Abstract
Objective
To evaluate quantitative chest computed tomography (CT) methods for the detection of air trapping (AT) and to assess its diagnostic performance for the diagnosis of bronchiolitis obliterans syndrome (BOS) in single lung transplant (SLT) patients.
Methods
Adult patients who had a SLT at a single transplant center and underwent CT scan after transplantation were retrospectively included. CT findings of air trapping were measured by three different methods: expiratory air-trapping index (ATIexp), mean lung density on expiratory acquisition (MLDexp) and expiratory to inspiratory ratio of mean lung density (E/I-ratio(MLD). Sensitivity, specificity and diagnostic accuracy of the three methods for the detection of BOS status evaluated by serial routine measures of pulmonary function tests (gold standard) were assessed.
Results
Forty-six SLT patients (52.2% females, mean age 58 ± 6 years) were included in the analysis, 12 (26%) patients with a diagnosis of BOS. Quantitative CT diagnosis of AT ranged from 26 to 35%. Sensitivity, specificity and accuracy of each method for the detection of BOS were 85.7%, 84.7% and 85.0% for ATIexp, 78.5%, 93.4% and 90.0% for MLD and 64.2%, 89.1% and 83.3% E/I-ratio(MLD), respectively.
Conclusion
Quantitative measures of AT obtained from standard CT are feasible and show high specificity and accuracy for the detection of BOS in SLT patients.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Long-term survival after lung transplantation (LT) is often hampered by chronic lung allograft dysfunction (CLAD), which is believed to reflect chronic rejection [1, 2]. CLAD, a term used to refer to all variants of pulmonary chronic dysfunction, affects up to 50% of lung transplant recipients within five years after surgery [3], and bronchiolitis obliterans syndrome (BOS) is the most common manifestation of CLAD [2]. Proposed by the Committee of the International Society for Heart and Lung Transplantation (ISHLT), the term BOS is a clinical description of bronchiolitis obliterans (BO), a histopathologic entity characterized by a fibroproliferative process of the small airways with multifocal obliteration of the terminal bronchiole [4]. Diagnostic criteria for BOS includes a persistent postoperative decline of the forced expiratory flow in one second (FEV1) by at least 20% in comparison to baseline for a minimum of 3 weeks. This decrease must not be explained by acute rejection, infection or other complications [3, 5, 6]. Transbronchial biopsy is frequently performed to detect infection or other entities that may be the cause of functional decline but it is rarely used to diagnose BO because of its low sensitivity [7]. In single lung transplant (SLT) patients, the interpretation of the decline of FEV1 for the diagnosis of BO is further confounded by the presence of a diseased native lung. [8].
Bilateral lung transplant has been favored in comparison to SLT because of perceived benefits in long-term survival and improvements in functional outcomes [9]. However, there has been a long-standing debate over the theme and considering all variables that occur in transplant practice, including disease indication, age of recipient, donor lung quality and organ scarcity, many centers around the world opt for SLT in a considerable number of patients [8, 10, 11]. Nonetheless, studies with this population have shown a tendency toward early onset of BOS when compared to patients with double lung transplant (DLT) [10,11,12,13]. Unlike DLT patients, SLT patients still have a diseased native lung, and its deterioration over time may render the correct interpretation of changes in recipient’s FEV1 values challenging. Therefore, identification of other tools to assess for BOS in addition to PFTs would be highly desirable.
Computed tomography (CT) is commonly used in post-lung transplant patients in association with pulmonary function tests to detect and monitor complications. Findings frequently seen in patients with BOS include bronchiectasis, bronchial wall thickening, mucus plugging and AT [14]. Air trapping, an established parameter to assess small airways disease [15, 16] is defined as less than normal increase in the attenuation of the lung parenchyma during expiration with lack of volume reduction [17]. In LT patients, the presence of AT on expiratory CT scans was postulated as an important sign of early chronic lung rejection [18,19,20,21]. Different CT measurements have been developed to assess post-lung transplant diagnosis of BOS [18,19,20,21], mainly in DLT, but no consensus yet has been reached on which method should be used.
The objectives of this study were to evaluate the presence of air trapping on chest CT using different quantitative methods, namely expiratory air-trapping index (ATIexp), mean lung density (MLD) on expiratory acquisition and expiratory to inspiratory ratio of mean lung density (E/I-ratio(MLD), and to assess the diagnostic performance of these quantitative methods for the diagnosis of BOS in SLT patients.
Materials and Methods
Patients
This institutional review board approved retrospective study was performed in SLT recipients from a single medical center with a mean of 40 lung transplants per year. All individuals older than 18 years of age who had undergone unilateral cadaveric lung transplantation between January 2012 and December 2018 and who underwent a CT scan at any time after transplantation were included. A CT scan is not routinely performed in asymptomatic patients at our lung transplant center, only when a surgical or clinical complication is suspected, or when an unexplained reduction in lung function occurs, including when BOS is suspected clinically or is under evaluation. Pulmonary function tests (PFT) are regularly performed during the routine postoperative visits. The presence of BOS and the BOS stages were classified according to the guidelines of the International Society for Heart and Lung Transplantation [5].
In order to avoid transitory postoperative CT findings and to allow a minimum of 4 spirometric tests performed, only outpatients CTs performed after the first 60 days post transplant were included. Patients with CT findings of lung infection, acute rejection, thoracic malignancy, previous lung surgery other than LT, bronchial narrowing or other reasons for a reduced FEV1, were excluded. In addition, patients with a restrictive pattern in lung function tests were also excluded (thus excluding restrictive CLAD). The maximum time interval allowed between PFT and CT was 30 days.
CT Protocol
All subjects underwent a paired unenhanced inspiratory and expiratory helical chest CT with 16 × 1.25 mm collimation (LightSpeed 16 Slice Pro, General Electric Healthcare Technologies, Waukesha, WI, USA). Scans were acquired in a caudocranial direction using the following parameters: tube voltage, 120 kV; a fixed tube current for inspiratory image, 200 mAs; a fixed tube current for expiratory images, 50 mAs; pitch, 1.375. Images were reconstructed in axial, sagittal and coronal planes with a standard reconstruction kernel, data matrix of 512 × 512, a field of view of 35–45 cm and a slice thickness and interval of 1.0 mm to achieve near-isotropic voxels.
Imaging Analysis
All examinations were analyzed in a commercially available workstation (Advantage Workstation 4.6, General Electric Healthcare Technologies, Waukesha, WI, USA). Inspiratory and expiratory CT images were evaluated using dedicated software designed for the assessment of segmented images from the chest wall, mediastinum, diaphragm, and airways. Automated segmentation of the transplanted lung from the chest wall and mediastinum was performed and the total lung volume and attenuation of all voxels included in the lung segmentation were quantified. The expiratory air-trapping index (ATIexp) was calculated as the percentage of lung voxels with attenuation of − 950 to − 856HU on expiratory CT images [15] (Fig. 1). Mean lung density (MLD) histograms were created for expiratory and inspiratory acquisitions for each subject, and the expiration and inspiration ratio of MLD (E/I-ratio(MLD) was calculated [22, 23]. Measurements were performed by 2 thoracic radiologists in consensus, with a minimum of 10 years of experience, blinded to all clinical information. Analysis of all measurements required approximately five minutes per patient.
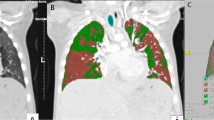
Coronal (1A) and axial (1B) slices of expiratory high resolution CT scan of the chest from a single lung transplant patient with BOS. Images 2A, 2B e 2C show the 3D-CT reconstruction of the lungs and images 3A, 3B e 3C show the same 3D-CT reconstruction highlighting areas of air trapping in the transplanted lung only (red color), represented by lung voxels with attenuation of − 950 to − 856 HU
Pulmonary Function Tests
All PFTs were performed with a MasterScreen IOS device (Viasys Health Care Global, Loma Linda, CA, USA), at the same location and by a team of technicians trained in pulmonary function testing and in accordance with the Brazilian Thoracic Association criteria and the American Thoracic Society criteria [24, 25]. Reference values were derived for the Brazilian population [26].
Statistical Analysis
Data were presented as mean ± standard deviation (SD), median or interquartile range or frequency and percentage. Using spirometric diagnosis of BOS as the gold standard, sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) were calculated for each of the quantitative CT findings: mean lung density expiration, expiration and inspiration ratio of MLD and expiratory air trapping index. Receiver operating characteristic (ROC) curve analysis was performed to evaluate the diagnostic capabilities of the quantitative CT findings in patients with BOS. In all cases, P values less than 0.05 were considered to be statistically significant. Statistical analyses were performed using the STATA v.15 (StataCorp, College Station, TX, USA).
Results
In total, 46 SLT patients were identified (mean age 58 ± 6 years; 24 females, 52.2%) and 12 patients fulfilled spirometric criteria for BOS. Sixty CTs were included in the study, 14 from patients with BOS. The average time between the diagnosis and CT scan was 7 months for those with BOS grade I, 14 months for those with BOS grade II and 38 months for patients with BOS grade III. Baseline characteristics of the study subjects are shown in Table 1. The most common pre-transplant diseases requiring SLT were pulmonary fibrosis and COPD and left SLT was more common than right SLT. The mean time of BOS diagnosis after transplantation was 48 (± 32) months. From the 134 CTs available during the study period, 74 were excluded due to the following reasons: fungal or bacterial infection (n = 38), bronchial stenosis (n = 9), CT with lack of an expiratory scan (n = 9) acute rejection (n = 8), lung function test with restrictive pattern (n = 4), thoracic tumor (n = 2), pneumothorax (n = 2) and inability to perform the quantitative analysis due to severe motion artifacts (n = 2).
After applying the Operating Characteristic Curve (ROC curve), best threshold for each method were found and the prevalence of air trapping based on MLDexp was 26% (95% CI 13–40), based on ATIexp was 35% (95% CI 21–50) and based on E/I-ratio(MLD) was 28% (95% CI 15–42). All the measurements demonstrated a good diagnostic performance for diagnosing BOS, with an accuracy above of 83% (Table 2). Using the density of − 737 HU as a cut-off, the sensitivity and specificity of MLDexp for predicting BOS was 78.5% and 93.4%, respectively. With a threshold index of 0.06, the ATIexp showed a sensitivity of 85.7% and a specificity of 84.7%. The third measure, E/I-ratio(MLD) demonstrated a sensitivity of 64.2% and a specificity of 89.1% for a threshold ratio of 0.93. All three methods demonstrated a high area under the ROC curve for the diagnosis of BOS (Fig. 2). There were cases in which the CT parameters were positive and clinical and spirometric follow up was negative for BOS, including 7 for ATIexp, 5 for E I ratio (MLD) and for 3 MLDexp.
Discussion
Bronchiolitis obliterans syndrome is a well-recognized complication that significantly limits long-term survival after lung transplantation [27]. The diagnosis of BOS by definition is only established in retrospect, challenging early identification of the disease. In addition, concern has been raised regarding the use of spirometry as the main diagnostic criteria due to the variability in both the spirometry test performance by the patient and spirometry interpretation by the physician that may influence the diagnosis of BOS [28]. Additional tools to help improve the diagnosis of BOS would be, therefore, desirable. In the present study, we demonstrated that quantitative CT assessment of air trapping in single lung transplant patients is feasible and demonstrates high specificity and accuracy for the detection of BOS.
Air trapping is an accepted indirect sign of small airway remodeling and is present in different obstructive airway diseases, including chronic obstructive pulmonary disease [29] and asthma [15]. In post-lung transplant patients, BOS due to obliterative bronchiolitis is characterized by the presence of AT [30]. Previous studies have evaluated the detection of AT with CT in this population [19, 30,31,32], however, a standardized quantitative method has yet to be validated. We tested three automated and easy to perform quantitative CT parameters in a consecutive sample of SLT patients and demonstrated a high prevalence of AT in the transplanted lung, ranging from 26 to 35%.
All three CT parameters, MLDexp, ATIexp and E/I-ratio(MLD), were highly specific for the detection of BOS with excellent accuracy. Only a few studies have assessed quantitative AT parameters in patients post-LT, but none in SLT patients. Solyanik et al. [18] recently evaluated density mapping, a voxel-wise image measurement method based on registration of the inspiration and expiration CT datasets, E/I-ratio (MLD) and a threshold-based method in expiration, similar to our ATIexp, to assess the accuracy for detection and quantification of pathologic AT in DLT patients. All three methods showed a good correlation to the ratio of residual volume to total lung capacity obtained by body plethysmography, however density mapping and E/I-ratio (MLD) were more suitable to detect AT, with a higher accuracy. Dettmer et al. [3] used a longitudinal analysis to evaluate quantitative measurements of lung volume and lung density in bilateral lung transplant. The authors demonstrated that increase in lung volume and decrease in lung density when compared to prior CTs showed a marked correlation with BOS onset, suggesting a potential role for CT in helping identifying patients with BOS at the first decrease in PFTs. Similarly to these investigations in DLT patients, our results add to the current knowledge that, in SLT patients, quantitative CT measurements of AT may also identify BOS with good accuracy.
Other methods for quantification of small airway disease have been evaluated more recently, including the ratio of expiratory and inspiratory MLD that can map the density of the whole lung and also allows regional assessment of the lung parenchyma [2, 18]. Parametric response mapping (PRM), a voxel-wise comparison of inspiratory and expiratory lung density seen on CT has also been tested for quantification of small airway disease in LT patients. The method showed value for monitoring the progression of BOS and for stratifying the risk of patient death in these patients [2, 33]. Verleden et al. used PRM to evaluate serial measurements from a stable to a diseased state (BOS) in the same patients and compared it to a control group. The authors found that functional small airways disease (corresponding to AT measures) increases at the time of BOS diagnoses and beyond [2]. We were unable to obtain serial measurements, nonetheless the quantitative methods we used indeed showed increased air trapping in patients with BOS. Although PRM may add important additional information, it is time-consuming and requires dedicated software analysis and a careful registration of the lungs. In addition, inadequate or variable inspiratory and expiratory efforts, the use of different CT scanners and settings may introduce important variability when using PRM for quantification of AT. All methods tested in the present study for the detection of AT and BOS are based on standard CT density measurements and are simple to perform.
In clinical practice, it is often not possible to differentiate between BOS and other etiologies at the time point of first decrease in lung function [3], a scenario even more challenging post-SLT, given the presence of one native diseased lung. The use of quantitative methods targeting the transplanted lung only, therefore, could allow more precise identification of BOS and lead to early management. In addition, although all three parameters tested showed good diagnostic performance in isolation, perhaps a combination of them could offer a better strategy to identify different forms of CLAD, integrating both lung volume and density parameters. This hypothesis, however, needs to be tested in a larger sample of LT patients.
The present study has limitations, including the retrospective design and the small sample size from a single transplant center. The diagnostic performance of the quantitative CT parameters to detect BOS was tested against the results of PFTs. Pulmonary function tests have known shortcomings, particularly intraindividual and interrater variability, however, it is the basis of the current strategy used for diagnosing BOS. In addition, CT studies that were not suitable for quantitative analysis were excluded. Nonetheless, studies with minor breathing artifacts or suboptimal inspiratory or expiratory efforts, findings that are relatively common in clinical practice, were allowed.
Conclusion
We demonstrated the feasibility of three quantitative parameters to detect AT in SLT patients obtained from standard non-contrast-enhanced CT studies. In addition, when compared to the current diagnostic criteria based on pulmonary function tests, all three parameters showed a high specificity and overall accuracy for the detection of BOS in this specific population. The addition of these easy to perform parameters to the diagnostic strategy for the detection of chronic lung allograft dysfunction may improve early diagnosis of BOS in single lung transplant patients and deserves further investigation.
References
Verleden GM, Raghu G, Meyer KC, Glanville AR, Corris P (2014) A new classification system for chronic lung allograft dysfunction. J Heart Lung Transplant 33:127–33
Verleden SE, Vos R, Vandermeulen E, Ruttens D, Bellon H, Heigl T et al (2016) Parametric response mapping of bronchiolitis obliterans syndrome progression after lung transplantation. Am J Transplant 16:3262–3269
Dettmer S, Suhling H, Klingenberg I, Otten O, Kaireit T, Fuge J et al (2018) Lobe-wise assessment of lung volume and density distribution in lung transplant patients and value for early detection of Bronchiolitis Obliterans Syndrome. Eur J Radiol. 106:137–44
Arcasoy SM, Kotloff RM. Lung transplantation. N Eng J Med. 1999; 340: 1081–91.
Meyer KC, Raghu G, Verleden GM, Corris PA, Aurora P, Wilson KC et al (2014) An international ISHLT/ATS/ERS clinical practice guideline: diagnosis and management of bronchiolitis obliterans syndrome. Eur Respir J 44(6):1479–1503
Cooper JD, Billingham M, Egan T, Hertz MI, Higenbottam T, Lynch J et al (1993) A working formulation for the standardization of nomenclature and for clinical staging of chronic dysfunction in lung allografts. International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 12:713–16
Chamberlain D, Maurer J, Chaparro C, Idolor L (1994) Evaluation of transbronchial lung biopsy specimens in the diagnosis of bronchiolitis obliterans after lung transplantation. J Heart Lung Transplant 13(6):963–971
Subramanian MP, Meyres BF (2018) Bilateral versus single lung transplantation: are two lungs better than one? J Thorac Dis 10(7):4588–4601
Schaffer JM, Singh SK, Reitz BA, Zamanian RT, Mallidi HR (2015) Single- vs double-lung transplantation in patients with chronic obstructive pulmonary disease and idiopathic pulmonary fibrosis since the implementation of lung allocation based on medical need. JAMA 313(9):936–948
Hadjiliadis D, Angel LF (2006) Controversies in lung transplantation: are two lungs better than one? Semin Respir Crit Care Med 27:561–566
Anyanwu AC, Rogers CA, Murday AJ (2000) Does Splitting the lung block into two single lung- grafts equate to doubling the societal benefit from bilateral lung donors? Comparisons between two single versus one bilateral lung transplant. Transpl Int 13:S201–S202
Gerbase MW, Spiliopoulos A, Rochat T, Archinard M, Nicod LP (2005) Health-related quality of life following single or bilateral lung transplantation. Chest 128:1371–1378
Neurohr C, Huppmann P, Thum D, Leuschner W, von Wulffen W, Meis T et al (2010) Potential functional and survival benefit of double over single lung transplantation for selected patients with idiopathic pulmonary fibrosis. Transpl Int 23:887–896
Ng YL, Paul N, Patsios D, Walsham A, Chung TB, Keshavjee S, et al (2009) Imaging of lung transplantation: review. AJR Am J Roentgenol 192(3 Suppl):S1–S19
Busacker A, Newell JD Jr, Keefe T, Hoffman EA, Granroth JC, Castro M, et al (2009) A multivariate analysis of risk factors for the air-trapping asthmatic phenotype as measured by quantitative CT analysis. Chest 135:48–56
Kauczor HU, Wielpütz MO, Owsijewitsch M, Ley-Zaporozhan J (2011) Computed tomographic imaging of the airways in COPD and asthma. J Thorac Imaging 26(4):290–300
Hansell DM, Bankier AA, MacMahon McLoud TC, Muller NL, Remy J (2008) Fleischner Society: glossary of terms for thoracic imaging. Radiology 246(3):697–722
Solyanik O, Hollmann P, Dettmer S, Kaireit T, Schaefer-Prokop C, Wacker F et al (2015) Quantification of pathologic air trapping in lung transplant patients using CT density mapping: Comparison with other CT air trapping measures. PLoS ONE 10:e0139102
Konen E, Gutierrez C, Chaparro C, Murray CP, Chung T, Crossin J et al (2004) Bronchiolitis obliterans syndrome in lung transplant recipients: can thinsection CT findings predict disease before its clinical appearance? Radiology 231:467–473
Berstad AE, Aaløkken TM, Kolbenstvedt A, Bjortuft O (2006) Performance of long-term CT monitoring in diagnosing bronchiolitis obliterans after lung transplantation. Eur J Radiol 58:124–131
Lee ES, Gotway MB, Reddy GP, Golden JA, Keith FM, Webb WR (2000) Early bronchiolitisobliteransfollowinglungtransplantation: accuracy of expiratory thin-section CT for diagnosis. Radiology 216:472–477
Eda S, Kubo K, Fujimoto K, Matsuzawa Y, Sekiguchi M, Sakai F (1997) The relations between expiratory chest CT using helical CT and pulmonary function tests in emphysema. Am J Respir Crit Care Med 155:1290–1294
Mets OM, Isgum I, Mol CP, Gietema HA, Zanen P, Prokop M et al (2012) Variation in quantitative CT air trapping in heavy smokers on repeat CT examinations. Eur Radiol 22:2710–2717
American Thoracic Society European Respiratory Society American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias This joint statement of the American Thoracic Society (ATS), and the European Respiratory Society (ERS) was adopted by the ATS board of directors, June 2001 and by the ERS Executive Committee, June 2001. Am J Respir Crit Care Med 2002; 165(2): 277–304
Pellegrino R, Viegi G, Brusasco V, Crapo RO, Burgos F, Casaburi R (2005) Interpretative strategies for lung function tests. Eur Respir J 26(5):948–968
Pereira CA, Sato T, Rodrigues SC (2007) New reference values for forced spirometry in white adults in Brazil. J Bras Pneumol 33(4):397–406
Burton CM, Carlsen J, Mortensen J, Andersen CB, Milman N, Iversen M (2007) Long-term survival after lung transplantation depends on development and severity of bronchiolitis obliterans syndrome. J Heart Lung Transplant 26(7):681–686
Kapila A, Baz MA, Valentine VG, Bhorade SM, AIRSAC Investigators (2015) Reliability of diagnostic criteria for bronchiolitis obliterans syndrome after lung transplantation: a survey. J Heart Lung Transplant 34(1):65–74
Matsuoka S, Kurihara Y, Yagihashi K, Hoshino M, Watanabe N, Nakajima Y (2008) Quantitative assessment of air trapping in chronic obstructive pulmonar disease using inspiratory and expiratory volumetric MDCT. AJR Am J Roentgenol 190(3):762–769
Bankier AA, Van Muylem A, Knoop C, Estenne M, Gevenois PA (2001) Bronchiolitis obliterans syndrome in heart-lung transplant recipients: diagnosis with expiratory CT. Radiology 218:533–539
Siegel MJ, Bhalla S, Gutierrez FR, Hildebolt C, Sweet S (2001) Post-lung transplantation bronchiolitis obliterans syndrome: usefulness of expiratory thin-section CT for diagnosis. Radiology. 220:455–62
Leung AN, Fisher K, Valentine V, Girgis RE, Berry GJ, Robbins RC et al (1998) Bronchiolitis obliterans after lung transplantation: detection using expiratory HRCT. Chest 113:365–370
Belloli EA, Degtiar I, Wang X, Yanik GA, Stuckey LJ, Verleden SE et al (2017) Parametric Response Mapping as an Imaging Biomarker in Lung Transplant Recipients. Am J Respir Crit Care Med 195:942–952
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nascimento, D.Z., Watte, G., Torres, F.S. et al. Utilization of Quantitative Computed Tomography Assessment to Identify Bronchiolitis Obliterans Syndrome After Single Lung Transplantation. Lung 199, 29–35 (2021). https://doi.org/10.1007/s00408-020-00417-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00408-020-00417-3