Abstract
Introduction
The mechanism of fast inspiratory flow rate (VI′) induced lung injury is unclear. As fast VI′ increases hysteresis, a measure of surface tension at the air–liquid interface, surfactant release or function may be important. This experimental study examines the contribution of impaired surfactant release or function to dynamic-VILI.
Methods
Isolated perfused lungs from male Sprague Dawley rats were randomly allocated to four groups: a long or short inspiratory time (Ti = 0.5 s; slow VI′ or Ti = 0.1 s; fast VI′) at PEEP of 2 or 10 cmH2O. Tidal volume was constant (7 ml/kg), with f = 60 breath/min. Forced impedance mechanics (tissue elastance (Htis), tissue resistance (Gtis) and airway resistance (Raw) were measured at 30, 60 and 90 min following which the lung was lavaged for surfactant phospholipids (PL) and disaturated PL (DSP).
Results
Fast VI′ resulted in a stiffer lung. Concurrently, PL and DSP were decreased in both tubular myelin rich and poor fractions. Phospholipid decreases were similar with PEEP. In a subsequent cohort, laser confocal microscopy-based assessment demonstrated increased cellular injury with increased VI′ at both 30 and 90 min ventilation.
Conclusion
Rapid VI′ may contribute to ventilator induced lung injury (VILI) through reduced surfactant release and/or more rapid reuptake despite unchanged tidal stretch.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ventilator-induced lung injury (VILI) has become recognized as a major adverse effect of ventilator assistance. Lung injury predisposes to VILI as associated lung collapse, inhomogeneity of aeration and reduced recruitability result in greater stress and strain on the lung [1]. Following pivotal basic and clinical research, current approaches to protective ventilation focus on static factors—tidal volume, plateau pressure and positive end-expiratory pressure (PEEP).
Inspiratory flow rate (VI′) and respiratory rate are dynamic factors that also contribute to VILI, and to the power that is dissipated during ventilation [1, 2]. While the respiratory rate and VI′ are related through the inspiratory time and inspiratory to expiratory (I:E) ratio, it appears that VI′ plays a greater role in VILI [3]. Indeed, analyzing the components of mechanical power, Gattinoni et al. suggest that tidal volume, driving pressure and VI′ have similar contributions [1, 2]. While the mechanisms of lung injury due to excessive volume and pressure have been extensively investigated [4], relatively little is known regarding lung injury due to high VI′.
Protti et al. [5] suggest that high VI′ lung injury may be due to amplified viscoelastic behavior. They report greater dynamic hysteresis and greater stress relaxation at high VI′ consistent with Eissa et al. [6] who had earlier reported an increase in stress relaxation both in the acute respiratory distress syndrome (ARDS) and with increasing VI′. While Protti et al. [5] suggested that this led to mechanical failure of the lung cytoskeleton, other factors including surface tension and inhomogeneity of ventilation also determine non-linear behavior and stress relaxation.
The amplitude, magnitude and rate of cell deformation led to stress failure and cell wounding of cultured alveolar type II epithelial cells, as measured by increased uptake of the membrane impermeable marker propidium iodide, and are associated with increased lipid trafficking [7,8,9]. As the wounded plasma membrane upregulates inflammatory gene expression and growth factor release, high strain or strain rate may lead to important changes prior to obvious cell injury. However, while these results have been confirmed in the whole lung where high strain amplitude (high VT) lung results in cell wounding [10], the whole lung effects of high strain rate (high VI′) have not been previously examined. Therefore, we used an isolated perfused lung model to examine the effects of high vs low VI′ on cell wounding and pulmonary surfactant release.
Materials and Methods
Animals
Specific pathogen-free male Sprague Dawley rats (body weight (BW) = 200 − 290 g) were used. All experiments were approved by the Flinders University Animal Welfare Committee and performed according to the National Health and Medical Research Council of Australia Guidelines on Animal Experimentation.
Isolated Perfused Lung Preparation
Rats were anesthetized with thiopentone sodium (120 mg/kg IP; Abbott Australasia, Kurnell, Australia) and a femoral vein and artery were catheterized. The trachea was cannulated, and the lungs ventilated via a computer controlled small animal ventilator (Flexivent, SCIREQ Scientific Respiratory Equipment, Montreal, Canada) with a mixture of 5% CO2, 20% O2, balanced with N2, at a tidal volume of 7 ml/kg BW, breathing frequency (f) of 60 min−1. The pulmonary artery and left atrium were cannulated and without interrupting the circulation, the lungs were perfused (flow rate of 20 ml/min, left atrial pressure of 2 cmH2O referenced to the top of the lungs) with Krebs bicarbonate solution (4.5% bovine serum albumin). The lungs and heart were removed from the chest, placed in a humidified chamber at 37 °C and allowed to stabilize for 2 min before the start of each protocol [11].
Isolated perfused rat lungs were randomly allocated to four groups (n = 10); Long or short inspiratory time (Ti = 0.5 s or Ti = 0.1 s) and PEEP of 2 or 10 cmH20. Airway, pulmonary arterial and left atrial pressures were monitored continuously using a MacLab System (AD Instruments, Australia).
Lung Mechanics
Forced impedance mechanics (airway (Newtonian) resistance, Raw; tissue resistance, Gtis; and tissue elastance, Htis) were measured at 30, 60 and 90 min by measuring the lung’s impedance (Z) using the computer-controlled ventilator, as previously [12]. Briefly, 2 min after a recruitment maneuver (2.5 × VT), impedance of the respiratory system was measured following a forced oscillation. The data were fitted to a constant phase model [13] where Z = Raw + jI + (Gtis–jHtis)/(2πf)α, where I is inertance, j is the imaginary unit, f is frequency, and α = (2/π)arc tan (Htis/Gtis). Inertance was negligible and is therefore not reported.
Preparation and Infusion of Radiolabels
Radiolabeled red blood cells (RBC), albumin and diethylenetriamine pentaacetic acid (DTPA) were prepared, as previously and added to the perfusate 10 min before the end of the experiment [11]. Activities were: 51Cr-RBC 5 µCi/100 g BW; 125I-albumin 0.1 µCi/100 g BW; 99mTc-DTPA 0.4 µCi/100 g BW.
Assessment of Lung Injury
The perfusate was sampled every 30 min for determination of gas tensions (ABL-5 Blood Gas Analyzer, Radiometer, Denmark) and samples stored at − 80 °C for analysis of cytokines. After 90 min ventilation the right upper lobe was resected for determination of wet-to-dry weight ratio and radiolabel analysis, as previously [11]. The remaining lung was degassed at 0.5 atm for 60 s and lavaged at 2 °C with 3 separate 32 ml/kg BW volumes of 0.9% sodium chloride, each instilled and withdrawn × 3. Percent recovery of lavage fluid was not different between treatment groups. The lung lavage fluid was centrifuged at 150×g for 5 min at 2 °C. A sample was taken from the supernatant, aliquoted and stored at − 80 °C until analysis for cytokines.
A further aliquot of lung lavage was taken for radiolabel analysis. Compartmentalization of radiolabels in lavage and upper right lobe tissue are expressed as a percentage volume of perfusate counts, as previously [11].
Surfactant Analysis
The remaining lavage supernatant was centrifuged at 1000×g for 25 min at 4 °C to separate the surface active tubular myelin rich (alv-1) fraction and the recycled tubular myelin poor (alv-2) fraction, which appear to show product-precursor relations with lamellar bodies [14]. Lipids were extracted [15], and total phospholipid (PL) content was determined by measuring the amount of inorganic phosphorous [16]. Disaturated phospholipids (DSP) were separated [17] and the phospholipid content determined [18].
Cytokine Determination
Cytokine concentrations in lung lavage and perfusate were analyzed using commercially available enzyme-linked immunosorbent assays (ELISA) for interleukin (IL)-6 and tumor necrosis factor (TNF)-α (PharmMingen Opt EIA, San Diego, CA), and ELISA developed in our laboratory using matched antibodies for IL-8 (GRO/KC) and monocyte chemotactic protein (MCP)-1 (Peprotech, Israel), as previously [19].
Computation of Mechanical Power
Mechanical power was computed as described by Gattinoni et al. [2]
where RR is the breathing rate, ΔV is the tidal volume, ELrs is the elastance of the respiratory system, I:E is the inspiratory-to-expiratory time ratio, and Raw is the airway resistance, and 0.098 is the conversion factor from L × cmH2O to joules (J).
Confocal Microscopy
In a second cohort of rats, laser confocal microscopy-based assessment of cell injury was undertaken [10]. Briefly, isolated perfused lungs were prepared, as above, and mechanically ventilated at PEEP 2 cmH2O and f 60 min−1 while perfused (flow rate 10 ml/min/kg, pulmonary artery pressure 10 cmH2O; left atrial pressure 0–4 cmH2O) with a red blood cell enriched Krebs bicarbonate solution containing 4 µg/ml propidium iodide (PI). The lungs were randomized: Group 1—Ti 0.5 VT 7 ml/kg 30 min; Group 2—Ti 0.1, VT 7 ml/kg 30 min; Group 3—Ti 0.5, VT 7 ml/kg 90 min; Group 4—Ti 0.1, VT 7 ml/kg 90 min; Group 5—Ti 0.5, VT 15 ml/kg, 30 min; Group 6—Ti 0.1, VT 15 ml/kg, 30 min. Once the ventilation protocol was complete, PI free Krebs was perfused through the lung for 3 min and the lungs and heart excised. The lungs were inflated with 10 ml air and placed in saline for confocal imaging.
Left upper lobe lung tissue was imaged using a Leica TCS SP5 confocal microscope (Leica Microsystems, Mannheim) by exciting the tissue with laser light (488 nm) and collecting emission wavelengths at 500–550 nm (auto florescence) and 600–720 nm (PI). Stacks of 8 images (1 µm apart) were taken up to a depth of 40 µm. The images were digitized (8-bit resolution; 512 × 512 pixels) and Image J was used to count the number of alveoli and PI+ cells per field.
Statistical Analysis
Due to their asymmetric nature all data were log-transformed before analysis. Results are expressed as mean ± SD and P ≤ 0.05 considered significant. Differences between groups were assessed by two-way or repeated-measures analysis of variance (ANOVA), as appropriate, and relationships between continuous variables by Pearson correlation (IBM SPSS 23.0, SPSS Inc., Chicago, IL).
Results
Respiratory Mechanics
Mechanical ventilation with fast VI′ (Ti = 0.1) increased peak airway pressure, tissue elastance and tissue resistance (Fig. 1a–d). High PEEP (10 cmH2O) independently increased peak airway pressure, and decreased airway and tissue resistance over the same period. However, there was no interactive effect of VI′ and PEEP on these parameters. Tissue elastance was unchanged by increasing PEEP (Fig. 1b) and airway resistance was unchanged by increased VI′ (Fig. 1c). Pulmonary arterial and venous return pressures remained unchanged by either VI′ or increasing PEEP (data not shown).
Respiratory mechanics of isolated perfused rat lungs during 90 min of slow (Ti = 0.5 s) and fast (Ti = 0.1 s) VI′ with PEEP of 2 or 10 cmH2O (n = 10). Data are presented as mean ± SD and differences between groups analyzed by repeated measures ANOVA. a Peak airway pressure, Ppk (VI′ P = 0.02; PEEP P ≤ 0.001; interaction P = 0.39; time P = 0.06). b Airway resistance, Raw (VI′ P = 0.57; PEEP P = 0.005; interaction P = 0.32; time P = 0.25). c Tissue elastance, Htis (VI′ P = 0.001; PEEP P = 0.24; interaction P = 0.11; time P = 0.09). d Tissue resistance, Gtis (VI′ P ≤ 0.001; PEEP P ≤ 0.001; interaction P = 0.84; time P = 0.01)
Surfactant Content
Alv-1 and Alv-2, total PL and DSP content decreased with increased VI′ (Table 1). Higher PEEP (10 cmH2O) had no effect.
Lung Injury
Although perfusate PO2 was unchanged by either VI′ or PEEP (Fig. 2a), PCO2 was lower and pH higher with higher PEEP (Fig. 2b, c). Wet/dry weight ratio was unchanged with either VI′ or PEEP (Fig. 2d). Consistent with this, flux of radio-labeled DTPA, albumin and RBC were unchanged by VI′ (Table 2). However, higher PEEP increased the flux of DTPA into the lung and lavage, as well as RBC into the lavage.
Lung injury parameters of isolated perfused rat lungs during 90 min of slow (Ti = 0.5 s) and fast (Ti = 0.1 s) VI′ with PEEP of 2 or 10 cmH2O (n = 10). Data are presented as mean ± SD and differences between groups analyzed by repeated measures ANOVA (a–c) or two-way ANOVA (d). a Perfusate oxygen pressure, PO2 (VI′ P = 0.76; PEEP P = 0.68; interaction P = 0.71; time P = 0.13). b Perfusate carbon dioxide pressure, PCO2 (VI′ P = 0.08; PEEP P ≤ 0.001; interaction P = 0.59; time P ≤ 0.001). c Perfusate pH (VI′ P = 0.58; PEEP P ≤ 0.001; interaction P = 0.58; time P ≤ 0.001). d Wet to dry lung weight ratio of isolated perfused rat lungs following 90 min of slow (Ti = 0.5 s) and fast (Ti = 0.1 s) VI′ with PEEP of 2 or 10 cmH2O (n = 10); (VI′ P = 0.48; PEEP P = 0.69; interaction P = 0.55)
Cytokines
All cytokines measured in the perfusate increased temporally (Fig. 3a–d). Perfusate IL-6 was increased over time with higher PEEP (Fig. 3a). However, was unchanged with increased VI′. There was no effect of VI′ or PEEP on lavage IL-6, or lavage or perfusate TNF-α, IL-8 or MCP-1 (Fig. 3b–h).
a–d Cytokine concentrations of perfusate from isolated perfused rat lungs during 90 min of slow (Ti = 0.5) and fast (Ti = 0.1) VI′ with PEEP of 2 or 10 cmH2O (n = 10). Data are presented as mean ± SD and differences between groups analyzed by repeated measures ANOVA. a IL-6 (VI′ P = 0.78; PEEP P = 0.03; interaction P = 0.16; time P ≤ 0.001). b TNF-α (VI′ P = 0.80; PEEP P = 0.36; interaction P = 0.54; time P ≤ 0.001). c IL-8 (VI′ P = 0.73; PEEP P = 0.11; interaction P = 0.54; time P ≤ 0.001). d MCP-1 (VI′ P = 0.98; PEEP P = 0.12; interaction P = 0.31; time P ≤ 0.001). e, f Cytokine concentrations of lung lavage from isolated perfused rat lungs following 90 min of slow (Ti = 0.5) and fast (Ti = 0.1) VI′ with PEEP of 2 or 10 cmH2O (n = 10). Data are presented as mean ± SD and differences between groups analyzed by two-way ANOVA. e IL-6 (VI′ P = 0.83; PEEP P = 0.65; interaction P = 0.48). f TNF-α (VI′ P = 0.53; PEEP P = 0.77; interaction P = 0.23). g IL-8 (VI′ P = 0.61; PEEP P = 0.35; interaction P = 0.61). h MCP-1 (VI′ P = 0.70; PEEP P = 0.57; interaction P = 0.57)
Mechanical Power
Computed mechanical power was correlated with measured indices of lung injury. Low mechanical power at PEEP 2 was positively associated with lung edema measured as lung wet to dry weight ratio, and negatively associated with Alv 2 DSP content (Fig. 4a, b). These relationships were not apparent at higher mechanical power resulting from PEEP 10 (Fig. 4f, g). Conversely, inflammatory cytokines, IL-6, TNF-α and IL-8 in bronchoalveolar lavage were positively correlated with mechanical power at PEEP 10 (Fig. 4h–j) but not at PEEP 2 (Fig. 4c–e).
Pearson correlations of mechanical power (J/min) calculated as per Gattinoni et al. [2] against indices of lung injury from isolated perfused rat lungs during 90 min of slow (Ti = 0.5) and fast (Ti = 0.1) VI′ with PEEP of 2 or 10 cmH2O (n = 10). a, f lung wet to dry weight ratio. b, g Alveolar surfactant (Alv 2) desaturated phospholipid (DSP). c, h interleukin (IL)-6. d, i tumor necrosis factor (TNF)-α. e, j IL-8. The r value represents the correlation coefficient, and P, the respective P value
PI+ Cells
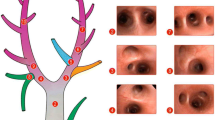
PI+ cells per alveolus were increased with increased VI′ at both 30- and 90-min ventilation at VT 7 ml/kg (Fig. 5a, c). However, increasing VT to 15 ml/kg for 30 min equalized the degree of cellular injury discernable by this method (Fig. 5d). As reported previously [10], there was considerable heterogeneity of injury with distinct field-to-field variability in the number of PI+ cells.
a, b Confocal images of subpleural alveoli (left, control— VI′ (Ti = 0.5); right, injury— VI′ (Ti = 0.1)). The red nuclei mark the injured cells (propidium iodide (PI)—positive nuclei). c, d Average number of PI+ cells per alveolus as assessed from ten random subpleural fields per animal. cVT—7 ml/kg: VI’ P ≤ 0.001; time P ≤ 0.001; interaction P = 0.007. d At 30 min: VI′ P = 0.001; VTP = 0.03; interaction P = 0.01. Data are presented as mean ± SD and differences between groups analyzed by two-way ANOVA
Discussion
In the isolated perfused rat lung, during lower VT ventilation fast VI′ led to impaired lung mechanics, without an increase in lung edema, deterioration in gas exchange or change in either perfusate or BAL cytokines. However, there was an associated increase in epithelial cell wounding measured as increased propidium iodide uptake, and both impaired lung stiffness and reduced pulmonary surfactant content of both total and disaturated phospholipids. In addition, computed mechanical power was correlated with various indices of lung injury, at low power (PEEP 2), and inflammation, at high power (PEEP 10), as seen previously [20].
The lack of lung edema with maintained oxygenation and inflammatory markers suggest that cell wounding and reduced pulmonary surfactant content are early changes that likely precede conventional parameters of lung injury. While others have reported lung injury and inflammation following fast VI′ [3, 5, 21, 22] it is possible that the absence of granulocytes in the lung perfusate modified the progression of lung injury in the current experiments. Pulmonary surfactant depletion using saline lavage is a common animal model of lung injury demonstrating marked changes in oxygenation, histology and lung inflammation [23]. However, consistent with the current fast VI′ data, in the absence of granulocytes only a modest increase in albumin permeability is reported [24].
While the 90-min experiment may not have been long enough for a change in cytokine release from alveolar cells, perfusate cytokines and chemokines increased over this time. Despite an increase in IL-6 with PEEP, there was no effect of increasing VI′ on any of the cytokines examined. This suggests that either exposure to the perfusate circuit or deterioration in the lung was occurring over the course of the experiment, as expected with the isolated perfused lung model. While the cell source of these proteins could be pulmonary endothelial cells or residual leukocytes including macrophages, it seems unlikely there was a contribution from epithelial cells given the lack of effect of VI′ on bronchoalveolar lavage proteins.
Examining high VT ventilation, Gajic et al. [10] found few changes in cell wounding, lung edema, lung histology and lung stiffness until VT was increased to 40 ml/kg (compared to control VT 6 ml/kg, 3 cmH2O PEEP). As surface tension accounts for around 70% of elastic recoil, increased lung stiffness might be due to either reduced aerated lung volume or reduced specific lung stiffness due to reduced surfactant function or content. In acute lung injury increased permeability results in inhibition of surface tension reduction by albumin resulting in, or potentiating, reduced aerated lung volume. In the isolated perfused lung used by both Gajic et al. [10] and in the current study, other factors may also inactivate surfactant function, or cell wounding and increased lipid trafficking due to excessive strain or strain rate may result in altered surfactant cycling within the alveolus.
In the current study we found increased tissue elastance and tissue resistance with fast VI′, associated with reduced pulmonary surfactant phospholipids. While reduced alveolar surfactant would produce increased surface tension and these mechanical changes, both the mechanism of reduced surfactant phospholipid content and alternative causes of these altered mechanics need to be considered. With regard to a direct effect of VI′ on surfactant, the authors are not aware of any specific prior data. Broader studies which have touched on these effects have seen either no effect [25] or increased elastance suggesting reduced surfactant [26], consistent with our findings. It has been postulated alveolar epithelial cell injury leads to altered cellular metabolism and diminished surfactant production, thereby resulting in increased lung stiffness [26, 27]. However, as we did not examine surfactant turnover in the alveolus it is not possible to speculate whether this is due to altered release, or changes in surfactant cycling, both of which might be more likely to occur given the previous suggestion of altered lipid cycling with stretch-induced cell wounding. High VI′ might have led to lung injury independently and increased lung stiffness, however, as the perfusate was free of granulocytes, and lung water, oxygenation and inflammatory proteins were unchanged, this seems less likely.
The isolated perfused lung preparation used allows cell wounding to be measured with PI in the intact organ, and direct comparison with prior work [10]. It also minimizes potential confounders such as non-pulmonary inflammation and hemodynamic effects attributable to differences in ventilation. While these advantages allow a more focused examination of normal lung effects and potential mechanism, they also reduce the direct translation to clinical effects that might be gained by work with intact circulations, lung injury models, and clinical research.
To separate slow from fast VI′ we used Ti of 0.5 s and 0.1 s with a constant VT of 7 ml/kg and a respiratory rate of 60 breaths/min. Again, these values were chosen to examine the effect and mechanism of fast VI′ in the rat lung, and do not suggest direct clinical translation. However, as Nakano et al. [28] reported a baseline Ti of 0.17 s with a VT of 5.8 ml/kg in control rats the range of VI′ examined appear appropriate, allowing some general conclusions to be drawn.
The association of cell wounding, reduced alveolar surfactant content and impaired lung mechanics with fast VI′ in the current study suggest a mechanism of strain rate related VILI. The associated decrease in surface tension would lead to amplified viscoelastic behavior consistent with Protti et al. [5], and this mechanism would likely be enhanced in an already injured lung with abnormal surface tension and heterogeneous aeration. Indeed, Gattinoni et al. [2] calculated that VI′ was of similar importance to VT in the manifestation of VILI. As VILI is modified by the underlying state of the lung there may be only minor impact in healthy lungs but in injured or inflamed lungs, particularly given the heterogeneous nature of lung injury, it is likely that there will be an impact of clinical inspiratory flows. In addition, the absence of perfusate granulocytes in the isolated lung model likely reduced evidence of associated lung injury [24, 29], however, this does suggest that high strain rate related cell wounding is a very early mechanical consequence.
These data support the suggestion of using low constant VI′, as opposed to decelerating flow waveforms which enforce an initial high VI′, as suggested by Gattinoni et al. [1]. However, further studies are needed to examine surfactant turnover in the alveolus, lipid cycling, the impact of granulocytes in the pulmonary circulation and the direct relevance to clinical practice.
References
Gattinoni L, Marini JJ, Collino F, Maiolo G, Rapetti F, Tonetti T, Vasques F, Quintel M (2017) The future of mechanical ventilation: lessons from the present and the past. Crit Care 21(1):183. https://doi.org/10.1186/s13054-017-1750-x
Gattinoni L, Tonetti T, Cressoni M, Cadringher P, Herrmann P, Moerer O, Protti A, Gotti M, Chiurazzi C, Carlesso E, Chiumello D, Quintel M (2016) Ventilator-related causes of lung injury: the mechanical power. Intensive Care Med 42(10):1567–1575. https://doi.org/10.1007/s00134-016-4505-2
Bersten AD, Bryan DL (2005) Ventilator-induced lung injury: do dynamic factors also play a role? Crit Care Med 33(4):907
Dreyfuss D, Saumon G (1998) Ventilator-induced lung injury: lessons from experimental studies. Am J Respir Crit Care Med 157(1):294–323. https://doi.org/10.1164/ajrccm.157.1.9604014
Protti A, Maraffi T, Milesi M, Votta E, Santini A, Pugni P, Andreis DT, Nicosia F, Zannin E, Gatti S, Vaira V, Ferrero S, Gattinoni L (2016) Role of strain rate in the pathogenesis of ventilator-induced lung edema. Crit Care Med 44(9):e838–e845. https://doi.org/10.1097/ccm.0000000000001718
Eissa NT, Ranieri VM, Corbeil C, Chasse M, Robatto FM, Braidy J, Milic-Emili J (1991) Analysis of behavior of the respiratory system in ARDS patients: effects of flow, volume, and time. J Appl Physiol (Bethesda, Md: 1985) 70(6):2719–2729
Tschumperlin DJ, Oswari J, Margulies AS (2000) Deformation-induced injury of alveolar epithelial cells. Effect of frequency, duration, and amplitude. Am J Respir Crit Care Med 162 (2 Pt 1):357–362. https://doi.org/10.1164/ajrccm.162.2.9807003
Vlahakis NE, Schroeder MA, Pagano RE, Hubmayr RD (2001) Deformation-induced lipid trafficking in alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol 280(5):L938–L946
Vlahakis NE, Schroeder MA, Pagano RE, Hubmayr RD (2002) Role of deformation-induced lipid trafficking in the prevention of plasma membrane stress failure. Am J Respir Crit Care Med 166(9):1282–1289. https://doi.org/10.1164/rccm.200203-207OC
Gajic O, Lee J, Doerr CH, Berrios JC, Myers JL, Hubmayr RD (2003) Ventilator-induced cell wounding and repair in the intact lung. Am J Respir Crit Care Med 167(8):1057–1063. https://doi.org/10.1164/rccm.200208-889OC
Davidson KG, Bersten AD, Barr HA, Dowling KD, Nicholas TE, Doyle IR (2000) Lung function, permeability, and surfactant composition in oleic acid-induced acute lung injury in rats. Am J Physiol Lung Cell Mol Physiol 279(6):L1091–L1102
Davidson KG, Bersten AD, Barr HA, Dowling KD, Nicholas TE, Doyle IR (2002) Endotoxin induces respiratory failure and increases surfactant turnover and respiration independent of alveolocapillary injury in rats. Am J Respir Crit Care Med 165(11):1516–1525
Hantos Z, Daroczy B, Suki B, Nagy S, Fredberg JJ (1992) Input impedance and peripheral inhomogeneity of dog lungs. J Appl Physiol 72(1):168–178
Nicholas TE, Power JH, Barr HA (1990) Effect of pattern of breathing on subfractions of surfactant in tissue and alveolar compartments of the adult rat lung. Am J Respir Cell Mol Biol 3(3):251–258
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37(8):911–917
Bartlett GR (1959) Phosphorus assay in column chromatography. J Biol Chem 234(3):466–468
Mason RJ, Nellenbogen J, Clements JA (1976) Isolation of disaturated phosphatidylcholine with osmium tetroxide. J Lipid Res 17(3):281–284
Power JH, Jones ME, Barr HA, Nicholas TE (1986) Analysis of pulmonary phospholipid compartments in the unanesthetized rat during prolonged periods of hyperpnea. Exp Lung Res 11(2):105–128
Dixon DL, Barr HA, Bersten AD, Doyle IR (2008) Intracellular storage of surfactant and proinflammatory cytokines in co-cultured alveolar epithelium and macrophages in response to increasing CO2 and cyclic cell stretch. Exp Lung Res 34(1):37–47
Samary CS, Silva PL, Gama de Abreu M, Pelosi P, Rocco PR (2016) Ventilator-induced lung injury: power to the mechanical power. Anesthesiology 125(5):1070–1071. https://doi.org/10.1097/aln.0000000000001297
Vaporidi K, Voloudakis G, Priniannakis G, Kondili E, Koutsopoulos A, Tsatsanis C, Georgopoulos D (2008) Effects of respiratory rate on ventilator-induced lung injury at a constant PaCO2 in a mouse model of normal lung. Crit Care Med 36(4):1277–1283. https://doi.org/10.1097/CCM.0b013e318169f30e
Bach KP, Kuschel CA, Hooper SB, Bertram J, McKnight S, Peachey SE, Zahra VA, Flecknoe SJ, Oliver MH, Wallace MJ, Bloomfield FH (2012) High bias gas flows increase lung injury in the ventilated preterm lamb. PLoS ONE 7(10):e47044. https://doi.org/10.1371/journal.pone.0047044
Matute-Bello G, Frevert CW, Martin TR (2008) Animal models of acute lung injury. Am J Physiol Lung Cell Mol Physiol 295(3):L379–L399. https://doi.org/10.1152/ajplung.00010.2008
Kawano T, Mori S, Cybulsky M, Burger R, Ballin A, Cutz E, Bryan AC (1987) Effect of granulocyte depletion in a ventilated surfactant-depleted lung. J Appl Physiol (Bethesda, Md: 1985) 62(1):27–33
Nicholas TE, Power JH, Barr HA (1982) Surfactant homeostasis in the rat lung during swimming exercise. J Appl Physiol 53(6):1521–1528. https://doi.org/10.1152/jappl.1982.53.6.1521
McClenahan JB, Urtnowski A (1967) Effect of ventilation on surfactant, and its turnover rate. J Appl Physiol 23(2):215–220. https://doi.org/10.1152/jappl.1967.23.2.215
Faridy EE, Permutt S, Riley RL (1966) Effect of ventilation on surface forces in excised dogs' lungs. J Appl Physiol 21(5):1453–1462. https://doi.org/10.1152/jappl.1966.21.5.1453
Nakano H, Magalang UJ, Lee SD, Krasney JA, Farkas GA (2001) Serotonergic modulation of ventilation and upper airway stability in obese Zucker rats. Am J Respir Crit Care Med 163(5):1191–1197. https://doi.org/10.1164/ajrccm.163.5.2004230
Dolinay T, Himes BE, Shumyatcher M, Lawrence GG, Margulies SS (2017) Integrated stress response mediates epithelial injury in mechanical ventilation. Am J Respir Cell Mol Biol 57(2):193–203. https://doi.org/10.1165/rcmb.2016-0404OC
Acknowledgements
The authors gratefully acknowledge Heather Barr, MSc, and Darren Kennedy, BSc for technical assistance, and Dr Jennifer Clarke for assistance with confocal imaging. This project was supported by the National Health and Medical Research Council (Grant #275565), the Australian and New Zealand College of Anaesthetists (Grant # 06/018) and the Flinders Medical Centre Foundation.
Funding
This project was supported by the National Health and Medical Research Council (Grant #275565), the Australian and New Zealand College of Anaesthetists (Grant # 06/018) and the Flinders Medical Centre Foundation.
Author information
Authors and Affiliations
Contributions
ADB, DLD conceived and designed the analysis. MK, KG collected the data. DLD performed the analysis. AB, DLD wrote the paper. AB, MK, KG, DLD revised and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
All experiments were approved by the Flinders University Animal Welfare Committee and performed according to the National Health and Medical Research Council of Australia Guidelines on Animal Experimentation.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bersten, A.D., Krupa, M., Griggs, K. et al. Reduced Surfactant Contributes to Increased Lung Stiffness Induced by Rapid Inspiratory Flow. Lung 198, 43–52 (2020). https://doi.org/10.1007/s00408-019-00317-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00408-019-00317-1