Abstract
Purpose
Insulin-like growth factor II mRNA-binding protein 3 (IMP3) is an oncofetal protein associated with several aggressive and advanced cancers. Whether IMP3 can predict invasion, and prognosis in patients with human lung adenocarcinoma (LAC) remains unclear.
Methods
Ninety-five LAC and 75 non-tumor lung tissue samples were included in a tissue microarray. IMP3 expression was assessed by immunohistochemical examination. Correlation between IMP3 expression levels, clinicopathological characteristics, and overall prognosis was evaluated. In a separate in vitro study, RNA interference method was applied for knockdown of IMP3 gene in human LAC cell lines. Invasive potential of LAC cells was then evaluated by transwell migration assay.
Results
IMP3 immunoreactivity was observed in 39 out of 95 (41.1 %) LAC patients, but not in non-tumor lung tissues. IMP3 expression levels were closely associated with histological grade (P = 0.037), TNM stage (P = 0.034), and lymph node metastasis (P = 0.011). Patients presenting with positive IMP3 expression (P = 0.000), an advanced TNM stage (P = 0.000), and lymph node metastasis (P = 0.001) had a worse overall survival, compared to those lacking these characteristics. Both IMP3 expression (hazard ratio [HR], 2.310; 95 % confidence interval [CI] 1.192–4.476; P = 0.013) and TNM stage (HR 2.338; 95 % CI 1.393–3.925; P = 0.001) were independent predictors of poor prognosis. The invasive potential of LAC cells was significantly inhibited by IMP3 knockdown.
Conclusion
IMP3 appears to play an important role in tumor invasion in patients with LAC and may serve as a useful prognostic biomarker in these patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lung cancer is by far the most common cancer and the leading cause of cancer-related death worldwide [1]. The incidence of lung adenocarcinoma (LAC) has increased in most countries in recent decades. LAC has replaced squamous cell carcinoma as the most frequently occurring histological type [2–4]. The 5-year survival rate for patients with advanced LAC is of the order of 5–20 % [5]. A key contributor to the dismal prognosis is that the most patients are diagnosed at an advanced stage, often with metastatic lesions. In addition, LAC is a radiologically, histomorphologically, and clinically heterogeneous tumor with a wide variability in malignant potential. The prognosis of LAC is notoriously unpredictable; the TNM stage of the tumor at the time of diagnosis or surgery has a relatively low reliability [6, 7]. Identification of novel bio-markers that could help predict the tendency for invasive growth and metastasis, overall survival, as well as for aiding clinical decision-making is a key research imperative.
Insulin-like growth factor II mRNA-binding protein 3 (IMP3) is a member of the IMP family that consists of IMP1, IMP2, and IMP3. It is also referred to as K homology domain containing-protein over-expressed in cancer (KOC), IGF2BP3, and L523S [8, 9]. IMP3 binds to the 5′ untranslated region of the IGF-II leader-3 mRNA as its translational activator, coding for IGF-II protein which regulates cell proliferation [9]. IMP3 is an oncofetal protein, which finds expression in developing epithelium, muscle tissue, and placenta during the early stages of human and mouse embryogenesis. Expression of IMP3 has not been demonstrated in normal adult human cells [10]. Recent studies have shown that IMP3 is an important cancer-specific gene associated with several types of cancers and that it is exclusively expressed in malignant tumors [11–15]. IMPs have been demonstrated to promote cell adhesion and invadopodia formation, suggesting its role in cell migration and invasion during cancer development and progression [16–18]. In several organ systems, IMP3 expression has been shown to correlate with a higher grade of tumor, increased risk of metastases, and poorer prognosis [19–22]. Our previous study also indicated that IMP3 was an independent prognostic factor in patients with cervical squamous cell carcinoma [15]. However, association of IMP3 expression in LAC has not been adequately researched. In previous studies, IMP3 expression was associated with histological differentiation, histological subtypes, and distant metastasis in LAC [19, 23, 24]. However, few studies have probed the association of IMP3 expression with survival. Further, the relationship between IMP3 expression and invasive potential of LAC cells is yet to be characterized at the cellular level.
In this study, we investigated IMP3 expression in human LAC tissue. The correlation between IMP3 expression, clinicopathological factors, and prognosis are examined. In a separate in vitro proof-of-concept study, we established cell sublines with stable knockdown of IMP3 gene to verify the role of IMP3 in tumor invasion.
Materials and Methods
Patients
IMP3 expression in 95 LAC and 75 non-tumor lung tissue samples was assessed by immunohistochemical (IHC) examination using commercially available tissue microarrays (Shanghai Outdo Biotech Co., Ltd. Shanghai, China). The operation time was from April 2004 to October 2007, and the follow-up time was July 2012. None of the patients in this study received chemotherapy or radiotherapy prior to surgery. This study was conducted according to the regulations of the local ethical committee. Among the study subjects, 52 were male and 43 were female (male:female ratio: 1.21:1). The mean age was 58.9 years (range 20–84 years). Tumor nodes metastasis (TNM) stages were determined according to the Union for International Cancer Control (UICC) 2002 classification [25]. The number of patients having stage I, II, III, and IV LAC was 49, 14, 24, and 8, respectively. LAC tissues were histologically graded as moderately or poorly differentiated, as per the World Health Organization criteria [26].
Immunohistochemical Analysis
Immunohistochemical examination was performed as previously described [15]. IMP3 immunoreactivity generally manifested as concentrated cytoplasmic staining. Each sample was semiquantitatively scored for both intensity and proportion of IMP3 expression. Intensity of staining was classified as follows: 0: none, 1: weak, 2: moderate, and 3: strong; the percentage of positive cells was graded using the following 4 categories: 0: <5 %; 1: ≥5 to <25 %; 2: ≥25 to <50 %; and 3: ≥50 %. A final immunoreactivity score for each sample was computed by adding the two individual scores. A final score <2 was considered as negative, while ≥2 was considered as positive.
Cell Culture
The human immortalized bronchial epithelial cells, 16HBE, and LAC cells, including, A549, GLC-82, H358, H322, and SPC-A1, were cultured in RPMI-1640 (HyClone, Logan, UT) supplemented with 10 % heat-inactivated fetal bovine serum (FBS) (Gibco, Los Angeles, CA, USA). All cell lines were obtained from the Department of Pathology at the Southern Medical University. Cells were cultured under humidified air containing 5 % CO2 at 37 °C.
Construction of IMP3 shRNA Lentiviral Vector and Transfection
Three target RNA interference (RNAi) sequences were designed based on the human IMP3 mRNA (GenBank Accession no. NM_006547) sequence and cloned into the pGC–LV–GFP vector (GeneChem, Shanghai, China). The RNAi sequence AGGAATTGACGCTGTATAA most effectively suppressed IMP3 mRNA in A549 and H322 cells, and was used in the subsequent experiments for endogenous IMP3 knockdown (A549-shIMP3, H322-shIMP3). Non-silencing (NS)-small interfering RNA (shRNA) (TTCTCCGAACGTGTCACGT) was also cloned into the pGC–LV–GFP vector and served as a negative control (A549-control, H322-control). The recombinant virus was packaged in 293T cells using a lentivector expression system. For cellular transfection, A549 and H322 cells were subcultured at 1 × 106/mL in 6-well culture plates and transfected with lentivirus-mediated IMP3-shRNA or NS-shRNA. The GFP expression level was detected via fluorescence microscopy (Olympus, Japan) to determine the infection efficiency at 72 h after transfection.
Western Blot and Real-Time Polymerase Chain Reaction
Western blot analysis, total RNA extraction, reverse transcription, and real-time PCR were carried out as previously described [15]. An anti-IMP3 antibody (Epitomics, USA) was diluted 1:3000. IMP3 forward and reverse primers were 5′-CGGAGACTGTTCATCTGTTTATCC-3′ and 5′-TTCTGTTGTTGGTGCTGC TTT-3′, respectively. GAPDH forward and reverse primers were 5′-GGAGCGAGA TCCCTCCAAAAT-3′ and 5′-GGCTGTTGTCATACTTCTCATGG-3′, respectively. The relative levels of gene expression were calculated using the 2−ΔΔCt method [27].
Transwell Migration Assay
A549 and H322 cells, with or without IMP3 knockdown, were plated at 1 × 106/mL in 100 µL serum-free medium in 24-well matrigel-coated transwell units with polycarbonate filters (8 µm pore size, Coring, USA). The lower chamber was loaded with 500 µL of RPMI-1640 containing 10 % FBS. After incubation for 6, 24, and 48 h under normal culture conditions, the top surface of the membrane was gently scrubbed with a cotton bud, fixed in 4 % paraformaldehyde, and stained with hematoxylin and eosin. The cells that had permeated through the membrane filters were counted using a light microscope and five microscopic fields (×200) were randomly selected to count the migrated cells.
Statistical Analysis
Statistical analysis was performed using SPSS software 13.0. Association between categorical variables was assessed using a χ 2 test. Correlation between IMP3 expression and the overall survival was evaluated using the Kaplan–Meier method and the log-rank test. A Cox regression proportional hazards model was used for multivariate analyses to determine the independent significance of relevant clinical covariates. The hazard ratio (HR) with 95 % confidence interval (CI) was measured to estimate the hazard risk for individual factors. Real-time PCR results were assessed by one-way analysis of variance (ANOVA). The results of transwell assay were assessed using two sample t tests. Two-tailed P values of <0.05 were considered statistically significant.
Results
IMP3 Expression in LAC and Non-tumor Lung Tissue Samples
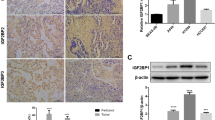
Immunostaining demonstrated that IMP3 expression was absent in all 75 non-tumor lung tissue specimens (Fig. 1a), while IMP3 expression was observed in 39 out of 95 (41.1 %) LAC tissue samples. The expression levels of IMP3 in LAC tissue samples varied between weak, moderate, and strongly positive (Fig. 1b–d).
Relationship Between IMP3 Expression and Clinicopathological Factors
Patients with LAC were categorized into IMP3 negative and IMP3 positive subgroups. IMP3 expression was present in 19.0 % (4/21), 42.6 % (20/47), and 55.6 % (15/27) of well, moderately, and poorly differentiated tumors, respectively. IMP3 expression increased significantly with histological grades (P = 0.037). Patients with stage III–IV LAC exhibited a greater IMP3 expression as compared to patients with I-II stages (P = 0.034). IMP3 expression significantly correlated with lymph node metastasis (P = 0.011). No significant correlation was observed between IMP3 expression and age (P = 0.659), gender (P = 0.785), and tumor size (P = 0.489) (Table 1).
Prognostic Significance of IMP3 Expression
The median survival time of LAC patients was 62.95 months and the 5-year survival was 54.7 %. Survival times were significantly decreased in LAC patients with IMP3 expression (P = 0.000), with advanced TNM stage (P = 0.000), and with lymph node metastasis (P = 0.001) (Fig. 2a–c) (Table S1). On univariate analysis, Cox proportional hazards model showed that advanced TNM stage (P = 0.000), lymph node metastasis (P = 0.002), and positive IMP3 expression (P = 0.000) correlated with a decreased overall survival (Table 2). However, multivariate analyses revealed that both TNM stage (HR 2.338; 95 % CI 1.393–3.925; P = 0.001) and IMP3 expression (HR 2.310; 95 % CI 1.192–4.476; P = 0.013) were significant independent predictors of survival (Table 2).
IMP3 Expression in LAC Cell Lines
IMP3 expression in 16HBE and LAC cells, including A549, GLC-82, H358, H322, and SPC-A1, were assessed by real-time PCR and Western blot analysis. As compared with 16HBE cells, significantly elevated IMP3 expression was observed in all LAC cell lines, except H358 (P = 0.001) (Table S2, Fig. 3a, b). In addition, IMP3 expression in A549 and H322 cells was detected by immunocytochemical (ICC) staining. Consistent with the results of immunostaining in tissue samples, ICC staining for IMP3 was concentrated cytoplasmic in LAC cells (Fig. 3c).
Establishment of Cell Sublines with Stable Knockdown of IMP3 Gene
Lentiviral vectors, labeled with GFP, were stably transfected onto A549 and H322 cells for constitutive knockdown IMP3 gene. After transfection, GFP+ cells were observed by fluorescence microscopy (Fig. 4a) and were sorted by flow cytometry. The RNAi efficiency of IMP3 gene was measured by real-time PCR (Table S3, Fig. 4b, c) and Western blot analysis (Fig. 4d, e). Both mRNA and protein levels of IMP3 expression were significantly down-regulated in the A549- and H322-shIMP3 groups as compared to those in the control groups.
IMP3 Gene Knockdown Inhibits LAC Cell Invasion
Transwell migration assay was performed at 6, 24, and 48 h to assess the invasive characteristics of cell sublines. The upper chamber contained 100 µL cell suspension with serum-free medium; and the lower chamber was loaded with 500 µL of RPMI-1640 containing 10 % FBS (Fig. 5a). The number of migrated cells in A549-shIMP3 group was significantly lower than that in the A549-control group. Similarly, significant differences were observed between H322-shIMP3 and H322-control groups. A significant effect in terms of time, group, and time-group interaction of A549 and H322 cell sublines was observed (Table S4, S5) (Fig. 5b, c).
Transwell migration assay of IMP3-shRNA transfected A549 and H322 cells. a The upper chamber contained 100 µL cell suspension with serum-free medium; lower chamber was loaded with 500 µL RPMI-1640 with 10 % FBS. b, c The migrated cells of all cell sublines were detected by transwell migration assay at 6, 24, and 48 h. The cells permeating the membrane filters were counted using a light microscope. Five microscopic fields (×200) were randomly selected to count the cells. FBS fetal bovine serum
Discussion
The effect of IMP3 on multiple tumors has recently been investigated. IMP3 is a 580 amino acid oncofetal RNA binding protein that contains two RNA recognition motifs and 4 K homology domains [28], and controls translation of IGF-II mRNA during cell proliferation. In patients with endometrial serous carcinoma and renal cell carcinoma, IMP3 has been shown to be a prognostic biomarker and was reportedly associated with an increased likelihood of metastasis after surgery and a shorter metastasis-free survival [13, 29, 30].
In patients with colon cancer, IMP3 expression was shown to correlate with cancer progression and was associated with an increased risk of metastasis and recurrence after colonectomy [21, 31]. Some in vitro studies have also revealed the importance of IMP3 in cancer progression. Liao et al. demonstrated that IMP3 knockdown slowed cell proliferation through an IGFII-dependent pathway in K562 human leukemia cells [32]. Depletion of IMP3 by RNA interference in hepatocellular carcinoma cell line, HA22T, decreased cell motility and attenuated the invasive characteristics as well as transendothelial migration. Microarray analysis revealed that IMP3 depletion was associated with downregulation of multiple genes involved in tumor invasion [33]. In a study by Vikesaa et al., IMPs appeared to induce cell adhesion and invasion by stabilizing CD44 mRNA [16]. However, in relation to human LAC, the prognostic value of IMP3 and its effect on tumor invasion remain unclear.
In the present study, IHC examination demonstrated IMP3 expression in 41.1 % (39/95) of LAC tissues, while it was absent in non-tumor lung tissue samples. Our findings are consistent with previous studies reporting IMP3 expression in 27–55 % of primary pulmonary adenocarcinoma cases [34]. Furthermore, we revealed demonstrated an association of IMP3 expression with histological grade and lymph node metastasis in LAC, which is also in line with results from earlier studies [19, 23, 24]. Of note, the expression level of IMP3, as determined by IHC analysis, increased with advancing disease state. The expression of IMP3 in patients with stage III–IV disease was significantly higher as compared to that of patients with stage I and II disease. Similar to our findings, Beljan et al. also reported evidence supporting the correlation of IMP3 expression with solid subtype and distant metastases, regardless of histological subtype of LAC [24]. Our findings indicate a potential role of IMP3 in the progression and invasion of LAC.
Early diagnosis of LAC is typically challenging, as early stages are often asymptomatic. LAC is a highly invasive malignancy, which spreads through blood and lymphatic vessels easily. The overall survival rate for patients with LAC continues to be poor in spite of the recent advances in its diagnosis and treatment. Therefore, the prediction of clinical prognosis for patient with LAC is very important, which may influence the choice of treatment strategies and outcomes.
In this study, we analyzed the correlation between clinicopathological factors and overall survival, and observed a significant correlation of IMP3 expression with the overall survival. IMP3 positive patients exhibited a poorer prognosis with a HR of 2.130 (95 % CI 1.192–4.476; P = 0.013) as compared to that of IMP3 negative patients. Based on these results, IMP3 expression appears to be a potential-independent biomarker for predicting poor prognosis of patients with LAC. There are two limitations identified in this study. First, the heterogeneity among LAC tissues may influence the result. The role of IMP3 gene in LAC should therefore be further verified. Second, we did not investigate the relationship between lung adenocarcinoma subtypes and IMP3 expression because the commercially available tissue microarrays were not able to discriminate subtypes. By collecting more specimens, the relationship between lung adenocarcinoma subclasses and IMP3 expression will be revealed in future studies.
Although the prognostic relevance of IMP3 in various malignancies has been confirmed, the mechanism underlying its correlation with tumor progression needs to be fully elucidated. Recent studies have shown that presence of IMP3 appeared to facilitate progression of pancreatic ductal adenocarcinoma by enhancing the pro-metastatic behavior of tumor cells [35]. Rivera et al. reported that IMP3 was capable of binding to the mRNAs of cyclin D1, cyclin D3, and cyclin G1, both in vivo as well as in vitro [36]. In breast cancer cells, IMP3 promoted invasion and migration through the epithelial-mesenchymal transition (EMT) [37]. In a separate in vitro study, we investigated IMP3 expression in LAC cell lines to further verify the role of IMP3 in tumor invasion. Not surprisingly, IMP3 expression was up-regulated in most LAC cells except H358, but weak in immortalized bronchial epithelial cells. The heterogeneity of different origins or IMP3 expression heterogeneity may explain the different expression levels among LAC cell lines. Furthermore, invasiveness of LAC cells was significantly inhibited after knockdown of IMP3 gene using RNAi method. The results imply a potential association of IMP3 over-expression with tumorigenesis.
In a phase I clinical trial of a vaccine targeting L523S (IMP3) in thirteen patients with non-small cell lung cancer, no dose-related toxic effects were observed. Although vaccination induced an immune response in only one patient, it is interesting to note that only two patients developed disease recurrence, while all remained alive during a median follow-up of 290 days. These findings support the case for further phase II investigation of the L523S vaccine [38]. Combined with the results in the current study, IMP3 may be a potential therapeutic target for LAC; however, further studies are warranted to provide more definitive evidence.
To conclude, IMP3 appears to play an important role in tumor invasion in patients with LAC and may serve as a prognostic indicator in these patients.
References
Jemal A, Bray F, Center MM et al (2011) Global cancer statistics. CA Cancer J Clin 61:69–90
Lortet-Tieulent J, Soerjomataram I, Ferlay J et al (2014) International trends in lung cancer incidence by histological subtype: adenocarcinoma stabilizing in men but still increasing in women. Lung Cancer 84:13–22
Nakamura H, Saji H (2014) Worldwide trend of increasing primary adenocarcinoma of the lung. Surg Today 44:1004–1012
Okada M (2013) Subtyping lung adenocarcinoma according to the novel 2011 IASLC/ATS/ERS classification: correlation with patient prognosis. Thorac Surg Clin 23:179–186
Gu W, Fang S, Gao L et al (2013) Clinic significance of microRNA-99a expression in human lung adenocarcinoma. J Surg Oncol 108:248–255
Chen W, Zheng R, Zhang S et al (2013) The incidences and mortalities of major cancers in China, 2009. Chin J Cancer 32:106–112
Chilosi M, Murer B (2010) Mixed adenocarcinomas of the lung: place in new proposals in classification, mandatory for target therapy. Arch Pathol Lab Med 134:55–65
Nielsen J, Christiansen J, Lykke-Andersen J et al (1999) A family of insulin-like growth factor II mRNA-binding proteins represses translation in late development. Mol Cell Biol 19:1262–1270
Liao B, Hu Y, Brewer G (2011) RNA-binding protein insulin-like growth factor mRNA-binding protein 3 (IMP-3) promotes cell survival via insulin-like growth factor II signaling after ionizing radiation. J Biol Chem 286:31145–31152
Findeis-Hosey JJ, Xu H (2011) The use of insulin like-growth factor II messenger RNA binding protein-3 in diagnostic pathology. Hum Pathol 42:303–314
Sitnikova L, Mendese G, Liu Q et al (2008) IMP3 predicts aggressive superficial urothelial carcinoma of the bladder. Clin Cancer Res 14:1701–1706
Jiang Z, Lohse CM, Chu PG et al (2008) Oncofetal protein IMP3: a novel molecular marker that predicts metastasis of papillary and chromophobe renal cell carcinomas. Cancer 112:2676–2682
Hoffmann NE, Sheinin Y, Lohse CM et al (2008) External validation of IMP3 expression as an independent prognostic marker for metastatic progression and death for patients with clear cell renal cell carcinoma. Cancer 112:1471–1479
Zheng W, Yi X, Fadare O et al (2008) The oncofetal protein IMP3: a novel biomarker for endometrial serous carcinoma. Am J Surg Pathol 32:304–315
Wei Q, Yan J, Fu B et al (2014) IMP3 expression is associated with poor survival in cervical squamous cell carcinoma. Hum Pathol 45:2218–2224
Vikesaa J, Hansen TV, Jonson L et al (2006) RNA-binding IMPs promote cell adhesion and invadopodia formation. EMBO J 25:1456–1468
Bell JL, Wachter K, Muhleck B et al (2013) Insulin-like growth factor 2 mRNA-binding proteins (IGF2BPs): post-transcriptional drivers of cancer progression? Cell Mol Life Sci 70:2657–2675
Hwang YS, Xianglan Z, Park KK et al (2012) Functional invadopodia formation through stabilization of the PDPN transcript by IMP-3 and cancer-stromal crosstalk for PDPN expression. Carcinogenesis 33:2135–2146
Findeis-Hosey JJ, Yang Q, Spaulding BO et al (2010) IMP3 expression is correlated with histologic grade of lung adenocarcinoma. Hum Pathol 41:477–484
Yuan RH, Wang CC, Chou CC et al (2009) Diffuse expression of RNA-binding protein IMP3 predicts high-stage lymph node metastasis and poor prognosis in colorectal adenocarcinoma. Ann Surg Oncol 16:1711–1719
Li D, Yan D, Tang H et al (2009) IMP3 is a novel prognostic marker that correlates with colon cancer progression and pathogenesis. Ann Surg Oncol 16:3499–3506
Gong Y, Woda BA, Jiang Z (2014) Oncofetal protein IMP3, a new cancer biomarker. Adv Anat Pathol 21:191–200
Bellezza G, Cavaliere A, Sidoni A (2009) IMP3 expression in non-small cell lung cancer. Hum Pathol 40:1205–1206
Beljan PR, Durdov MG, Capkun V et al (2012) IMP3 can predict aggressive behaviour of lung adenocarcinoma. Diagn Pathol 7:165
Dhwc S (2002) International union against cancer (UICC): TNM classification of malignant tumors. Wiley, New York
Travis WD, Brambilla E, Müller-Hermelink HK et al. (2004) Pathology and genetics of tumours of the lung, pleura, thymus and heart, vol 10. Lyon: IARC Press. World Health Organization Classification of Tumors
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods 25(4):402–408
Mueller-Pillasch F, Lacher U, Wallrapp C et al (1997) Cloning of a gene highly overexpressed in cancer coding for a novel KH-domain containing protein. Oncogene 14:2729–2733
Jiang Z, Chu PG, Woda BA et al (2006) Analysis of RNA-binding protein IMP3 to predict metastasis and prognosis of renal-cell carcinoma: a retrospective study. Lancet Oncol 7:556–564
Zheng W, Yi X, Fadare O et al (2008) The oncofetal protein IMP3: a novel biomarker for endometrial serous carcinoma. Am J Surg Pathol 32:304–315
Yuan RH, Wang CC, Chou CC et al (2009) Diffuse expression of RNA-binding protein IMP3 predicts high-stage lymph node metastasis and poor prognosis in colorectal adenocarcinoma. Ann Surg Oncol 16:1711–1719
Liao B, Hu Y, Herrick DJ et al (2005) The RNA-binding protein IMP-3 is a translational activator of insulin-like growth factor II leader-3 mRNA during proliferation of human K562 leukemia cells. J Biol Chem 280:18517–18524
Jeng YM, Chang CC, Hu FC et al (2008) RNA-binding protein insulin-like growth factor II mRNA-binding protein 3 expression promotes tumor invasion and predicts early recurrence and poor prognosis in hepatocellular carcinoma. Hepatology 48:1118–1127
Findeis-Hosey JJ, Xu H (2012) Insulin-like growth factor II-messenger RNA-binding protein-3 and lung cancer. Biotech Histochem 87:24–29
Pasiliao CC, Chang CW, Sutherland BW et al (2015) The involvement of insulin-like growth factor 2 binding protein 3 (IMP3) in pancreatic cancer cell migration, invasion, and adhesion. BMC Cancer 15:266
Rivera VT, Boudoukha S, Simon A et al (2014) Post-transcriptional regulation of cyclins D1, D3 and G1 and proliferation of human cancer cells depend on IMP-3 nuclear localization. Oncogene 33:2866–2875
Su P, Hu J, Zhang H et al (2014) IMP3 expression is associated with epithelial-mesenchymal transition in breast cancer. Int J Clin Exp Pathol 7:3008–3017
Nemunaitis J, Meyers T, Senzer N et al (2006) Phase I trial of sequential administration of recombinant DNA and adenovirus expressing L523S protein in early stage non-small-cell lung cancer. Mol Therapy 13:1185–1191
Acknowledgments
We thank Professor Hong Shen for providing LAC cell lines. This study was supported by National Natural Science Foundation of China (Grant Number: 81470013) and Natural Science Foundation of Guangdong Province (Grant Number: 2015A030313244).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Jinhai Yan and Qingzhu Wei have contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yan, J., Wei, Q., Jian, W. et al. IMP3 Predicts Invasion and Prognosis in Human Lung Adenocarcinoma. Lung 194, 137–146 (2016). https://doi.org/10.1007/s00408-015-9829-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00408-015-9829-0