Abstract
Purpose
Fibroproliferation and excess deposition of extracellular matrix (ECM) are the pathologic hallmarks of idiopathic pulmonary fibrosis (IPF), a chronic progressive disorder with high mortality and suboptimal treatment options. Although the etiologic mechanisms responsible for the development and progression of IPF remain unclear, cell-ECM interactions and growth factors are considered important. Cilengitide is a cyclic RGD pentapeptide with anti-angiogenic activity that targets αvβ3, αvβ5 and α5β1, integrins known to mediate cell-ECM interactions and activate the pro-fibrotic growth factor Transforming Growth Factor beta (TGF-β).
Methods
Cilengitide was studied in vitro with the use of NIH/3T3 cells and primary lung fibroblasts, and in vivo in the well-characterized bleomycin-induced lung injury model. The extent of ECM deposition was determined by RT-PCR, Western blot, histologic analysis and hydroxyproline assay of lung tissue. Bronchoalveolar lavage analysis was used to determine cell counts.
Results
Cilengitide treatment of cultured fibroblasts showed decreased adhesion to vitronectin and fibronectin, both integrin-dependent events. Cilengitide also inhibited TGF-β-induced fibronectin gene expression and reduced the accumulation of mRNAs and protein for fibronectin and collagen type I. Both preventive and treatment effects of daily injections of cilengitide (20 mg/kg) failed to inhibit the development of pulmonary fibrosis as determined by histological analysis (Ashcroft scoring), bronchoalveolar lavage (BAL) fluid cell counts, and hydroxyproline content.
Conclusions
Overall, our data suggest that, despite its in vitro activity in fibroblasts, daily injections of cilengitide (20 mg/kg) did not inhibit the development of or ameliorate bleomycin-induced pulmonary fibrosis in mice.
Similar content being viewed by others

Avoid common mistakes on your manuscript.
Introduction
Idiopathic pulmonary fibrosis is a largely sporadic but sometimes hereditary progressive lung disease affecting millions of patients worldwide [1]. Despite advances in diagnosis and the recent approval of two new drugs for treatment, the median survival for patients after diagnosis remains 3–5 years, and no current therapies are able to halt or reverse the progression of disease. Although the underlying mechanism of pulmonary fibrosis remains incompletely understood, significant inroads have been made into the biology of extracellular matrix (ECM) and fibroproliferation. The orderly structure of epithelial and mesenchymal cell layers is disrupted in pulmonary fibrosis due to inflammation, oxidative stress, and excessive ECM protein expression and turnover [2]. This leads to thickening of the pulmonary interstitium and irreversible obliteration of the alveolar spaces necessary for proper lung function.
ECMs not only provide structural support, but also provide cues that influence cellular migration, differentiation and other functions through cell-ECM interactions. Signaling receptors, termed integrins, are cell surface receptors formed by non-covalent interactions between α and β integrin subunits that, when activated, transduce signals capable of impacting complex processes ranging from alveolar maturation to vasculogenesis. Of these receptor complexes, the αv integrins appear to play roles in tissue fibrosis, thereby unveiling another set of potential therapeutic targets [3]. For example, αvβ5 integrin expression was found to be increased in fibrotic foci of lung tissue harvested from patients with idiopathic pulmonary fibrosis [4]. In other work, knockdown of the αv integrin gene attenuated fibrosis in a mouse model of lung fibrosis induced by bleomycin [5]. Furthermore, knockdown of the β6 gene, an integrin subunit often found complexed with αv, protects from bleomycin-induced pulmonary fibrosis [6]. Increased expression of αvβ3 and αvβ5 has been detected in models of cardiac and skin fibrosis as well, and treatment of primary fibroblasts from scleroderma patients with anti-αvβ5 and anti-αvβ5 antibodies reduced collagen I expression [7,8,9]. Together, these investigations suggest that interventions targeting αv integrin activity could alter the development and/or progression of pulmonary fibrosis.
Cilengitide, a cyclic arginine-glycine-aspartic acid (RGD) pentapeptide that mimics the binding motifs of prominent ECM proteins such as fibronectin and vitronectin, is designed to inhibit ligand binding to αv integrins [10]. Specifically, it inhibits αvβ3, αvβ5 and α5β1 at subnanomolar and nanomolar concentrations, respectively. Preclinical studies in mouse models of glioblastoma led to clinical trials of cilengitide as an anti-tumor agent and many other studies have investigated the ability of cilengitide to inhibit angiogenesis [11]. However, there are fewer preclinical studies of cilengitide in fibrosis models. In a study by Li et al., cilengitide inhibited TGF-β1 activity and collagen I (αI) expression in a mouse model of Crohn’s disease [12]. Patsenker and colleagues demonstrated that treatment with cilengitide decreased hepatic angiogenesis in a model of liver fibrosis [13]. The effects of cilengitide in the lung are unknown.
In the current study, we investigated the ability of cilengitide to attenuate experimental pulmonary fibrosis. To our knowledge, this study represents the first characterization of the effects of cilengitide in an experimental model of pulmonary fibrosis.
Materials and Methods
Experimental Reagents
Cilengitide was purchased from MedChem Express (Monmouth Junction, NJ). Recombinant mouse TGF-β1 was purchased from R&D Systems (Minneapolis, MN). Recombinant human vitronectin was purchased from Peprotech (Rocky Hill, NJ) and human plasma fibronectin was purchased from Sigma-Aldrich (St. Louis, MO). NIH/3T3 murine fibroblasts were purchased from American Type Culture Collection (Rockville, MD). Bleomycin was purchased from Sigma-Aldrich (St. Louis, MO).
Luciferase Expression Assay
To evaluate the effect of cilengitide on TGF-β1-stimulated fibronectin promoter activity, NIH/3T3 fibroblasts stably transfected with a reporter plasmid containing the human fibronectin (FN) gene promoter driving the luciferase (LUC) gene were utilized [14]. Cells were plated at 5000 per 100 μL in Dulbecco’s modified Eagle’s medium (DMEM) with 4.5 g/L glucose supplemented with 10% heat-inactivated fetal bovine serum (FBS) and 1% antibiotic-antimycotic solution. After 24 h, media was replaced with DMEM (0.1% FBS) before stimulation with TGF-β1 and/or cilengitide. After 24 h, cells were lysed with Luciferase Cell Culture Lysis Reagent and mixed with Luciferase Assay Reagent (Promega, Madison, WI). Luminescence, proportional to fibronectin promoter activity, was quantified using a Labsystems Luminoskan Ascent Luminometer (Helsinki, Finland).
Cell Adhesion Assay and Cell Viability Assay
To evaluate cilengitide inhibition of fibroblast adhesion, 96 or 12 well plates were coated with 5 μg/ml human recombinant vitronectin or human plasma fibronectin. NIH/3T3 or primary lung fibroblasts, isolated as previously described [15], were plated using different concentrations of cilengitide and allowed to adhere. After 1 h, non-adherent cells were washed away. P-nitrophenyl n-acetyl β-D-glucosaminide substrate (7.5 mM in 0.1 M sodium citrate) was added and incubated at 37 °C overnight. After the addition of quenching buffer (50 mM glycine, 5 mM EDTA, pH 10), colorimetric results correlated to a standard curve were obtained using a Beckman Coulter AD 340C Absorbance Detector (Beckman Coulter, Indianapolis, IN) at 405 nm. Cell viability was measured by trypsinization, harvesting, resuspension in trypan blue and measuring with the Bio-Rad TC20 Automated Cell Counter (Bio-Rad, Hercules, CA).
Measurement of mRNA
The Quick-RNA MiniPrep kit (Zymo Research, Irvine, CA) was used for RNA isolation from fibroblasts. The iScript cDNA Synthesis Kit (Bio-Rad, Hercules, CA) was used to generate cDNA, Real-time quantitative PCR (qPCR) was used to measure collagen (I), fibronectin, alpha smooth muscle actin and 18s mRNA expression using TaqMan probes (TaqMan® Gene Expression Assay Mm00801666-g1, Mm01256744_m1, Mm00725412_s1, Mm03928990_g1), according to the manufacturer’s protocol. Step One Plus Real-Time PCR System (Applied Biosystems, Wilmington, DE) was used with the parameters: 50 °C for 2 min, 95 °C for 20 s, followed by 40 cycles of 95 °C for 1 s and 60 °C for 20 s. The use of iTaq Universal SYBR Green Supermix (Bio-Rad, Hercules, CA) was also used with the following parameters: 95 °C for 3 min, then 40 cycles of 95 °C for 15 s, 60 °C for 1 min. The mouse fibronectin primers were forward: (5′- GACTGTACTTGTCTAGGCGAAG) and reverse (5′-GTTTCCTCGGTTGTCCTTCT). Results were analyzed using Step One Software version 2.3 (Applied Biosystems, Wilmington, DE). The amplification curves were analyzed by the mathematical equation of the second derivative, and the amount of mRNA expression was normalized to the housekeeping gene 18s mRNA. The 2-ΔΔCT method was used to calculate relative quantification [16].
Measurement of Protein
NIH/3T3 fibroblasts were plated into 100 mm dishes (8 × 105 cells/dish) in Dulbecco’s modified Eagle’s medium (DMEM) with 4.5 g/L glucose supplemented with 10% heat-inactivated fetal bovine serum (FBS) and 1% antibiotic-antimycotic solution. After 24 h, media was replaced with DMEM (0.1% FBS) before stimulation with TGF-β1 (2 ng/ml) and/or cilengitide (10–1000 nM). After 48 h, cells were harvested in RIPA buffer (Thermofisher, Waltham, MA) and homogenized using a Bead Mill 4 cell disruptor (Thermofisher, Waltham, MA). Total protein was measured using Bradford protein dye reagent (Bio-Rad, Hercules, CA) and quantified using a Beckman DU800 spectrophotometer (Beckman Coulter, Brea, CA) at OD 595. Samples (20 μg) were loaded onto a 5% SDS polyacrylamide gel and electrophoresed at 150 V for 3 h. Afterwards, protein was transferred onto 0.2 μm nitrocellulose membrane (Bio-Rad, Hercules, CA) at 25V for 2.0 h using a Trans-Blot SD semi-dry transfer cell (Bio-Rad, Hercules, CA). Membranes were blocked for 1 h at room temperature in Odyssey Blocking Buffer (Li-COR Biosciences, Lincoln, NE) and incubated overnight at 4 °C with primary antibody against GAPDH (Sigma, St. Louis, MO, G9545; 1:10,000), fibronectin (Sigma, F3648, 1:1000), or collagen (Invitrogen, PA5-95137; 1:1000). Afterwards, membranes were washed and incubated with a secondary antibody (Li-COR, 1:20,000) for 1 h at room temperature. Membranes were washed and scanned using a Li-COR Odyssey CLx imaging system and analyzed in Image Studio Lite (LI-COR).
Animals and Experimental Design
Eight-week-old male C57BL/6 mice (Jackson Laboratory, Bar Harbor, ME) were housed in a pathogen-free barrier facility. All animal procedures were approved by the University of Louisville’s Institutional Animal Care and Use Committee. Mice were anesthetized, the trachea were exposed and bleomycin was instilled at 1 U/kg body weight. For assessment of prevention of pulmonary fibrosis, we began daily cilengitide and control treatment (20 mg/kg in 100 μL PBS, i.p.) 24 h after bleomycin instillation. For amelioration of pulmonary fibrosis, cilengitide and control treatment began 7 days after bleomycin instillation. The dosage of cilengitide was determined from the evaluation of previous studies where similar doses of cilengitide inhibited TGF-β1 activity and collagen I (αI) expression in a model of Crohn’s disease [12] and decreased hepatic angiogenesis in a model of liver fibrosis [13]. All mice were sacrificed 21 days after bleomycin instillation.
Histologic Analysis
All lungs were flushed with cold PBS, instilled with 10% buffered formalin and the whole left lung was excised and fixed for 24 h. Afterwards, the left lung was dehydrated and paraffin-embedded using standard procedures. Coronal sections 5 μm thick were cut from the central part of the lung and stained with Masson’s trichrome. Evaluation of the extent of fibrosis was performed by five laboratory investigators blinded to the experimental groups and scored using the Ashcroft scale [17].
Bronchoalveolar Lavage Analysis
After euthanasia, sterile PBS (800 μL) was flushed into the lungs and gently aspirated. This bronchoalveolar lavage fluid (BALF) was then subject to a total cell count using a Bio-Rad TC20 cell counter.
Hydroxyproline Assay
The excised right upper lobes of the mice were snap frozen in liquid nitrogen, minced with a razor blade, weighed, and incubated in 500 μL of 6 N HCl at 55 °C for 18 h. The mixture was filtered, 80 μL were mixed with 400 μL of chloramine T solution for 20 min at room temperature. Samples were mixed with 400 μL of Ehrlich’s solution and incubated at 65 °C for 15 min. Colorimetric results, correlated to a concurrently run hydroxyproline standard curve, were obtained by spectrophotometric analysis at 550 nm.
Statistics
Data are presented as means ± standard error and analyzed using Graphpad Prizm. Two-tailed Student’s T-test was used to determine significance between groups with p < 0.05 considered statistically significant.
RESULTS
Cilengitide Inhibits Fibroblasts Function In Vitro
Fibroblasts are a primary mediator of pulmonary fibrosis, and express αv integrins that are inhibited by cilengitide. We utilized two in vitro assays, a cell based luciferase reporter assay and a cell adhesion assay, to verify that cilengitide can inhibit the cellular actions of fibroblasts. For the luciferase reporter assay, we used NIH/3T3 murine fibroblasts stably transfected with a plasmid expressing the luciferase gene under control of the human fibronectin promoter. We stimulated these fibroblasts with recombinant mouse TGF-β1 in the presence or absence of cilengitide. As expected, TGF-β1 was able to stimulate luciferase production by 39%, but with addition of 1000 nM cilengitide, this increase in luciferase production was reduced to only 11% (Fig. 1a); cell viability was not affected (Fig. 1b). Cilengitide also inhibited TGF-β1-induced accumulation of mRNAs coding for fibronectin and collagen type I in NIH/3T3 murine fibroblasts (Fig. 1c, d). Similar observations were made in primary lung fibroblasts (not shown). Cilengitide also inhibited TGF-β1-induced accumulation of both fibronectin and collagen type I protein in NIH/3T3 murine fibroblasts (Fig. 1e, f).
Cilengitide inhibits fibroblast adhesion and TGFβ-induced ECM expression. (a) Fibronectin promoter-luciferase gene-transfected NIH 3T3 cells were incubated with cilengitide in the presence or absence of TGF-β1 and analyzed for luciferase gene expression by a luminescence assay. (b) The viability of the fibronectin promoter-luciferase gene-transfected NIH 3T3 cells incubated with cilengitide in presence or absence of TGF-β1 was measured, results are represented as percentage viable cells detected. (c) Collagen Type (I) mRNA accumulation was examined in TGF-β stimulated fibroblasts using Real-time quantitative PCR (qPCR). Amplification curves were analyzed by the mathematical equation of the second derivative, and the amount of mRNA expression was normalized to the housekeeping gene 18s mRNA expression. Results expressed as fold increase in mRNA expression compared to non-treated controls. (d) Fibronectin mRNA accumulation was detected in TGF-β stimulated fibroblasts. (e) Collagen Type (I) protein expression was examined in TGF-β stimulated fibroblasts treated with or without cilengitide using Western blot. (f) Fibronectin protein expression was examined in TGF-β stimulated fibroblasts treated with or without cilengitide using Western blot. (g) Assay of NIH 3T3 cell adhesion to vitronectin-coated 96 or 12 well plates in the presence of cilengitide. (h) Viability of NIH 3T3 cells adhered to vitronectin-coated 12 well plate in the presence of cilengitide was measured, results are represented as percentage viable cells detected. (i) Assay of NIH 3T3 cell adhesion to fibronectin-coated 12 well plate in the presence of cilengitide. (j) Viability of NIH 3T3 cells adhered to fibronectin-coated 12 well plate in the presence of cilengitide was measured, results are represented as percentage viable cells detected. Data are expressed as means ± standard error. * represent values significantly different from controls where p < 0.05
To test cell adherence, NIH/3T3 fibroblasts were exposed to varying doses of cilengitide, while cultured on vitronectin-coated plates and allowed to adhere for 1 h. We found that 1000 nM cilengitide significantly inhibited cell adhesion to vitronectin by 52% without affecting cell viability (Fig. 1g, h); similar observations were made for adhesion to fibronectin (Fig. 1i, j). Collectively, our results demonstrate that cilengitide can inhibit fibroblast cell adhesion and ECM protein expression.
Cilengitide Does Not Prevent Bleomycin-Induced Pulmonary Fibrosis
Having established that cilengitide has inhibitory activity towards fibroblasts, we then tested its actions in a well-established murine model of pulmonary fibrosis induced by bleomycin. To answer the question whether cilengitide could prevent the development of fibrosis, we instilled 8-week-old C57BL/6 mice with 1 U/kg of intra-tracheal bleomycin and 24 h later began daily intraperitoneal cilengitide injections (Fig. 2a). Twenty-one days after the initial bleomycin instillation, the mice were euthanized. We first stained the left lung tissue with trichrome and scored results using the Ashcroft scale (Fig. 2b, c). There was no significant difference in the amount of fibrosis between the vehicle and cilengitide groups. We performed a hydroxyproline assay on the right upper lobes, but also did not detect any difference between treatment groups (Fig. 2d). From the BALF analysis, we did not find any significant differences in total cell counts (Fig. 2e). Overall, we did not find any difference between bleomycin-instilled mice treated with 21 days of cilengitide or vehicle control.
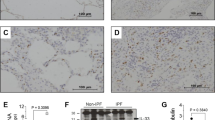
Cilengitide does not prevent bleomycin-induced fibrosis. (a) Schematic of cilengitide prevention in bleomycin-induced pulmonary fibrosis time course. (b) Histologic section of left lungs from representative vehicle or cilengitide-treated mice. (c) Ashcroft scoring of histologic lung sections. (d) Hydroxyproline assay of the right upper lobe, normalized as grams hydroxyproline per 100 g of lung tissue. (e) Bronchoalveolar lavage fluid total cell counts
Cilengitide Does Not Ameliorate Bleomycin-Induced Pulmonary Fibrosis
Although we were unable to prevent the development of pulmonary fibrosis with immediate cilengitide treatment, we persevered to ask if cilengitide could at least ameliorate the extent of fibrosis. To this end, we waited 7 days before commencing treatment (Fig. 3a). Again, we did not observe any difference in Ashcroft-scored histologic sections of lung tissue (Fig. 3b, c), hydroxyproline content (Fig. 3d) or cell counts of the BALF (Fig. 3e) between treatment groups. Overall, cilengitide was not able to ameliorate the pulmonary fibrotic effects of bleomycin.
Cilengitide does not ameliorate bleomycin-induced pulmonary fibrosis. (a) Schematic of cilengitide amelioration in bleomycin-induced pulmonary fibrosis time course. (b) Histologic section of left lungs from representative vehicle or cilengitide-treated mice. (c) Ashcroft scoring of histologic lung sections. (d) Hydroxyproline assay of the right upper lobe, normalized as grams hydroxyproline per 100 g of lung tissue. (e) Bronchoalveolar lavage fluid total cell counts
Discussion
In this report, we demonstrate that cilengitide can inhibit fibroblast adhesion to vitronectin and fibronectin, as well as attenuate TGF-β-induced fibronectin gene transcription and fibronectin and collagen type I mRNA and protein accumulation without affecting cell viability. We then utilized a well-characterized model of pulmonary fibrosis to corroborate these actions in vivo. Unfortunately, we were not successful in preventing the development of pulmonary fibrosis nor were we able to ameliorate the fibrotic response with cilengitide. Previous studies from other investigators have demonstrated promise for inhibition of integrins as a therapeutic avenue in pulmonary fibrosis. Our negative findings may be the result of suboptimal pharmacodynamics of drug administration, or the limits of the pharmacokinetics of cilengitide itself.
Fibroblast-vitronectin adhesion and ECM expression are important events in fibroproliferation that can be subject to inhibition [18]. It is also the primary ligand for αvβ3 and αvβ5 integrins [19, 20]. Our results showing inhibition of fibroblast adhesion to vitronectin in vitro is evidence that cilengitide has direct activity on fibroblasts. Fibronectin is another glycoprotein produced and assembled in the ECM during wound healing by macrophages and fibroblasts in response to TGF-β [21, 22]. That we were also able to inhibit another fibroblast process, TGF-β-induced fibronectin expression, with cilengitide further supported its therapeutic potential in deranged fibrosis.
This potential to prevent the development of fibrosis in bleomycin-induced pulmonary fibrosis is what we tested next. The rationale for prevention was based on multiple previous studies that implicate integrins in the pathogenesis of pulmonary fibrosis. Expression of αvβ5 integrin was found in fibrotic foci of lung biopsies from patients with idiopathic pulmonary fibrosis [4]. Scleroderma fibroblasts exhibit increased expression of αvβ3 and αvβ5 integrins [9]. The genetic deletion of αv integrin subunit in mice prevented bleomycin-induced fibrosis and small molecule inhibition of all αv containing integrins achieved a similar endpoint [5]. Although the most promising integrin target for prevention of pulmonary fibrosis is the more extensively characterized αvβ6, and the genetic knockout of β3 and β5 subunits did not prevent development of bleomycin-induced pulmonary fibrosis, a small molecule inhibition study focusing on αvβ3 and αvβ5 with cilengitide has never, to our knowledge, been conducted. Furthermore, there is evidence that integrin-knockout and small molecule integrin inhibition produce different effects [23]. Therefore cilengitide effects on pulmonary fibrosis may differ from integrin-knockout studies. Finally, cilengitide has been studied primarily in the context of angiogenesis inhibition, but a preclinical study in Crohn’s disease and TNBS colitis demonstrated anti-fibrotic activity [12]. Although we were optimistic that cilengitide possessed anti-fibrotic activity, in bleomycin-induced pulmonary fibrosis, this turned out to be premature.
The negative results of our two cilengitide treatment trials may be attributed to suboptimal route of administration, frequency and dose of cilengitide, or to the organ-specific biology of αv integrins. Because an aforementioned study utilized daily intraperitoneal cilengitide injections and achieved decreased collagen production by intestinal muscle cells after TNBS treatment, we utilized a similar cilengitide dose and route in our own investigation [12]. However, intraperitoneal injection may not be the optimal route for pulmonary-specific cilengitide action. Pirfenidone, a currently approved drug for IPF, prevents bleomycin-induced pulmonary fibrosis when administered intravenously [24]. Nintedanib, another approved IPF drug, demonstrated anti-fibrotic activity in bleomycin-induced pulmonary fibrosis via gavage administration [25]. During human clinical trials, cilengitide was also administered intravenously [26]. However, cilengitide ultimately failed to prevent glioblastoma progression in a phase 3 trial, thus the in vivo pharmacokinetics of cilengitide are still incompletely understood [27]. Although cilengitide did not show a reduction in fibrosis at the dose tested, others have demonstrated increased efficacy in its effect with adjunct treatments. In a study by Kim et al., the authors showed that the combined treatment with cilengitide and belotecan, a camptothecin anti-tumor analog, increased apoptosis of tumor cells in vitro and decreased angiogenesis in vivo to significantly reduce tumor volume and increase overall survival [28]. Bon and colleagues showed that ECM accumulation during fibroblast-epithelial cell interactions in a renal co-culture assay system was inhibited by the use of the anti-fibrotic drug nintedanib and partially abrogated by TGFβ neutralization [29]. However, they also discovered that the use of nintedanib and TGFβ neutralization did not return ECM levels to baseline unless they included the use of anti-integrin blocking antibodies and small molecule inhibitors in their system. They found a major role of the αV integrins in the ECM accumulation process. This highlights the possibility of the use of adjunct treatments together, such as pirfenidone or nintedanib and αV integrin inhibitors, such as cilengitide, as a more efficacious treatment strategy.
We chose to use one-a-day dosing, but this may have limited Cilengitide’s efficacy against fibrosis considering its relatively short half-life of up to 5 h in humans [26]. Furthermore, although we demonstrated that 1000 nM cilengitide was effective against fibroblasts in vitro, the appropriate corresponding doses for treatment in vivo are difficult to assess. Cilengitide stimulates angiogenesis at low doses, but inhibits angiogenesis at high dose, therefore our limited in vivo results may be the result of suboptimal dosing as well [30]. In addition, fibroblasts are not the only cell type in the lung that express αvβ3 and αvβ5 integrins; thus, effects on fibroblasts may not directly correlate to effects on leukocytes and other cells [31]. Finally, the inhibition-biology of integrins still requires further understanding. Pan αv integrin inhibition can attenuate fibrosis across multiple solid organs, but individual targeting of various β subunits have produced only partial or contradictory results [5]. Of course, one other possibility is that αv integrins do not play a major role in our model, but this deserves further exploration. Overall, our negative results highlight the challenges of targeting in vivo cellular processes with peptide RGD integrin inhibitors.
Further understanding of the biology of αv integrin family and additional optimization of cyclic RGD peptides may lead to more efficacious drugs in the future.
References
Thannickal VJ, Henke CA, Horowitz JC, Noble PW, Roman J, Sime PJ, Zhou Y, Wells RG, White ES, Tschumperlin DJ (2014) Matrix biology of idiopathic pulmonary fibrosis: a workshop report of the national heart, lung, and blood institute. Am J Pathol 184(6):1643–1651. https://doi.org/10.1016/j.ajpath.2014.02.003
Watson WH, Ritzenthaler JD, Roman J (2016) Lung extracellular matrix and redox regulation. Redox Biol 8:305–315. https://doi.org/10.1016/j.redox.2016.02.005
Jenkins RG, Su X, Su G, Scotton CJ, Camerer E, Laurent GJ, Davis GE, Chambers RC, Matthay MA, Sheppard D (2006) Ligation of protease-activated receptor 1 enhances alpha(v)beta6 integrin-dependent TGF-beta activation and promotes acute lung injury. J Clin Invest 116(6). https://doi.org/10.1172/JCI27183.1606
Scotton CJ, Krupiczojc MA, Königshoff M, Mercer PF, Lee YC, Kaminski N, Morser J, Post JM, Maher TM, Nicholson AG, Moffatt JD, Laurent GJ, Derian CK, Eickelberg O, Chambers R (2009) Increased local expression of coagulation factor X contributes to the fibrotic response in human and murine lung injury. J Clin Invest 119(9):2550–2563
Henderson NC, Arnold TD, Katamura Y, Giacomini MM, Rodriguez JD, McCarty JH et al (2013) Targeting of αv integrin identifies a core molecular pathway that regulates fibrosis in several organs. Nat Med 19(12):1617–1624. https://doi.org/10.1038/nm.3282
Munger JS, Huang X, Kawakatsu H, Griffiths MJ, Dalton SL, Wu J et al (1999) The integrin αvβ6 binds and activates latent TGFβ1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell 96(3):319–328. https://doi.org/10.1016/S0092-8674(00)80545-0
Sarrazy V, Koehler A, Chow ML, Zimina E, Li CX, Kato H, Caldarone CA, Hinz B (2014) Integrins αvβ5 and αvβ3 promote latent TGF-β1 activation by human cardiac fibroblast contraction. Cardiovasc Res 102(3):407–417. https://doi.org/10.1093/cvr/cvu053
Asano Y, Ihn H, Yamane K, Jinnin M, Tamaki K (2006) Increased expression of integrin αvβ5 induces the myofibroblastic differentiation of dermal fibroblasts. Am J Pathol 168(2):499–510. https://doi.org/10.2353/ajpath.2006.041306
Asano Y, Ihn H, Yamane K, Jinnin M, Mimura Y, Tamaki K (2005) Increased expression of integrin α v β 3 contributes to the establishment of autocrine TGF-β signaling in scleroderma fibroblasts. J Immunol 175(11):7708–7718. https://doi.org/10.4049/jimmunol.175.11.7708
Mas-Moruno C, Rechenmacher F, Kessler H (2010) Cilengitide: the first anti-angiogenic small molecule drug candidate. Design, synthesis and clinical evaluation. Anti Cancer Agents Med Chem 10(10):753–768. https://doi.org/10.2174/187152010794728639
Nabors LB, Mikkelsen T, Rosenfeld SS, Hochberg F, Akella NS, Fisher JD et al (2007) Phase I and correlative biology study of cilengitide in patients with recurrent malignant glioma. J Clin Oncol 25(13):1651–1657. https://doi.org/10.1200/JCO.2006.06.6514
Li C, Flynn RS, Grider JR, Murthy KS, Kellum JM, Akbari H, Kuemmerle JF (2013) Increased activation of latent TGF-β1 by αVβ3 in human Crohn’s disease and fibrosis in TNBS colitis can be prevented by cilengitide. Inflamm Bowel Dis 19(13):2829–2839. https://doi.org/10.1097/MIB.0b013e3182a8452e16
Patsenker E, Popov Y, Stickel F, Schneider V, Ledermann M, Sägesser H, Niedobitek G, Goodman SL, Schuppan D (2009) Pharmacological inhibition of integrin alphavbeta3 aggravates experimental liver fibrosis and suppresses hepatic angiogenesis. Hepatology 50(5):1501–1511. https://doi.org/10.1002/hep.23144
Michaelson JE, Ritzenthaler JD, Roman J (2002) Regulation of serum-induced fibronectin expression by protein kinases, cytoskeletal integrity, and CREB. Am J Physiol Lung Cell Mol Physiol 282:291–301. https://doi.org/10.1152/ajplung.00445.2000
Sueblinvong V, Neujahr DC, Mills ST, Roser-Page S, Ritzenthaler JD, Guidot D, Rojas M, Roman J (2012) Predisposition for disrepair in the aged lung. Am J Med Sci 344(1):41–51. https://doi.org/10.1097/MAJ.0b013e318234c132
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 25(4):402–408. https://doi.org/10.1006/meth.2001.1262
Ashcroft T, Simpson JM, Timbrell V (1988) Simple method of estimating severity of pulmonary fibrosis on a numerical scale. J Clin Pathol 41(4):467–470. https://doi.org/10.1136/jcp.41.4.467
Pohl WR, Conlan MG, Thompson AB, Ertl RF, Romberger DJ, Mosher DF, Rennard SI (1991) Vitronectin in bronchoalveolar lavage fluid is increased in patients with interstitial lung disease. Am Rev Respir Dis 143(6):1369–1375. https://doi.org/10.1164/ajrccm/143.6.1369
Roberts MS, Woods AJ, Shaw PE, Norman JC (2003) ERK1 associates with αvβ3 integrin and regulates cell spreading on vitronectin. J Biol Chem 278(3):1975–1985. https://doi.org/10.1074/jbc.M208607200
Hermann P, Armant M, Brown E, Rubio M, Ishihara H, Ulrich D, Caspary RG et al (1999) The vitronectin receptor and its associated CD47 molecule mediates proinflammatory cytokine synthesis in human monocytes by interaction with soluble CD23. J Cell Biol 144(4):767–775. https://doi.org/10.1083/jcb.144.4.767
Ignotzs RA, Massague J (1986) Transforming growth factor stimulates the expression of fibronectin and collagen and their incorporation into the extracellular atrix. J Biol Chem 261(9):4337–4345
Hocevar BA, Brown TL, Howe PH (1999) TGF-β induces fibronectin synthesis through a c-Jun N-terminal kinase-dependent, Smad4-independent pathway. EMBO J 18(5):1345–1356
Kim S, Bakre M, Yin H, Varner JA (2002) Inhibition of endothelial cell survival and angiogenesis by protein kinase A. J Clin Invest 110(7):933–941. https://doi.org/10.1172/JCI0214268
Oku H, Shimizu T, Kawabata T, Nagira M, Hikita I, Ueyama A, Matsushima S, Torii M, Arimura A (2008) Antifibrotic action of pirfenidone and prednisolone: different effects on pulmonary cytokines and growth factors in bleomycin-induced murine pulmonary fibrosis. Eur J Pharmacol 590:400–408. https://doi.org/10.1016/j.ejphar.2008.06.046
Wollin L, Maillet I, Quesniaux V, Holweg A, Ryffel B (2014) Antifibrotic and anti-inflammatory activity of the tyrosine kinase inhibitor nintedanib in experimental models of lung fibrosiss. J Pharmacol Exp Ther 349(2):209–220. https://doi.org/10.1124/jpet.113.208223
Eskens FALM, Dumez H, Hoekstra R, Perschl A, Brindley C, Böttcher S, Wynendaele W, Drevs J, Verweij J, van Oosterom AT (2003) Phase I and pharmacokinetic study of continuous twice weekly intravenous administration of Cilengitide (EMD 121974), a novel inhibitor of the integrins αvβ3 and αvβ5 in patients with advanced solid tumours. Eur J Cancer 39(7):917–926. https://doi.org/10.1016/S0959-8049(03)00057-1
Stupp R, Hegi ME, Gorlia T, Erridge SC, Perry J, Hong YK et al (2014) Cilengitide combined with standard treatment for patients with newly diagnosed glioblastoma with methylated MGMT promoter (CENTRIC EORTC 26071-22072 study): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol 15(10):1100–1108. https://doi.org/10.1016/S1470-2045(14)70379-1
Kim YH, Lee JK, Kim B, DeWitt JP, Lee JE, Han JH, Kim SK, Oh CW, Kim CY (2013) Combination therapy of cilengitide with belotecan against experimental glioblastoma. Int J Cancer 133(3):749–756. https://doi.org/10.1002/ijc.28058 Epub 2013 Feb 27
Bon H, Hales P, Lumb S, Holdsworth G, Johnson T, Qureshi O, Twomey BM (2019) Spontaneous extracellular matrix accumulation in a human in vitro model of renal fibrosis is mediated by αV integrins. Nephron 142(4):328–350. https://doi.org/10.1159/000499506
Tucci M, Stucci S, Silvestris F (2014) Does cilengitide deserve another chance? Lancet Oncol 15(13):e584–e585. https://doi.org/10.1016/S1470-2045(14)70462-0
Luzina IG, Todd NW, Nacu N, Lockatell V, Choi J, Hummers LK, Atamas SP (2009) Regulation of pulmonary inflammation and fibrosis through expression of integrins αVβ3 and αVβ5 on pulmonary T lymphocytes. Arthritis Rheum 60(5):1530–1539. https://doi.org/10.1002/art.24435
Funding
This work was supported by a Grant from the Department of Veterans Affairs grant (BX000216) to JR.
Author information
Authors and Affiliations
Contributions
Concept and design: MZ, ETG, JDR, JR; Analysis and interpretation: MZ, ETG, JDR, JR; Drafting manuscript and important intellectual input: MZ, JDR, JR.
Corresponding author
Ethics declarations
Conflict of interest
Jesse Roman receives support for research related to clinical trials from industry (e.g., Genentech, Boehringer Ingelheim, Galapagos). He also serves as consultant on DSMB and steering committees for Genentech, Novartis and Boehringer Ingelheim. All other authors, no potential conflict of interest was reported.
Ethical Approval
All animal procedures were approved by the University of Louisville’s Institutional Animal Care and Use Committee.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ritzenthaler, J.D., Zhang, M., Torres-Gonzalez, E. et al. The Integrin Inhibitor Cilengitide and Bleomycin-Induced Pulmonary Fibrosis. Lung 198, 947–955 (2020). https://doi.org/10.1007/s00408-020-00400-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00408-020-00400-y