 and 3 can be reconciled with a cooling rate of 25 °C/Ma even without recourse to experimental uncertainties in D, simply by assuming that profile 3 reflects a High-T flux threshold corresponding to 75 % of the equilibrium flux (Fig. 6b). Profile 4 can also be matched with the same cooling rate, but with a 10 % flux limit, i.e., loss of Zr was severely restricted from this grain. Profile 2 is still difficult to explain because it cuts across models, as do many profiles of Zr, Nb, and U concentrations that are relatively flat in the rutile grains investigated.
The Low-T zero-flux models fit other aspects of the data: Shutting off Zr loss from rutile grains at ~550 °C flattens profiles in the outer few microns and helps explain why so many profiles trend toward relatively high Zr concentrations on the rim (Figs. 3, 4, 5, S4). That is, Equilibrium and High-T flux threshold models all imply that Zr should tend toward 0 ppm at the crystal surface, yet we observe trends toward 0 ppm in a minority of natural profiles—notably profile 1 and arguably profile 3 in sample A14-57b (Fig. 6). If a flux cutoff occurred, constraints on its temperature might be derived from the average Zr-in-rutile temperature at the rim, which is ~625 °C (~250 ppm). Diffusion of Zr during cooling below the threshold temperature for zero flux (550 °C in our models) reverses the temporal trend toward 0 ppm Zr at the crystal rim. For example, the equilibrium rim Zr concentration would be ~85 ppm at 550 °C. In the Low-T zero-flux model, a grain would initially evolve toward 0 ppm at the rim, but imposition of a flux cutoff at 550 °C, combined with internal diffusion within the rutile crystal, would raise the rim concentration to ~150 ppm (c. 590 °C) in the cooled rock. Below 550 °C, Zr diffuses down its concentration gradient from the rutile core toward the rim, but is unable to leave the crystal, so the rim concentration increases. If the zero-flux threshold temperature for rutile was 625 °C, temperatures and Zr contents at the rim of the rutile would be even higher. Thus, the zero-flux threshold temperature implied by our data must be below 625 °C. A cutoff temperature of ~550 °C may be appropriate as it yields models that span the range of observed rim compositions. Carlson (2012) proposed a nearly identical mechanism to explain REE profiles in high-grade garnets. The main difference compared to our data is that REE increase toward garnet rims, whereas Zr in rutile decreases. So, the zero-flux limit that Carlson imposed at the garnet crystal surface in his models resulted in a decrease in REE concentrations at the garnet rim as REE diffused back toward the garnet core. A higher temperature threshold was also assumed (767 °C).
Comparison of diffusion rates
Presuming that the profiles we measured formed over a temperature range of 675–550 °C and represent typical interior and rim Zr concentrations, we can compare our inferred ratios of diffusivities to predictions from experiments (Cherniak et al. 2007; Marschall et al. 2013). Results for geochemically similar species compare well (Figs. S5–6): At 675–550 °C, experimentally predicted (D Zr/D Hf) = 0.1–8 (average ~ 0.95) versus sample A14-29 (D Zr/D Hf) = ~0.2 to ~5 (accounting for factor of ~4 potential inaccuracy; 1.08 ± 0.09 for regression precision alone); predicted (D Nb/D Ta) = 1.3–24 (average ~ 6) versus A14-29 (D Nb/D Ta) = ~0.4 to ~8 (accuracy; 1.94 ± 0.25 for precision). A value for D Zr/D Hf ~ 1 is expected as Zr and Hf are geochemically similar (same charge and nearly identical ionic radii) and according to Cherniak et al. (2007) have indistinguishable experimental D’s and activation energies. Our data support this view to much lower temperatures than can be investigated experimentally. For D Nb/D Ta, experimental data at 850–1250 °C yield D Nb/D Ta = 1.6 to 18, overlapping results at considerably lower temperature. Taken together, D Nb/D Ta must be consistently small (between 1 and 10) over a wide range of temperatures, implying that Nb and Ta have similar activation energies. The fact that D Nb exceeds D Ta supports models that argue for diffusion-induced fractionation of Nb from Ta during formation of continental crust (Marschall et al. 2013). The diffusivity of U has not been previously determined, but profiles of U concentration are markedly steeper than profiles of Zr concentration (Figs. 5, S4–5). These differences suggest that U diffuses substantially more slowly than Zr (Figs. S5–6). Insofar as DPb ~ D Zr at 600–700 °C (Cherniak 2006), resetting or closure of U–Pb ages of rutile will be dominated by diffusive Pb loss rather than diffusive redistribution of U. If, like other trace elements, Pb loss from rutile depends on flux limitations within the matrix, intracrystalline Pb diffusion rates alone may not control the U–Pb closure temperature for rutile and individual grains may retain older ages than expected.
For D Zr/D Nb and D Zr/D Ta, experimental errors from Cherniak et al. (2007) and Marschall et al. (2013), when formally propagated down-temperature, expand to errors in D-ratios of ~2 orders of magnitude and encompass our data, which have precisions of <1 order of magnitude. That is, depth profiling data from natural rutile can potentially limit D-ratios at temperatures of 550–675 °C more narrowly than experiments. At 625 °C, A14-29 (D Zr/D Nb) is about ten times higher than experimentally predicted (D Zr/D Nb), so either Zr diffuses faster or Nb diffuses slower at metamorphic conditions than expected. Comparison between Zr and Ta is reconcilable within the accuracy of our data [A14-29 (D Zr/D Ta) is approximately two times experimental (D Zr/D Ta)]. Possibly, the activation energy for Nb diffusion (nominally 378 ± 20 kJ) should be higher and more similar to Ta (392 ± 72 kJ), yielding lower D Nb and higher D Zr/D Nb at metamorphic conditions. A faster D Zr could also be reconciled with our data and might explain profile 1 in sample A14-57b (Fig. 6; analysis 25, Fig. S2), which was collected ⊥c, but can be explained only assuming faster diffusivities (nominally∥c).
Implications for geospeedometry—putting on the brakes
Geospeedometry (Lasaga 1983) generally attempts to invert diffusion profiles to infer cooling histories. Key requirements of the inversion include assessing how diffusivities and boundary concentrations vary with T, normally assuming partitioning equilibrium between the surface of the mineral of interest and the matrix assemblage. Some authors attempt to model specific individual profiles (e.g., Ganguly et al. 2000), whereas others fit histories to averaged data (e.g., Smye and Stockli 2014). Yet our data are best explained not through simple partitioning equilibrium, but rather through a combination of spatially variable processes that include variations in flux rates through the matrix (all elements), major differences in partition coefficient between rutile and the matrix (Nb increases toward the rim in some grains and decreases in others), and possibly even wholesale shutdown of diffusive fluxes (all elements) within the matrix. Factors such as proximity and reactivity of Nb- and Zr-bearing phases (amphibole, zircon, etc.) probably play a major role in how trace elements partition on rutile rims and flux into or out of grains. One consequence of this complexity is that the boundary condition is difficult to define, and indeed, it must have varied among different grains within an individual sample. Boundary condition ambiguity profoundly compromises quantification of the true cooling history (Spear 2004). Another consequence of flux limitations is that profiles are flatter with higher concentrations and higher preserved temperatures near rutile rims than would be expected if the rutile maintained equilibrium partitioning with matrix minerals during cooling.
Flatter profiles and higher concentrations imply that Equilibrium models will systematically overestimate calculated cooling rates. For example, profile 4 in sample A14-57b can be fit using an Equilibrium model only with cooling rates far in excess of 100 °C/Ma (Fig. 6a), whereas a High-T flux threshold model fits the same data with a cooling rate of only 25 °C (Fig. 6b). An Equilibrium model applied to profile 2 would imply a steadily increasing cooling rate that ultimately exceeds 100 °C/Ma (Fig. 6a), whereas a Low-T zero-flux model again fits the data at 25 °C/Ma (Fig. 6c). In general, matrix flux limitations retard reequilibration of a mineral grain with its surrounding matrix, and Equilibrium models, applied to such profiles, will imply faster cooling. Only the most diffusively relaxed profiles (e.g., profile 1, Fig. 6) should be interpreted in terms of cooling rate in the context of an Equilibrium model. Such profiles still provide only maximum limits on cooling rate.
The characteristics of our depth profiles are not unique. Chronologic depth profiles from high-temperature rutile from the Ivrea zone show consistent decreases in U–Pb age (basically, Pb loss at the crystal surface; Smye and Stockli 2014). Yet individual profiles are, in fact, statistically distinct (Fig. S7). Rutile exhibits moderate Pb diffusion rates (Cherniak 2000) with a typical closure temperature estimated at 650–700 °C (100 µm radius, dT/dt = 25 °C/Ma), and peak metamorphic conditions for the Ivrea zone were ≥850 °C (Luvizotto and Zack 2009). Thus, different rutile grains should have expelled all radiogenic Pb initially and yield identical chronologic profiles. The disparities among different profiles suggest that the boundary condition on the rim of each rutile grain was not the same and that the inferred cooling rate, determined from average data, is likely overestimated.
Implications for thermometry and closure temperatures
Analysis of Zr contents in granulite-facies rutile sometimes reveals much higher concentrations and arguably flatter concentration profiles compared to expectations from diffusive loss of Zr in equilibrium with zircon and quartz (e.g., Luvizotto and Zack 2009; Meyer et al. 2011; Kooijman et al. 2012; Ewing et al. 2013; Pauly et al. 2016). For example, using diffusion data from Cherniak et al. (2007), a typical Zr-in-rutile closure temperature should be ~700 °C (radius = 100 µm; dT/dt = 25 °C/Ma), whereas temperatures in excess of 900 °C are sometimes recovered. Taylor-Jones and Powell (2015) proposed that slow Si diffusion in rutile retards Zr diffusion, leading to higher Zr contents and higher closure temperatures. Our observations regarding diffusivities and flux limitations have direct implications for this hypothesis. Here, we re-evaluate the slow Si (diffusion) hypothesis, first framing it in the context of thermodynamic equilibria and chemical potential gradients, much as Taylor-Jones and Powell (2015) do, then expanding to consideration of flux limitations. Even making similar assumptions as Taylor-Jones and Powell (2015), we conclude that slow Si diffusion (if it exists) does not explain higher Zr-in-rutile temperatures and is not relevant for modeling diffusion of Zr in rutile.
We follow Taylor-Jones and Powell (2015) in assuming Zr and Si contents of rutile increase with increasing temperature, and that Si diffusion in rutile is far slower than Zr. Silicon content probably also increases with increasing pressure (Taylor-Jones and Powell 2015), but for simplicity, we describe reequilibration in terms of decreasing temperature, in part because pressure and temperature often decrease together in rocks. Silicon may also diffuse faster than Zr, because Si occupies interstitial positions (Golden et al. 2015). Other cations that occupy interstitial positions in rutile (Fe, Ti, etc.) generally diffuse faster via an interstitialcy mechanism than non-interstitial cations that diffuse via a vacancy mechanism (Ba, Pb, etc.; Nowotny et al. 2008; van Orman and Crispin 2010). However, if Si diffusion is fast, it cannot rate limit Zr diffusion. So our consideration of the Taylor-Jones and Powell slow Si hypothesis requires that we assume that Si diffuses slowly. We also assume that Zr substitutes directly for Ti in octahedral sites (Fromkneckt et al. 1996) and is not somehow crystal-chemically coupled to Si substitution. In the following discussion, we use the terms ZrO2 and SiO2 in discussing chemical equilibria and thermodynamics (with chemical potential of ZrO2 and SiO2 implicitly referenced to baddeleyite and quartz), and Zr and Si when discussing diffusing species.
We assume that for rutile at high temperature (T 0), ZrO2 and SiO2 contents are in equilibrium with zircon and quartz in the matrix (Fig. 7a); normally baddeleyite is not stable so the activity of ZrO2 is <1.0. With decreasing temperature (T 1, Fig. 7b), both SiO2 and ZrO2 contents in rutile decrease to maintain equilibrium with zircon and quartz in the matrix. At the rutile rim, both Zr and Si decrease, but, in the rutile core, both SiO2 and ZrO2 concentrations are higher than at the rim. Chemical potential of trace constituents is proportional to concentration (via Henry’s law):
where γ 1 and γ 2 are Henry’s law constants. Thus, \(\mu_{{{\text{ZrO}}_{ 2} }}\)(Rt, core) > \(\mu_{{{\text{ZrO}}_{ 2} }}\)(Rt, rim) and \(\mu_{{{\text{SiO}}_{2} }}\)(Rt, core) > \(\mu_{{{\text{SiO}}_{2} }}\)(Rt, rim). Because the rim is in equilibrium with zircon, these relationships imply that \(\mu_{{{\text{ZrSiO}}_{4} }}\)(Rt, core) > \(\mu_{{{\text{ZrSiO}}_{4} }}\)(Rt, rim), i.e., that the rutile core is supersaturated in zircon (and quartz) during cooling. The chemical potential gradients in ZrO2 and SiO2 between core and rim drive diffusive loss of Zr and Si from the rutile crystal (arrows, Fig. 7). With a further decrease in temperature (T 2), if nucleation barriers prevent supersaturated minerals from exsolving, Zr and Si will simply continue to diffuse outward (Fig. 7c), increasing the degree of zircon and quartz supersaturation in the rutile core, perhaps even crossing to baddeleyite supersaturation. Even if SiO2 were completely immobile, \(\mu_{{{\text{ZrO}}_{ 2} }}\)(Rt, core) would always exceed \(\mu_{{{\text{ZrO}}_{ 2} }}\)(Rt, rim) and Zr would diffuse down its chemical potential gradient toward the crystal rim. SiO2 immobility in the rutile interior does supersaturate it in quartz and zircon, but does not rate limit Zr diffusion.
Schematic diagrams illustrating the high-temperature evolution of Zr and Si profiles in rutile and the saturation index during cooling; saturation index for a component i equals log10(a i/a eq), where a i is the activity of i and a eq is the activity of i at equilibrium. For a component such as zircon, saturation index would be calculated as the product of the activities of SiO2 and ZrO2 in the rutile interior divided by the activities at the rutile rim. a Initial temperature (T 0): ZrO2 and SiO2 concentrations (X) and activities (a) are constant and reflect equilibrium saturation (saturation index = 0) with quartz and zircon. b–c As temperature drops (T 0 → T 1 → T 2), rim \(X_{{{\text{ZrO}}_{2} }}\) and \(X_{{{\text{SiO}}_{2} }}\) drop, compressing the composition dependence of \(a_{{{\text{ZrO}}_{2} }}\) and \(a_{{{\text{SiO}}_{2} }}\) (right-hand scales). The resulting concentration gradients drive Zr and Si diffusion (at different rates) toward rim. The interior of rutile, with high \(X_{{{\text{ZrO}}_{2} }}\) and \(X_{{{\text{SiO}}_{2} }}\) becomes increasingly supersaturated with zircon and quartz, and the core may reach baddeleyite supersaturation. Zr and Si diffuse down their respective concentration (chemical potential) gradients, independent of other components. d–e Precipitation of zircon in rutile core (gray band) decreases Zr and Si values, such that the product \(X_{{{\text{ZrO}}_{2} }}\)·\(X_{{{\text{SiO}}_{2} }}\) (core) = \(X_{{{\text{ZrO}}_{2} }}\)·\(X_{{{\text{SiO}}_{2} }}\) (rim). For very low \(X_{{{\text{SiO}}_{2} }}\) concentrations in the core, \(a_{{{\text{SiO}}_{2} }}\) < 1, whereas for very high \(X_{{{\text{SiO}}_{2} }}\) concentrations, \(a_{{{\text{SiO}}_{2} }}\) > 1
Our view departs from Taylor-Jones and Powell (2015), who argue that \(\mu_{{{\text{ZrO}}_{ 2} }}\)(Rt) is defined solely in relation to the thermometer equilibrium (Eq. 1), not with respect to the mole fraction of ZrO2 in rutile (Eq. 4b). In this view, if Si diffuses slowly, the interior of the grain no longer maintains equilibrium, and there is no chemical potential gradient to drive diffusive loss of Zr. Our view is that the gradient in Zr defines a gradient in \(\mu_{{{\text{ZrO}}_{ 2} }}\) via Eq. 4b and that the equilibrium among quartz, rutile, and zircon acts at the rim of the rutile to anchor Zr concentrations and \(\mu_{{{\text{ZrO}}_{ 2} }}\) at the grain boundary. The driving force for Zr diffusion, then, is the Zr concentration gradient. While we agree that slow Si diffusion would lead to disequilibrium concentrations of SiO2 (Fig. 7b–e), we disagree that this implies there is no chemical potential gradient to drive Zr diffusion.
Exsolution of zircon from rutile has been reported from a few ultra-high-temperature rocks (e.g., Kooijman et al. 2012; Ewing et al. 2013; likely Meyer et al. 2011) and presents a useful endmember for testing models of ZrO2 and SiO2 reequilibration. The impact of exsolution depends in part on how Si concentrations change with temperature, which is not known. Consequently, we consider two cases where the mole fraction of SiO2 (\(X_{{{\text{SiO}}_{2} }}\)) in the rutile core is either much less than \(X_{{{\text{ZrO}}_{2} }}\) (Fig. 7d) or much greater than \(X_{{{\text{ZrO}}_{2} }}\) (Fig. 7e). Zircon nucleation forces equilibration via the relationships:
where the activity (a i) of each component is assumed to be linearly proportional to its mole fraction (Xi, i.e., Henry’s law for rutile and Raoult’s law for zircon), \(\Delta \mu^{o}\) is the chemical potential difference among endmembers at the P–T condition of interest, and the constant activity coefficients are gathered together into a single term that can be combined with \(\Delta \mu^{o}\) to yield \(\Delta \mu^{o\prime}\), which has the same functional form as \(\Delta \mu^{o}\). Prior to zircon nucleation (Fig. 7c), \(X_{{{\text{ZrO}}_{2} }}\) and \(X_{{{\text{SiO}}_{2} }}\) in the rutile interior exceed values in equilibrium with zircon. So, assuming zircon is essentially pure ZrSiO4, its precipitation drives decreases in \(X_{{{\text{SiO}}_{2} }}\) and \(X_{{{\text{ZrO}}_{2} }}\) adjacent to the newly formed zircon until they reach equilibrium with zircon, and:
Prior to zircon precipitation if \(X_{{{\text{SiO}}_{2} }}\) ≪ \(X_{{{\text{ZrO}}_{2} }}\) (e.g., Pauly et al. 2016), precipitation of zircon with a Si/Zr molar ratio of 1:1 will cause \(X_{{{\text{SiO}}_{2} }}\) and \(a_{{{\text{SiO}}_{2} }}\) to decrease faster than \(X_{{{\text{ZrO}}_{2} }}\) and \(a_{{{\text{ZrO}}_{2} }}\). At the point where \(a_{{{\text{SiO}}_{2} }}\) in the rutile interior reaches 1.0 (quartz saturation), \(a_{{{\text{ZrO}}_{2} }}\) may still exceed \(a_{{{\text{ZrO}}_{2} }}\) on the mineral rim, and the left side of Eq. 7 exceeds the right side. If so, \(a_{{{\text{SiO}}_{2} }}\) in the rutile interior will decrease below 1.0 (Fig. 7d). Zirconium now diffuses down two different \(\upmu_{{{\text{ZrO}}_{ 2} }}\) gradients: (a) toward the rim, which maintains equilibrium with zircon and quartz (fixing \(a_{{{\text{SiO}}_{2} }}\) = 1), and (b) toward the boundary with exsolved zircon, which maintains equilibrium with zircon and balances \(X_{{{\text{ZrO}}_{2} }}\) against local \(X_{{{\text{SiO}}_{2} }}\) < 1 (Fig. 7d). Matrix zircon and exsolved zircon represent Zr sinks, and Zr diffuses toward both. Qualitatively, Zr behaves similarly if \(X_{{{\text{SiO}}_{2} }}\) ≫ \(X_{{{\text{ZrO}}_{2} }}\) (Fig. 7e), but \(a_{{{\text{SiO}}_{2} }}\) > 1, \(X_{{{\text{ZrO}}_{2} }}\) is lower, and Zr is removed more effectively in the rutile interior. Thus, Zr reduction in the rutile interior occurs through both precipitation of zircon and diffusion. Slow diffusion of Si does not directly influence Zr diffusion or its closure temperature, rather it helps stabilize zircon in rutile interiors, providing a proximal sink that more effectively lowers Zr in the host rutile.
If limited Si mobility alone does not explain high Zr contents in rutile, what does? Perhaps most revealing, proximity to matrix zircon and exsolution of zircon or baddeleyite correlate with lower Zr contents in rutile (Kooijman et al. 2012; Ewing et al. 2013). As proposed by others (Meyer et al. 2011; Taylor-Jones and Powell 2015), grain boundary diffusion rates or proximity to zircon grains in the matrix may limit loss of Zr from rutile. Although the spatial resolution of most studies is generally insufficient to identify near-rim profile shapes, qualitatively this hypothesis parallels the expectations of flux-limited models—if grain boundaries limit flux, and zircon is too far away, rutile will be unable to expel its Zr. As our data–model comparisons show (Fig. 6), flux limitations better explain flat near-rim profiles and high concentrations than unrealistically high cooling rates. Natural D Zr could be similar to estimates derived from experiments or even faster, but the actual Zr content in a specific rutile grain depends on factors related to scales of equilibration. Boundary conditions and intracrystalline diffusion, not intracrystalline diffusion alone, control closure temperatures.
Conclusions
-
Irreconcilable differences among composition depth profiles (Figs. 3, 4, 5, S4) indicate that trace elements (Zr, Nb, and U) in natural rutile crystals do not maintain equilibrium with matrix minerals along crystal surfaces during cooling. A likely minimum cutoff temperature for efficient equilibration is c. 550 °C.
-
Composition profile shapes suggest that D Zr ~ D Hf ~ 10D Nb ~ 20D Ta ~ 40D U at an average temperature of ~625 °C.
-
Slow grain boundary diffusion, detachment of SiO2, and/or attachment of ZrSiO4 most likely impede equilibration; consequent flux limitations explain disparate composition profiles among rutile grains (Fig. 6).
-
Equilibrium models applied to average composition profiles will overestimate cooling rates.
-
High Zr-in-rutile temperatures observed in UHT rocks do not reflect inherently slow intracrystalline Zr diffusion rates or thermodynamic coupling with slow-diffusing Si (Fig. 7), but rather the degree to which the surfaces of crystals are able to maintain equilibrium with matrix minerals.
References
Bebout GE, Barton MD (1993) Metasomatism during subduction; products and possible paths in the Catalina Schist, California. Chem Geol 108:61–92
Bebout GE, Barton MD (2002) Tectonic and metasomatic mixing in a high-T, subduction-zone mélange—insights into the geochemical evolution of the slab-mantle interface. Chem Geol 187:79–106
Carlson WD (2012) Rates and mechanism of Y, REE, and Cr diffusion in garnet. Am Mineral 97:1598–1618
Carlson WD, Gordon CL (2004) Effects of matrix grain size on the kinetics of intergranular diffusion. J Metamorph Geol 22(8):733–742. doi:10.1111/j.1525-1314.2004.00545.x
Chambers JA, Kohn MJ (2012) Titanium in muscovite, biotite, and hornblende: modeling, thermometry, and rutile activities of metapelites and amphibolites. Am Mineral 97:543–555
Cherniak DJ (2000) Pb diffusion in rutile. Contrib Mineral Petrol 139:198–207
Cherniak DJ (2006) Zr diffusion in titanite. Contrib Mineral Petrol 152:639–647
Cherniak DJ, Manchester J, Watson EB (2007) Zr and Hf diffusion in rutile. Earth Planet Sci Lett 261:267–279
Ewing TA, Hermann J, Rubatto D (2013) The robustness of the Zr-in-rutile and Ti-in-zircon thermometers during high-temperature metamorphism (Ivrea-Verbano Zone, northern Italy). Contrib Mineral Petrol 165:757–779
Ferry JM, Watson EB (2007) New thermodynamic models and revised calibrations for the Ti-in-zircon and Zr-in-rutile thermometers. Contrib Mineral Petrol 154:429–437
Fromkneckt R, Khubeis I, Meyer O (1996) La-, Sn- and Hf-implanted in TiO2 single crystals: lattice disorder and lattice site location. Nucl Instr Meth Phys Res B116:109–112
Ganguly J, Dasgupta S, Cheng W, Neogi S (2000) Exhumation history of a section of the Sikkim Himalayas, India: records in the metamorphic mineral equilibria and compositional zoning of garnet. Earth Planet Sci Lett 183:471–486
Golden EM, Giles NC, Yang S, Halliburton LE (2015) Interstitial silicon ions in rutile TiO2 crystals. Phys Rev B 91:134110
Grove M, Bebout GE, Jacobson CE, Barth AP, Kimbrough DL, King RL, Zou H, Lovera OM, Mahoney BJ, Gehrels GE (2008) The Catalina Schist: evidence for middle Cretaceous subduction erosion of southwestern North America. Geol Soc Am Spec Pap 436:335–361
Kohn MJ (1999) Why most “dry” rocks should cool “wet”. Am Mineral 84:570–580
Kohn MJ, Corrie SL (2011) Preserved Zr-temperatures and U-Pb ages in high-grade metamorphic titanite: evidence for a static hot channel in the Himalayan orogen. Earth Planet Sci Lett 311:136–143
Kohn MJ, Corrie SL, Markley C (2015) The fall and rise of metamorphic zircon. Am Mineral 100:897–908
Kooijman E, Smit MA, Mezger K, Berndt J (2012) Trace element systematics in granulite facies rutile: implications for Zr geothermometry and provenance studies. J Metamorph Geol 30(4):397–412. doi:10.1111/j.1525-1314.2012.00972.x
Lasaga AC (1983) Geospeedometry: an extension of geothermometry. In: Saxena SK (ed) Kinetics and equilibrium in mineral reactions. Springer, New York, pp 81–114
Luvizotto GL, Zack T (2009) Nb and Zr behavior in rutile during high-grade metamorphism and retrogression: an example from the Ivrea-Verbano Zone. Chem Geol 261:303–317
Luvizotto GL, Zack T, Meyer HP, Ludwig T, Triebold S, Kronz A, Münker C, Stockli DF, Prowatke S, Klemme S, Jacob DE, von Eynatten H (2009) Rutile crystals as potential trace element and isotope mineral standards for microanalysis. Chem Geol 261:346–369
Marschall HR, Dohmen R, Ludwig T (2013) Diffusion-induced fractionation of niobium and tantalum during continental crust formation. Earth Planet Sci Lett 375:361–371
Mattinson JM (1986) Geochronology of high pressure—low temperature Franciscan metabasites: a new approach using the U-Pb system. Geol Soc Am Mem 164:95–106
Meyer M, John T, Brandt S, Klemd R (2011) Trace element composition of rutile and the application of Zr-in-rutile thermometry to UHT metamorphism (Epupa Complex, NW Namibia). Lithos 126:388–401
Nowotny MK, Sheppard LR, Bak T, Nowotny J (2008) Defect chemistry of titanium dioxide. Application of defect engineering in processing TiO2-based photocatalysts. J Phys Chem C 112:5275–5300
Paton C, Hellstrom J, Woodhead J, Hergt J (2011) Iolite: freeware for the visualisation and processing of mass spectrometric data. J Analyt Atom Spec 26:2508–2518
Pauly J, Marschall HR, Meyer H-P, Chatterjee N, Monteleone B (2016) Prolonged Ediacaran-Cambrian metamorphic history and short-lived high-pressure granulite-facies metamorphism in the H.U. Sverdrupfjella, Dronning Maud Land (East Antarctica): evidence for continental collision during Gondwana Assembly. J Petrol 57:185–228
Penniston-Dorland SC, Bebout GE, Pogge von Strandmann PAE, Elliott T, Sorensen SS (2012) Lithium and its isotopes as tracers of subduction zone fluids and metasomatic processes: evidence from the Catalina Schist, California, USA. Geochim Cosmochim Acta 77:530–545
Platt JP (1975) Metamorphic and deformational processes in the Franciscan Complex, California: some insights from the Catalina Schist terrane. Geol Soc Am Bull 86:1337–1347
Platt JP (1976) The petrology, structure and geologic history of the Catalina Schist terrain, southern California. Univ Calif Publ Geol Sci 112:1–111
Sasaki J, Peterson NL, Hoshino K (1985) Tracer impurity diffusion in single-crystal rutile (TiO2−x ). J Phys Chem Solids 46:1267–1283
Sheppard LR, Atanacio AJ, Bak T, Nowotny J, Prince KE (2007) Bulk diffusion of niobium in single-crystal titanium dioxide. J Phys Chem B 111:8126–8130
Sheppard LR, Atanacio AJ, Bak T, Nowotny J, Nowotny MK, Prince KE (2009) Niobium diffusion in niobium-doped titanium dioxide. J Solid State Electrochem 13(7):1115–1121. doi:10.1007/s10008-008-0717-x
Smye AJ, Stockli DF (2014) Rutile U-Pb age depth profiling: a continuous record of lithospheric thermal evolution. Earth Planet Sci Lett 408:171–182
Sorensen SS (1988) Petrology of amphibolite-facies mafic and ultramafic rocks from the Catalina Schist, southern California: metasomatism and migmatization in a subduction zone metamorphic setting. J Metamorph Geol 6:405–435
Sorensen SS, Barton MD (1987) Metasomatism and partial melting in a subduction complex: catalina Schist, southern California. Geology 15:115–118
Spear FS (2004) Fast cooling and exhumation of the Valhalla metamorphic core complex, southeastern British Columbia. Int Geol Rev 46:193–209
Taylor-Jones K, Powell R (2015) Interpreting zirconium-in-rutile thermometric results. J Metamorph Geol 33(2):115–122. doi:10.1111/jmg.12109
Thomas JB, Watson EB, Spear FS, Shemella PT, Nayak SJ, Lanzirotti A (2010) TitaniQ under pressure: the effect of pressure and temperature on the solubility of Ti in quartz. Contrib Mineral Petrol 160:743–759
Tomkins HS, Powell R, Ellis DJ (2007) The pressure dependence of the zirconium-in-rutile thermometer. J Metamorph Geol 25:703–713
van Orman JA, Crispin KL (2010) Diffusion in oxides. Rev Mineral Geochem 72(1):757–825
Watson EB, Wark DA, Thomas JB (2006) Crystallization thermometers for zircon and rutile. Contrib Mineral Petrol 151:413–433
Wark DA, Watson EB (2006) TitaniQ: a titanium-in-quartz geothermometer. Contrib Mineral Petrol 152:743–754
Yardley BWD (1981) Effect of cooling on the water content and mechanical behavior of metamorphosed rocks. Geology 9:405–408
Zack T, Foley SF, Rivers T (2002) Equilibrium and disequilibrium trace element partitioning in hydrous eclogites (Trescolmen, Central Alps). J Petrol 43:1947–1974
Zack T, Moraes R, Kronz A (2004) Temperature dependence of Zr in rutile: empirical calibration of a rutile thermometer. Contrib Mineral Petrol 148:471–488
Acknowledgments
This research was funded by NSF Grants EAR-1419865 to MJK and EAR-1419871 to SPD, and by a Government of Brazil undergraduate fellowship to JCSF. We thank Frank Spear for discussions about diffusion modeling approaches, Thomas Zack for providing the R10 rutile standard, Phil Piccoli for EPMA analysis of the Rietfontein rutile, and Marion Lytle for help with LA-ICP-MS analysis, Othmar Müntener for editorial handling, and Mike Jollands and Horst Marschall for extensive and educational reviews that substantially improved the quality of this study. The Catalina Island Conservancy is acknowledged for logistics and support of sample collection of the Catalina Schist.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Prof. Othmar Müntener.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kohn, M.J., Penniston-Dorland, S.C. & Ferreira, J.C.S. Implications of near-rim compositional zoning in rutile for geothermometry, geospeedometry, and trace element equilibration. Contrib Mineral Petrol 171, 78 (2016). https://doi.org/10.1007/s00410-016-1285-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00410-016-1285-1