Abstract
Introduction
Bronchiolitis obliterans (BO) is a chronic airway disease following an insult to the lower respiratory tract. Lung clearance index (LCI) measures ventilation inhomogeneity and has been studied in cystic fibrosis (CF). We aimed to evaluate LCI in BO and to compare it to LCI in CF patients.
Methods
LCI was measured in BO patients, compared to CF patients, and correlated with spirometry and CT findings.
Results
Twenty BO patients and 26 CF patients (with similar mean age and BMI) underwent evaluation. FEV1 % and FEF25–75 % predicted were significantly lower in the BO group (60.5 ± 17.8 vs. 72.7 ± 20.7, p = 0.041, and 42.8 ± 22.8 vs. 66.4 ± 37.4, p = 0.017, respectively). In both groups, LCI was inversely correlated with FVC %, FEV1 %, and FEF25–75 % predicted. LCI % was slightly higher (190.4 ± 63.5 vs. 164.9 ± 39.4, p = 0.1) and FRC gas % (measured by multiple breath washout) was significantly higher in the BO group (92.5 ± 35.9 vs. 71.3 ± 18, p = 0.014). The strength of statistical association between the lower FEF25–75 % values and the higher LCI values was stronger in BO patients.
Conclusions
Similar to CF, LCI may provide estimation of ventilation inhomogeneity in BO. The results indicate greater small airway involvement and air trapping in BO. Further prospective longitudinal studies evaluating the correlation of LCI measurements with multiple clinical and physiological parameters should be performed to assess the clinical benefit of LCI measurement in BO.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Bronchiolitis obliterans (BO) is a form of chronic obstructive lung disease following an insult to the lower respiratory tract, mainly post adenovirus. Characteristic pathological changes are fibrotic inflammatory processes surrounding the lumen of the bronchiole, which result in concentric narrowing and obliteration of the small airways. Consequently, a characteristic mosaic pattern of an inhomogeneous attenuation pattern develops, with abnormal lung function tests [1].
The diagnostic criteria for BO include a history of an acute lower airway insult, permanent obstructive impairments, characteristic computed tomography (CT) findings (hyperinflation, bronchial wall thickening, bronchiectasis, mosaic perfusion due to air trapping, with or without atelectasis), and exclusion of other chronic obstructive lung diseases. Due to the irregular distribution of the lesions, histological confirmation is not required [2]. In early lung disease, ventilation heterogeneity results from regional differences in small caliber airways. Inert gas washout is a technique that involves measuring the elimination of a non-absorbed gas as it is exhaled during tidal breathing [3].
Lung clearance index (LCI), a simple marker of deranged ventilation, can be calculated from multiple breath washout (MBW) curves, and it has gained increased interest in recent years. It provides a global measurement of ventilation inhomogeneity and thus may detect early airway disease in children. As LCI requires only tidal breathing, it is particularly useful for studies in the young age group [4, 5] who cannot perform reliable spirometry; LCI has repeatedly been shown to be superior to FEV1 in monitoring early lung disease [5, 6] and may serve as a tool for clinical studies to detect subtle changes [4, 7].
Recently, it has been recognized as an outcome measure for CF clinical trials [7, 8].
BO is a rare but severe classic small airways disease with mosaic pattern of ventilation inhomogeneity. There is no consensus on treatment beyond supportive measures and no optimal way to assess progression or response to treatment. Recently [9] in a pilot study, ventilation/perfusion scintigraphy was suggested to assess disease severity and to predict patients’ outcome.
Our aim was to assess whether LCI could serve as a non-invasive, irradiation-free, marker of deranged physiology in this disease. Few studies evaluated LCI in BO following bone marrow transplantation (BMT) and lung transplantation; LCI was shown to worsen with time, correlating with graft-versus-host disease (GVHD) [10] and post-lung transplantation BO [11–14]. BO post transplantation are specific entities with a progressive nature. Post-infectious BO is considered a non-progressive disease after stabilization of the initial insult. However, the course of the disease varies with pulmonary exacerbations. During the exacerbations, there is a decline in lung function. We could not find studies evaluating LCI in BO of other etiologies. Our aim was to assess LCI in BO not related to BMT or lung transplantation and to compare LCI of BO patients to that of CF patients and to parameters of lung function tests and imaging. Our hypothesis was that in BO, LCI would be higher, indicating higher degree of ventilation inhomogeneity.
Methods
Participants
This was a single-center, cross-sectional retrospective study. The institutional board reviewed and approved the study.
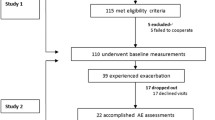
The study population consisted of consecutive patients aged >6 years diagnosed with BO or CF, followed at our institute. Measurements were performed at the Pediatric Pulmonology Institute. BO patients were diagnosed on the basis of clinical, lung function, and radiographic criteria, as suggested by Yu [15]. The patients performed spirometry, LCI measurements, and CT scans as part of their routine follow-up. LCI and lung function measurements were performed on the same visit. Due to ethical reasons, we assessed the CT scans that were performed closest to the date of the evaluation. Patients who had symptoms consistent with a pulmonary exacerbation, during the visit or the preceding week, were excluded. The BO group included patients with various etiologies. Patients with post-BMT BO were excluded. The LCI measurements of BO and CF patients were compared.
Demographic variables were extracted anonymously from the patients’ files, including age, gender, body mass index (BMI), and CF-related conditions (pancreatic insufficiency and CF-related diabetes).
LCI
MBW measurements were performed using the Easy-One Pro, MBW Module (NDD Medical Technologies, Zurich, Switzerland) as first described by Fuchs et al. in 2008 [6]. The system consists of a side stream ultrasonic transducer for temperature- and humidity-independent sampling of the molar mass (MMss), a mainstream ultrasonic transducer for flow sampling, and a side stream infrared CO2 analyzer (DUET ETCO2 Module, Welch Allyn OEM Technologies, Beaverton OR) to correct the MMss signal for exhaled CO2.
WBreath software (NDD, Switzerland) was used for data acquisition, storage, and analysis. LCI was calculated as the cumulative expired volume during the washout phase divided by the functional residual capacity (FRC), which is the number of FRC turnovers required to washout the nitrogen [7]. An increased LCI (>7) indicated more FRC turnovers required for the washout, reflecting ventilation inhomogeneity [5, 16, 17]. As previously described, FRC was calculated as the cumulative expired volume of nitrogen divided by the difference between end-tidal gas concentration at the start and end of the washout [18]. FRC measured by MBW (FRC gas [17]) represents the volume of lung gas at end expiration, which is in direct communication with the airways (excluding gas trapped in unventilated lung regions). It is, therefore, often lower than FRC by plethysmography.
As recommended, the minimum re-equilibration time between trials was the time needed for washout in the previous trial [5].
All MBW tests underwent visual quality control before analysis, ensuring regular tidal breathing before washout and absence of a leak. At least three technical acceptable MBW tests were required for inclusion of the participants in the final analysis.
Spirometry
Spirometry was performed in accordance with the ATS/ERS (American Thoracic Society/European Respiratory Society) Task Force, using a KoKo spirometer (n-Spire Healthcare, Inc., Longmont CO) [19]. Results were expressed as absolute values and percent predicted (mean ± SD) derived from Polgar and Quanjer [20].
CT Scoring
CT scans were performed every 3 years as part of the routine follow-up of our patients and were scored by a trained radiologist. In the patients with a clinical suspicion of BO, only expiratory views were performed to reduce the risk of irradiation exposure. In 1991, Bhalla et al. proposed a CT scoring system, which has been utilized to quantify the structural changes in the lungs of CF patients [21]. The modified Bhalla score has been previously used in post-infectious BO [1]. The score includes the extent of bronchiectasis and number of segments involved, peribronchial thickening, mucus plugs, sacculations and abscesses, bullae, emphysema, and collapse and consolidation. The total score ranges from 9 to 25, with a higher score indicating more severe changes.
Statistical Analysis
The primary outcome parameters were LCI measurements in CF and BO groups. Secondary outcome parameters included lung functions and CT scores of these groups.
Statistical analysis was performed using SPSS version 21.
Sample size calculation was based on the estimated mean LCI values of 7 ± 1.1 in CF and 8 ± 1.1 in BO. Thus, mean differences of LCI between the two groups of 1 unit (SD = 1.1) with a power of 80 %, and type I error of 5 %, led us to recruit at least 19 patients in each group. Differences between the two groups (BO vs. CF patients) in the quantitative parameters were performed with Student’s t test. Categorical parameters were presented as proportions and compared using Fisher’s exact test. Pearson correlation was used for relation between LCI and several lung function measurements. Multi-variate linear regression analysis was used to assess the contribution of potential confounding factors in predicting LCI. Linear regression was used to determine the predictors (FEV1 % or FEF25–75 %) associated with the level of LCI. Comparison of the slopes of regression lines between CF versus BO patients was done according to the model previously described [22, 23] (computing the difference between the two slopes divided by the standard error of the difference between the slopes, thus \(t = \frac{{b_{1} - b_{2} }}{{s_{{b_{1} - b_{2} }} }}\) on (N–4) degrees of freedom).
A value of p < 0.05 was considered significant.
Results
Forty-six patients participated in this study, with 20 BO (65 % male) and 26 CF (50 % male) patients. Their mean ages were 15.1 ± 8.3 and 15.9 ± 7.5 years, respectively. Among the BO patients, 14 had post-infectious BO and two were post burn.
Four patients that fulfilled diagnostic criteria for BO and underwent evaluation were eventually diagnosed as primary ciliary dyskinesia (PCD). After nasal NO measurements became available in our center, these patients had more than two measurements with diagnostic low nasal NO values (nasal NO during plateau <77 nl/min on two occasions >2 months apart with CF exclusion). Two of them had ciliary biopsy and the other two were found to have mutations consistent with PCD. Thus, these patients fulfilled the criteria for PCD, according to the PCD foundation consensus [24].
As these patients underwent evaluation as BO patients, we included them in the analysis. However, when omitting the four patients from the analysis, the results were similar (Table 1). Among the CF patients, 17 had pancreatic insufficiency (65 %) and five had CF-related diabetes (19 %).
The demographic characteristics, spirometry, LCI, and CT scores of the patients are presented in Table 1. There were no statistical differences in age, gender, and BMI between the groups.
FEV1 % and FEF25–75 % were significantly lower in the BO group (60.5 ± 17.8 vs. 72.7 ± 20.7, p = 0.041, and 42.8 ± 22.8 vs. 66.4 ± 37.4, p = 0.017, respectively).
LCI percent predicted (%) was slightly higher in the BO group, but did not reach statistical significance (190.4 ± 63.5 vs. 164.9 ± 39.4, p = 0.1). FRC gas % was significantly higher in the BO group, 92.5 ± 35.9 vs. 71.3 ± 18, p = 0.014. In the BO group, three patients had FEV1 above 80 % predicted, of which two had LCI >7 (FEV1 of 81 and 87 % and LCI of 13.65 and 7.18, respectively). In the CF group, six of 10 patients with FEV1 above 80 % had LCI >7 (FEV1 107, 82, 88, 92, 96, and 88 % and LCI 7.94, 7.68, 7.05, 8.98, 8.07, and 8.71, respectively).
CT scans were performed in 15 BO and 24 CF patients. The CT Bhalla score was similar between the groups, 8.3 ± 2.6 vs. 9.6 ± 6.1, p = 0.46.
The correlation coefficients between LCI and CT and parameters of spirometry are presented in Table 2. As can be seen, in both groups LCI was inversely correlated with FVC %, FEV1 %, and FEF25–75 %. CT scores correlated with LCI and LCI % in both groups (r = 0.579, p = 0.024, r = 0.636, p = 0.011 in BO, and r = 0.706, p = 0.001, r = 0.666, p = 0.001 in CF, respectively). CT scores were also inversely correlated with FVC %, FEV1 %, and FEF25–75 % in CF patients; however, in BO the correlation was not significant.
Multi-variate linear regression analysis was used to assess the contribution of potential confounding factors in predicting LCI. As FEV1 % and FEF25–75 % were highly correlated (r = 0.90, p < 0.0001), we chose FEF25–75 %. None of our BO patients had pseudomonas positive culture and therefore it was omitted. The following confounding factors were included: BMI, gender, group, time lag between CT and evaluation, CT score, and FEF25–75 % (Table 3). LCI could be predicted only by FEF25–75 % and CT score Dependent Variable: LCI % = 160.86 + (−0.617 × FEF25–75 %) + 3.56 × CT score.
Linear regression, presented in Fig. 1, found a significantly higher slope of the line (rate of decrease in LCI as related to FEF25–75) in BO patients compared with CF patients.
For CF: LCI = 205.4 + (−0.61 × FEF25–75 %); for BO: LCI = 265.6 + (−1.76 × FEF25–75 %), (p = 0.025).
Discussion
In this single-center preliminary study, we have shown that LCI, a marker of impaired gas mixing, is significantly elevated in patients with BO and correlates with spirometry parameters of airway obstruction. To the best of our knowledge, this is the first study to evaluate LCI in BO not related to BMT.
The most common form of BO in children occurs following a severe lower respiratory tract infection [25]. As mentioned earlier, LCI is a sensitive measure of early obstructive lung disease. As the range of normal values is narrow across a wide age range (from 6 years of age) and is almost unaffected by height or gender, it is appropriate for use in longitudinal studies, especially in children. There is an emerging need for a sensitive surrogate marker of early obstructive disease [26]. The use of FEF25–75 % as an early predictor of BO post lung transplant has been suggested [27]. However, there is considerable concern regarding the sensitivity and variability of this parameter in children [4]. LCI has the ability to identify early airway dysfunction in the “silent years,” while FEV1 is still within the normal range [3–5].
Several studies have shown the superiority of LCI over spirometry in CF, with better correlation with CT findings [7, 28]. Consequently, LCI has gained increased interest in use in clinical trials in CF [7, 29]. In PCD, Irving et al. found no correlation between LCI and spirometry or CT findings [30]; in contrast, Boon et al. found that LCI correlated well with CF CT score and was more sensitive than FEV1 [31].
Originally, LCI measurement required a mass spectrometer which has been considered the gold standard gas analyzer [17]. Nowadays, devices based on ultrasonic flow meters measure the molar mass of the respired gas. This technique, also used in our study, has been found to be reliable and feasible with a regular breathing pattern [32, 33]; validation against the mass spectrometer system demonstrated good agreement [6]. Fuchs et al. found good repeatability and reproducibility in healthy volunteers and CF patients [5, 16]. Recently, this method was used to assess early lung disease in alpha-1-antitrypsin deficiency [34]. Although MBW is not considered the gold standard for assessment of gas trapping, Gustafsson et al. concluded that it can be assessed with acceptable precision [18]. Moreover, Heinze et al. suggested that FRC measured by MBW is clinically acceptable, compared with FRC measured by helium dilution and plethysmography [35].
In the current study, LCI was slightly higher in the BO group, and FRC gas % was significantly higher. The difference may reflect relatively more air trapping in relation to the degree of ventilation inhomogeneity in BO. However, due to a small number of patients type 1 error cannot be excluded.
When comparing the slope of regression line of FEF25–75 % versus LCI %, we found a greater magnitude in BO; this implies a greater degree of ventilation inhomogeneity with lower FEF25–75 % in BO patients.
Noteworthy, four BO patients were diagnosed as having PCD, in accordance with the PCD foundation consensus [24]. PCD was not described in the literature as an etiologic factor for BO. Moreover, only those four, out of 40 PCD patients followed in our institute, showed signs of BO. We believe that this finding is of clinical importance and suggest that nasal NO be measured in patients with BO without definite etiologic factors.
In this study, we found similar CT Bhalla scores in the BO and CF patients. There was a good correlation between CT and LCI; CT correlated with spirometry better in CF than in BO. Several studies investigated CT findings in BO, mainly post BMT. The main findings were air trapping, bronchial wall thickening, bronchiectasis [1, 25], as well as mosaic attenuation and ground glass [36]. A composite CT scoring system to detect early bronchiolitis obliterans following lung transplant was described but was not validated in CF [37]. In post-infectious BO, the Bhalla modified score in early life anticipated future lung functions [1].
The main limitation of our study is the relatively small number of patients. Few patients had normal spirometry; therefore, we could not conclude that LCI is more sensitive than FEV1. Bhalla score was validated for CF only. FRC was measured by MBW and was not compared to whole-body plethysmography, thus FRC gas could have been underestimated in MBW. This was a cross-sectional study and correlations of LCI measurements with multiple clinical and physiological parameters (e.g., exercise capacity, 6 min walk test, and blood gas) were not done.
In conclusion, LCI correlated well with spirometry and may be useful in BO patients. The strength of statistical association between the lower FEF25–75 % values and the higher LCI values in BO patients compared with CF patients suggests more small airways involvement and greater ventilation inhomogeneity in BO. These findings suggest that LCI measurement in BO may serve as a potential marker of disease severity and an indicator of exacerbations or therapeutic interventions. Further prospective longitudinal studies including a larger number of patients with BO across various ages assessing LCI in clinically stable and during pulmonary exacerbation are needed.
References
Mattiello R, Sarria EE, Mallol J et al (2010) Post-infectious bronchiolitis obliterans: can CT scan findings at early age anticipate lung function? Pediatr Pulmonol 45:315–319. doi:10.1002/ppul.21115
Moonnumakal SP, Fan LL (2008) Bronchiolitis obliterans in children. Curr Opin Pediatr 20:272–278. doi:10.1097/MOP.0b013e3282ff62e9
Horsley AR, Gustafsson PM, Macleod KA et al (2008) Lung clearance index is a sensitive, repeatable and practical measure of airways disease in adults with cystic fibrosis. Thorax 63:135–140. doi:10.1136/thx.2007.082628
Horsley A (2009) Lung clearance index in the assessment of airways disease. Respir Med 103:793–799. doi:10.1016/j.rmed.2009.01.025
Fuchs SI, Eder J, Ellemunter H, Gappa M (2009) Lung clearance index: normal values, repeatability, and reproducibility in healthy children and adolescents. Pediatr Pulmonol 44:1180–1185. doi:10.1002/ppul.21093
Fuchs SI, Sturz J, Junge S et al (2008) A novel sidestream ultrasonic flow sensor for multiple breath washout in children. Pediatr Pulmonol 43:731–738. doi:10.1002/ppul.20825
Kent L, Reix P, Innes JA et al (2014) Lung clearance index: evidence for use in clinical trials in cystic fibrosis. J Cyst Fibros 13:123–138. doi:10.1016/j.jcf.2013.09.005
Subbarao P, Milla C, Aurora P et al (2015) Multiple-breath washout as a lung function test in cystic fibrosis. A cystic fibrosis foundation workshop report. An Am Thorac Soc 12:932–939. doi:10.1513/AnnalsATS.201501-021FR
Xie BQ, Wang W, Zhang WQ et al (2014) Ventilation/perfusion scintigraphy in children with post-infectious bronchiolitis obliterans: a pilot study. PLoS One. doi:10.1371/journal.pone.0098381
Lahzami S, Schoeffel RE, Pechey V et al (2011) Small airways function declines after allogeneic haematopoietic stem cell transplantation. Eur Respir J 38:1180–1188. doi:10.1183/09031936.00018311
Lahzami S, Schoeffel RE, Pechey V et al (2011) Small airways function declines after allogeneic haematopoietic stem cell transplantation. Eur Respir J 38:1180–1188. doi:10.1183/09031936.00018311
Uhlving HH, Mathiesen S, Buchvald F et al (2015) Small airways dysfunction in long-term survivors of pediatric stem cell transplantation. Pediatr Pulmonol 50:704–712. doi:10.1002/ppul.23058
Van Muylem A, Antoine M, Yernault JC et al (1995) Inert gas single-breath washout after heart-lung transplantation. Am J Respir Crit Care Med 152:947–952. doi:10.1164/ajrccm.152.3.7663808
Van Muylem A, Paiva M, Estenne M (2001) Involvement of peripheral airways during methacholine-induced bronchoconstriction after lung transplantation. Am J Respir Crit Care Med 164:1200–1203. doi:10.1164/ajrccm.164.7.2102113
Yu J (2015) Postinfectious bronchiolitis obliterans in children: lessons from bronchiolitis obliterans after lung transplantation and hematopoietic stem cell transplantation. Korean J Pediatr 58:459–465. doi:10.3345/kjp.2015.58.12.459
Fuchs SI, Ellemunter H, Eder J et al (2012) Feasibility and variability of measuring the lung clearance index in a multi-center setting. Pediatr Pulmonol 47:649–657. doi:10.1002/ppul.21610
Robinson PD, Latzin P, Verbanck S et al (2013) Consensus statement for inert gas washout measurement using multiple- and single-breath tests. Eur Respir J 41:507–522. doi:10.1183/09031936.00069712
Gustafsson PM, Källman S, Ljungberg H, Lindblad A (2003) Method for assessment of volume of trapped gas in infants during multiple-breath inert gas washout. Pediatr Pulmonol 35:42–49. doi:10.1002/ppul.10221
Miller MR, Hankinson J, Brusasco V et al (2005) Standardisation of spirometry. Eur Respir J 26:319–338. doi:10.1183/09031936.05.00034805
Quanjer PH, Borsboom GJ, Brunekreef B et al (1995) Spirometric reference values for white European children and adolescents: polgar revisited. Pediatr Pulmonol 19:135–142
Bhalla M, Turcios N, Aponte V et al (1991) Cystic fibrosis: scoring system with thin-section CT. Radiology 179:783–788. doi:10.1148/radiology.179.3.2027992
http://www.statstodo.com/comp2 Comparing 2 regression lines explained. http://www.statstodo.com/comp2regs_exp.php. Accessed 24 Jul 2016
Wuensch KL (2016) Comparing correlation coefficients, Slopes, and Intercepts pp 1–4
Shapiro AJ, Zariwala MA, Ferkol T et al (2016) Diagnosis, monitoring, and treatment of primary ciliary dyskinesia: pCD foundation consensus recommendations based on state of the art review. Pediatr Pulmonol 51:115–132. doi:10.1002/ppul.23304
Bandeira T, Negreiro F, Ferreira R et al (2011) Clinical, radiological, and physiological differences between obliterative bronchiolitis and problematic severe asthma in adolescents and young adults: the early origins of the overlap syndrome? Pediatr Pulmonol 46:573–580. doi:10.1002/ppul.21405
Jensen R, Stanojevic S, Gibney K et al (2013) Multiple breath nitrogen washout: a feasible alternative to mass spectrometry. PLoS One 8:1–7. doi:10.1371/journal.pone.0056868
Rosen JB, Smith EO, Schecter MG et al (2012) Decline in 25 to 75% forced expiratory flow as an early predictor of chronic airway rejection in pediatric lung transplant recipients. J Heart Lung Transplant 31:1288–1292. doi:10.1016/j.healun.2012.09.010
Rowan SA, Bradley JM, Bradbury I et al (2014) Lung clearance index is a repeatable and sensitive indicator of radiological changes in bronchiectasis. Am J Respir Crit Care Med 189:586–592. doi:10.1164/rccm.201310-1747OC
Subbarao P, Stanojevic S, Brown M et al (2013) Lung clearance index as an outcome measure for clinical trials in young children with cystic fibrosis: a pilot study using inhaled hypertonic saline. Am J Respir Crit Care Med 188:456–460. doi:10.1164/rccm.201302-0219OC
Irving SJ, Ives A, Davies G et al (2013) Lung clearance index and high-resolution computed tomography scores in primary ciliary dyskinesia. Am J Respir Crit Care Med 188:545–549. doi:10.1164/rccm.201304-0800OC
Boon M, Vermeulen FL, Gysemans W et al (2015) Lung structure-function correlation in patients with primary ciliary dyskinesia. Thorax 70:339–345. doi:10.1136/thoraxjnl-2014-206578
Schmidt A, Yammine S, Proietti E et al (2015) Validation of multiple-breath washout equipment for infants and young children. Pediatr Pulmonol 50:607–614. doi:10.1002/ppul.23010
Fuchs SI, Gappa M (2010) Equipment for multiple breath washout. Eur Respir J 35:458–459. doi:10.1183/09031936.00159509
Fuchs SI, Schwerk N, Pittschieler K et al (2016) Lung clearance index for monitoring early lung disease in alpha-1-antitrypsin deficiency. Respir Med 116:93–99. doi:10.1016/j.rmed.2016.04.015
Heinze H, Schaaf B, Grefer J et al (2007) The accuracy of the oxygen washout technique for functional residual capacity assessment during spontaneous breathing. Anesth Analg 104:598–604. doi:10.1213/01.ane.0000255044.05784.95
Chan PW, Muridan R, Debruyne JA (2000) Bronchiolitis obliterans in children: clinical profile and diagnosis. Respirology 5:369–375
de Jong PA, Dodd JD, Coxson HO et al (2006) Bronchiolitis obliterans following lung transplantation: early detection using computed tomographic scanning. Thorax 61:799–804. doi:10.1136/thx.2005.053249
Acknowledgements
The authors acknowledge the statistical help of Mrs. R. Leiba from the Medical Statistics Unit, Rambam Health Care Campus. The study was partially funded by the Israeli Lung Association.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Michal Gur and Karin Yaacoby-Bianu have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Gur, M., Yaacoby-Bianu, K., Ilivitzki, A. et al. Lung Clearance Index (LCI) in Patients with Bronchiolitis Obliterans: A Preliminary Report and Comparison to Cystic Fibrosis Patients. Lung 194, 1007–1013 (2016). https://doi.org/10.1007/s00408-016-9934-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00408-016-9934-8