Abstract
Introduction
Patients with limited disease small-cell lung cancer (SCLC) receive radiochemotherapy followed by prophylactic cranial irradiation. The prognosis of these patients remains poor with a median survival of 16–24 months. Systemic inflammation was suggested as an important prognostic factor for outcomes. This study investigated the impact of systemic inflammation measured with neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) at first diagnosis in patients with limited disease SCLC for outcomes.
Methods
Data of 65 patients receiving radiochemotherapy for limited disease SCLC were analyzed. NLR and PLR were obtained from blood sample at first diagnosis of SCLC and 12 characteristics including gender, age, ECOG, T-category, N-category, pack years, smoking during radiotherapy, respiratory insufficiency, hemoglobin levels during radiotherapy, radiation dose (<56 vs. ≥56 Gy), concurrent radiochemotherapy, and prophylactic cranial irradiation (PCI) were evaluated for local control, metastasis-free survival, and overall survival.
Results
Survival rates at 1, 2, and 3 years were 71, 45, and 28%, respectively. Median survival time was 20 months. Independent factors for improved survival were NLR < 4 (p = 0.03), ECOG 0–1 (p = 0.002), and PCI (p = 0.015). Lower T-category was an independent positive factor of local control (p = 0.035). Improved metastasis-free survival was associated with NLR < 4 (p = 0.011), ECOG 0–1 (p = 0.002), N-category 0–1 (p = 0.048), non-smoking during radiotherapy (p = 0.009), and PCI (p = 0.006).
Conclusion
NLR was found to be an independent prognostic factor for overall survival. The evaluation of NLR can help identify patients with poor prognosis and appears a useful prognostic marker in clinical practice. A prospective analysis is warranted to confirm these findings.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lung cancer is the leading cause of cancer-related deaths worldwide [1]. Small-cell lung cancer (SCLC) represents up to 20% of all lung cancers and is clinically aggressive and has a dismal prognosis [1, 2]. Only 30% of SCLC patients present with limited disease and can be treated with curative intent [3]. The standard treatment for limited disease SCLC is radiochemotherapy followed by prophylactic cranial irradiation. However, median survival rates range only from 16 to 24 months. There is a need to identify prognostic factors to predict patients’ outcome and to identify particularly high-risk patients.
Inflammation including its impact on tumor development has been extensively investigated. Systemic inflammation has been suggested to be responsible for cancer-related symptoms such as pain, dysphagia, cachexia, and poor prognosis [4]. Several systemic inflammatory response (SIR) markers including levels of albumin, C-reactive protein (CRP), fibrinogen, and serum cancer markers were already analyzed for potential impact on survival, tumor progression, and development of metastases [5, 6]. Several studies have investigated the potential role of cytokines such as IL-1, IL-6, or TNF as important cross-talk factors that can trigger the development of metastases [7]. However, these factors are not used in clinical routine and prognostic relevance remains unclear. In several types of cancer including colorectal, pancreas, and non-small-cell lung cancer (NSCLC), the prognostic impact of neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) has been established [8–16]. NLR and PLR can be easily obtained by blood samples. NLR and PLR have not yet been properly investigated as potential prognostic factors in SCLC. The aim of this study was to investigate the impact of systemic inflammation as assessed with NLR and PLR at the time of diagnosis in patients with limited disease SCLC.
Patients and Methods
In this retrospective study, we analyzed 65 patients with limited disease SCLC treated with radiochemotherapy between 2006 and 2014. The data were obtained from the patient files, treating physicians, and the patients being alive at the last follow-up. The patients have not been enrolled in prospective trials. All patients received chemotherapy consisting of two to six courses of etoposide (120 mg/m2 on days 1–3) plus either cisplatin (60–80 mg/m2 on day 1) or carboplatin (AUC 6 on day 1). Median radiation dose administered in an equivalent dose in 2 Gy fractions (EQD2) was 56 Gy (range 40–64 Gy). Prophylactic cranial irradiation (PCI) followed radiochemotherapy was administered to 72% of all patients.
The neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) obtained at NLR and PLR were obtained from blood samples at the first diagnosis of SCLC by complete blood cell count (CBC). NLR was calculated dividing the number of neutrophil by lymphocyte number. Similarly, PLR was calculated dividing the platelet number by lymphocyte number. According to the previous reports, cut-off levels for NLR and PLR were determined [14, 16]. NLR was categorized in two groups (<4 and ≥4) and PLR > 180 was defined as elevated.
These inflammatory markers and 12 characteristics were analyzed for independent association with local control, metastasis-free survival, and overall survival. Further characteristics were gender, age (≤64 vs. ≥65 years), ECOG (0–1 vs. 2–3), T-category (T1- 2 vs. T3-4), N-category (N0-1 vs. N2-3), pack years (<40 vs. ≥40 years), smoking during radiotherapy (yes vs. no), respiratory insufficiency (yes vs. no), hemoglobin levels during radiotherapy (<12 vs. ≥12 g/dl), EQD2 (<56 Gy vs. ≥56 Gy), concurrent radiochemotherapy (yes vs. no), and PCI (yes vs. no). Hemoglobin levels were generally evaluated once a week during radiotherapy. Since the overall treatment time of radiotherapy was 4 to 6 weeks, 4 to 6 hemoglobin levels were obtained for each patient. The majority of the hemoglobin levels (≥3 of 4 levels, ≥3 of 5 levels, ≥4 of 6 levels) were either <12 or ≥12 g/dL. The situation that 2 of 4 levels or 3 of 6 levels were each <12 g/dL and ≥12 g/dL did not occur.
The distribution of these characteristics is shown in Table 1. All 12 characteristics were included in the univariate analysis (Kaplan–Meier method plus log-rank test [17]), and those achieving significance (p < 0.05) additionally in a multivariate analysis (Cox regression).
Results
The results of the univariate analysis for local control are summarized in Table 2. On univariate analysis, lower T-category (T-category 1–2) was associated with improved local control at 3 years (62% vs. 41%, p = 0.04). On multivariate analysis, T-category proved to be an independent factor [p = 0.035; HR 1.84 (95% Cl 1.04–3.86)].
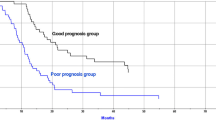
On univariate analysis, a better metastasis-free survival was found for NLR < 4 (p = 0.011, Fig. 1), ECOG 0–1 (p = 0.002), N-category 0–1 (p = 0.048), non-smoking during radiotherapy (p = 0.009), and administration of PCI (p = 0.006) (Table 3). These factors were included in the multivariate analysis. A trend for improved metastasis-free survival was found for lower NLR (p = 0.063; HR 2.19 [95% Cl 0.96–5.06)] and lower N-category [p = 0.062; HR 3.4 (95% Cl 0.95–21.9)].
Overall survival rates at 1, 2, and 3 years were 71%, 45%, and 28%, respectively, and the median survival time was 20 months (Table 4). The corresponding median overall survival times are summarized in Table 5. Improved overall survival was associated with a NLR < 4 (p = 0.001, Fig. 2), ECOG 0–1 (p < 0.001), non-smoking during radiotherapy (p = 0.007), no respiratory insufficiency before radiotherapy (p = 0.03), and PCI (p < 0.001). On multivariate analysis, NLR < 4 (p = 0.03; HR 2.05 [95% Cl 1.06–3.95)], ECOG 0–1 (p = 0.002; HR 3.41 [95% Cl 1.57–7.36)], and PCI [p = 0.015; HR 2.56 (95% Cl 1.21–5.34)] were independently associated with improved overall survival.
Discussion
SCLC is an aggressive type of cancer with a relatively poor prognosis. The link between inflammation and lung cancer has been investigated in the last few decades. Systemic inflammation is a promoter of tumorigenesis. Accumulating evidence shows that tumor-promoting inflammation is responsible for disease growth and progression by creating a tumor-promoting micro-environment [18]. Several systemic inflammatory response (SIR) markers including levels of albumin, C-reactive protein (CRP), fibrinogen, and serum cancer markers have been analyzed for potential impact on survival, tumor progression, and development of metastases [5, 6]. In different types of cancer including gastric, colorectal, and pancreas, the prognostic impact of Neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) has been established [8–16]. Recent studies revealed that elevated pretreatment NLR was associated with poor prognosis in NSCLC patients. This effect was evident in patients with early stage [13], advanced stage [11], treatment with stereotactic radiation therapy [8], and concurrent radiochemotherapy [12]. However, in contrast to NSCLC, there is a lack of data for patients with SCLC. The present study is the first to investigate the impact of pretreatment NLR and PLR on outcome for limited disease SCLC.
According to the results of this study, NLR proved to be an independent predictor of overall survival. A NLR of ≥4 resulted in significantly worse outcomes than a NLE of <4. This finding agrees well with the results of the retrospective study of 187 patients receiving chemotherapy alone for SCLC reported by Kang et al. [16]. In that study, the median overall survival time was worse in the high-NLR group (11.2 vs. 9.2 months, p = 0.019). In addition, the authors found that high NLR was associated with unfavorable factors such as poor performance status, advanced stage, and lower response rate. In contrast to the study of Kang et al., our present study was performed in patients treated with radiochemotherapy and focused particularly on limited disease SCLC. To our knowledge, there has been no specific analysis so far of patients with limited disease SCLC who were treated with radiochemotherapy.
In the present study, “traditional” prognostic factors such as performance status, T-category, and N-category were associated with outcomes, which had already been widely recognized for SCLC [19, 20]. In addition, in the current study, smoking during radiotherapy led to reduced OS and MFS in the corresponding patients when compared to patients having stopped smoking before radiotherapy. This effect was previously reported for both patients with NSCLC and those with SCLC [21, 22]. In a retrospective study of 181 patients irradiated for NSCLC, smoking during radiotherapy had a significantly negative impact on locoregional control on both univariate (p < 0.001) and multivariate analysis (p = 0.029) [21]. Two-year locoregional control rates were 34% in patients who continued smoking during radiotherapy and 59% in those patients who stopped smoking prior to irradiation, respectively. In a retrospective series of 215 patients with SCLC, Videtic et al. found an improved median survival in patients who quit smoking prior to radiotherapy when compared to those patients who continued smoking during their radiation treatment (18 vs. 13.6 months, p = 0.002) [22]. It appears likely that patients who did not stop smoking also had a history of more pack years, which may have caused a hidden bias. In the present study, 57% of the patients who did stop and 39% of those patients who did not stop smoking prior to radiotherapy had ≤40 pack years, respectively. However, the correlation was not significant (p = 0.36, Chi-square test). The retrospective nature of our data and the relatively small number of patients should be considered when interpreting the results. However, prospective studies regarding the prognostic impact of NLR and PLR for SCLC are not available.
In conclusion, NLR is an independent prognostic factor for overall survival of patients with limited SCLC. The evaluation of NLR could be used to identify patients with particularly poor prognoses and be a useful prognostic marker in clinical practice. A prospective analysis is warranted to confirm these findings.
References
Siegel RL, Miller KD, Jemal A (2015) Cancer statistics, 2015. CA Cancer J Clin 65:5–29
Pietanza MC, Byers LA, Minna JD et al (2015) Small cell lung cancer: will recent progress lead to improved outcomes? Clin Cancer Res 21:2244–2255
Jeremic B, Dobric Z, Casas F (2011) Limited-disease small-cell lung cancer. Advances in radiation oncology in lung cancer. Springer, Heidelberg
Alifano M, Mansuet-Lupo A, Lococo F, et al. (2014) Systemic inflammation, nutritional status and tumor immune microenvironment determine outcome of resected non-small cell lung cancer. PloS ONE 9:e106914
Zhou T, Zhan J, Hong S et al (2015) Ratio of C-reactive protein/albumin is an inflammatory prognostic score for predicting overall survival of patients with small-cell lung cancer. Sci Rep 18(5):10481
He X, Zhou T, Yang Y et al (2015) Advanced lung cancer inflammation index, a new prognostic score, predicts outcome in patients with small-cell lung cancer. Clin Lung Cancer 16:e165–e171
Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144:646–674
Cannon NA, Meyer J, Iyengar P et al (2015) Neutrophil–lymphocyte and platelet–lymphocyte ratios as prognostic factors after stereotactic radiation therapy for early-stage non–small-cell lung cancer. J Thorac Oncol 10:280–285
Walsh S, Cook E, Goulder F et al (2005) Neutrophil-lymphocyte ratio as a prognostic factor in colorectal cancer. J Surg Oncol 91:181–184
Asaoka T, Miyamoto A, Maeda S et al (2016) Prognostic impact of preoperative NLR and CA19-9 in pancreatic cancer. Pancreatology 16:434–440
Berardi R, Rinaldi S, Santoni M, Newsom-Davis T, Tiberi M, Morgese F, Caramanti M, Savini A, Ferrini C, Torniai M, Fiordoliva I, et al. (2016) Prognostic models to predict survival in patients with advanced non-small cell lung cancer treated with first-line chemo-or targeted therapy. Oncotarget 7(18):26916–26924
Tang H, Ma H, Peng F et al (2016) Prognostic performance of inflammation-based prognostic indices in locally advanced non-small-lung cancer treated with endostar and concurrent chemoradiotherapy. Mol Clin Oncol 4:801–806
Pinato D, Shiner R, Seckl M et al (2014) Prognostic performance of inflammation-based prognostic indices in primary operable non-small cell lung cancer. Br J Cancer 110:1930–1935
Gu X, Sun S, Gao X-S et al (2016) Prognostic value of platelet to lymphocyte ratio in non-small cell lung cancer: evidence from 3,430 patients. Sci Rep 30(6):23893
Mizunuma M, Yokoyama Y, Futagami M et al (2015) The pretreatment neutrophil-to-lymphocyte ratio predicts therapeutic response to radiation therapy and concurrent chemoradiation therapy in uterine cervical cancer. Int J Clin Oncol 20:989–996
Kang MH, Go SI, Song H et al (2014) The prognostic impact of the neutrophil-to-lymphocyte ratio in patients with small-cell lung cancer. Br J Cancer 111:452–460
Kaplan E, Meier P (1958) Nonparametric estimation from incomplete observation. J Am Stat Assoc 53:457–481
Ma X, Yan F, Deng Q, et al. (2015) Modulation of tumorigenesis by the pro-inflammatory microRNA miR-301a in mouse models of lung cancer and colorectal cancer. Cell Discov 19(1):15005
Nakamura H, Kato Y, Kato H (2004) Outcome of surgery for small cell lung cancer–response to induction chemotherapy predicts survival. Thorac Cardiovasc Surg 52:206–210
Vallieres E, Shepherd FA, Crowley J et al (2009) The IASLC lung cancer staging project: proposals regarding the relevance of TNM in the pathologic staging of small cell lung cancer in the forthcoming (seventh) edition of the TNM classification for lung cancer. J Thorac Oncol 4:1049–1059
Rades D, Setter C, Schild SE et al (2008) Effect of smoking during radiotherapy, respiratory insufficiency, and hemoglobin levels on outcome in patients irradiated for non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 71:1134–1142
Videtic GM, Truong PT, Ash RB et al (2005) Does sex influence the impact that smoking, treatment interruption and impaired pulmonary function have on outcomes in limited stage small cell lung cancer treatment? Can Respir J 12:245–250
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None of the authors has any conflict of interest to declare in relation to this work.
Ethical Standards
All procedures performed were in accordance with ethical standards and the Helsinki declaration from 1964 and its later amendments.
Informed Consent
Due to its retrospective design, informed consent specifically for this study was not required.
Rights and permissions
About this article
Cite this article
Käsmann, L., Bolm, L., Schild, S.E. et al. Neutrophil-to-Lymphocyte Ratio Predicts Outcome in Limited Disease Small-cell Lung Cancer. Lung 195, 217–224 (2017). https://doi.org/10.1007/s00408-017-9976-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00408-017-9976-6