Abstract
Patients with treatment-resistant schizophrenia (TRS), particularly those resistant to clozapine (CTRS), pose a clinical challenge due to limited response to standard antipsychotic treatments. Inflammatory factors like tumor necrosis factor-alpha (TNF-α), interleukin 2 (IL-2), and interleukin 6 (IL-6) are implicated in schizophrenia’s pathophysiology. Our study examines cognitive function, psychopathological symptoms and inflammatory factors in TRS patients, focusing on differences between CTRS and non-CTRS individuals, as well as healthy controls. A cohort of 115 TRS patients and 84 healthy controls were recruited, assessing IL-2, IL-6 and TNF-α. The Positive and Negative Syndrome Scale (PANSS) was applied to assess psychopathological symptoms, while the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) was applied to assess cognitive functioning. CTRS patients showed lower visuospatial constructional score (p = 0.015), higher PANSS scores, higher levels of IL-2 and reduced TNF-α than non-CTRS patients (p < 0.05). Notably, IL-2 was independently associated with psychopathology symptoms in CTRS patients (Beta = 0.268, t = 2.075, p = 0.042), while IL-6 was associated with psychopathology symptoms in non-CTRS patients (Beta = – 0.327, t = − 2.109, p = 0.042). Sex-specific analysis in CTRS patients revealed IL-2 associations with PANSS total and positive symptoms in females, and TNF-α associations with PANSS positive symptoms in males. Furthermore, IL-2, IL-6, and TNF-α displayed potential diagnostic value in TRS patients and CTRS patients (p < 0.05). Clozapine‑resistant symptoms represent an independent endophenotype in schizophrenia with distinctive immunoinflammatory characteristics, potentially influenced by sex.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Schizophrenia, a chronic and debilitating disorder, affects approximately 0.75% of the Chinese population, posing significant burdens on patients and their families [19]. Among patients with schizophrenia, a considerable (about 1/5 to 1/3) are classified as having “treatment-resistant schizophrenia (TRS)” or “refractory schizophrenia” when they failed to respond to two or more antipsychotic medications [33, 39]. Despite significant advancements in schizophrenia treatment, TRS remains a challenging problem due to its higher clinical severity, safety concerns with available treatments, increased health care costs [21]. TRS patients experience an earlier onset of symptoms, have a poorer prognosis, require frequent hospitalizations, and exhibit diminished real-life functioning. [6]. Treatment-resistant disease might be a distinct subtype of schizophrenia and not merely a more severe form [49]. However, the underlying neurological and pathophysiological mechanisms contributing to TRS remain largely unknown.
Clozapine (CLZ), an atypical antipsychotic, serves as the gold standard and the only evidence-based effective treatment for TRS [32, 38]. Nevertheless, even with adequate blood levels of clozapine, a substantial portion (approximately 40−70%) of TRS patients remain resistant to its effects [5, 41]. Consequently, TRS patients who do not respond well to clozapine monotherapy are identified as having clozapine-resistant treatment-refractory schizophrenia (CTRS) [17, 20]. The varying responsiveness to antipsychotic drugs suggests that TRS represents a distinct biological subtype with a pathophysiology distinct from treatment-responsive schizophrenia [9, 33]. Thus, there is a critical need for further investigations to elucidate the characteristics of patients with TRS and CTRS.
The immune system and inflammation have been implicated in the pathophysiology of schizophrenia [22, 29]. Abnormalities in proinflammatory markers, such as tumor necrosis factor-α (TNF-α), interleukin 2 (IL-2), and interleukin 6 (IL-6), along with a chronic low-grade inflammatory state, have been associated with psychopathological symptoms and cognitive deficits in schizophrenia [15, 30, 36]. The effects of antipsychotic drugs on inflammatory factors have shown considerable heterogeneity. Studies have revealed that antipsychotic drugs may inhibit microglia activation, a source of pro-inflammatory markers like nitric oxide and TNF [1]. Nonetheless, existing evidence remains inconclusive on whether antipsychotic drugs impact serum inflammatory factors levels [8]. Furthermore, limited research has investigated alterations in blood levels of inflammatory factors in TRS or CTRS patients.
Emerging evidence supports the significant role of sex in explaining heterogeneity in clinical presentation, disease course, treatment response, and tolerance in schizophrenia [44, 46]. However, investigations into sex-related differences in the prevalence and clinical correlates of inflammatory factors among TRS patients treated with CLZ remain scarce. Existing literature on sex-related differences in CLZ tolerance primarily focuses on metabolic disturbances [24]. Women tend to exhibit higher long-term BMI and elevated blood glucose, possibly due to higher CLZ plasma concentrations [3]. Conversely, men taking olanzapine are at increased risk for other metabolic abnormalities, such as elevated triglycerides and lower HDL cholesterol levels [2]. Therefore, further studies are needed to explore the sex-related differences in the prevalence and clinical correlates of inflammatory factors in TRS patients.
Taken together, in order to determine if CTRS patients exhibit more abnormalities in proinflammatory markers and poorer cognitive performance than those without clozapine‑resistant symptoms, the current study was conducted to investigate the differences among CTRS, TRS patients, and healthy controls, focusing on the analysis of clinical, psychopathological and inflammatory markers. The aims of our study were: (1) to compare the inflammatory factors (IL-2, IL-6 and TNF-α), psychopathological symptoms and cognitive function among TRS patients with and without clozapine‑resistant symptoms, as well as healthy controls; and (2) to explore the relationship between inflammatory factors and clinical characteristics in TRS patients with and without clozapine resistant symptoms, respectively. In particular, we hypothesized that CTRS patients would have poorer cognitive performance, as well as unique proinflammatory markers changes, than patients without clozapine‑resistant symptoms.
Methods
Subjects
All patients were recruited from the Shanghai Pudong New Area Mental Health Center specializing in psychiatric care between September 6, 2018, and August 1, 2021, through medical records and referrals from treating psychiatrists. The final sample included 115 TRS patients (male/female: 58/57; average age: 47.90 ± 8.56 years) and 84 healthy controls (male/female: 44/40; average age: 44.36 ± 7.21 years). Additionally, healthy controls were recruited by word of mouth from the same area. None of the healthy controls exhibited a personal or family history of mental disorders had fever, infection, or received any drugs that affect the immune-inflammatory system within the last month. In accordance with the recently published guidelines of the Treatment Response and Resistance in Psychosis Working Group, patients were regarded as treatment-resistant after failing 2 or more trials of different antipsychotics at adequate doses and for a duration of ≥ 6 weeks, with objective assessments of adherence [18]. TRS patients were further divided into clozapine-resistant (CTRS) and non-clozapine-resistant (non-CTRS) groups based on their response to clozapine, as defined by clinical evaluations and standardized criteria. This study was approved by the Institutional Review Board of Shanghai Pudong New Area Mental Health Center (No.2018008), and all participants signed informed consent. The protocol was registered on clincialtrials.gov before participant enrollment (ID: NCT03652974).
The inclusion criteria were: (1) a diagnosed with schizophrenia according to the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV), confirmed using the Structured Clinical Interview for DSM-IV (SCID-I/P); (2) aged between 18 and 65 years old, Han ethnicity; (3) had received at least two antipsychotics with different mechanisms of action at appropriate doses and an adequate duration, and recently achieved stabilization on a stable clozapine (i.e., receiving a minimum of 400 mg/d or more for at least 6 months) to ensure a reasonable response to clozapine monotherapy; (4) a review of the patient’s past medical history revealed that the patient had persistent psychotic symptoms that were never effectively controlled. The exclusion criteria were: (1) pregnant or breastfeeding women; (2) severe physical diseases; (3) any other major Axis I disorder; (4) substance abuse/dependence except for tobacco.
Clinical assessments
Recruiters used self-designed questionnaires to collect demographic characteristics (sex, age, BMI, education, marital status) and history of disease and medication use. Psychopathological symptoms were assessed using the PANSS [40, 48], while cognitive functioning was evaluated with the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) [37]. Both assessors underwent thorough training on these scales, and the calculated interobserver correlation coefficients (ICC) were above 0.8. In addition, the assessors assessing psychopathological symptoms were blinded to the laboratory parameters. Blinding was implemented to ensure unbiased evaluations and to prevent any potential influence of the knowledge about laboratory results on the assessment of symptoms. The assessors were not provided with any information regarding the laboratory findings during the evaluation process.
Blood sampling and measurements of inflammatory factors
Peripheral venous blood samples were drawn at 8 am from subjects following an overnight fast and centrifuged at 4000 rpm for 10 min at room temperature to analyze plasma inflammatory factors levels. Plasma was extracted and stored at − 80 ℃ until assay. For the measurement of levels of IL-2, IL-6 and TNF-α, enzyme-linked immunosorbent assay (ELISA) was utilized (Jiangsu Meimian Industrial Co., Ltd, MM-0055H1, MM-0049H1 and MM-0122H1) following the manufacturer’s instructions. ELISA samples and standards for IL-2, IL-6 and TNF-α were analyzed in duplicate, with the average of two readings representing a separate dilution. The absorbance at 450 nm was measured using an automated microplate reader, and the plasma levels of IL-2, IL-6 and TNF-α were expressed in pg/ml. Detection limit of IL-2, IL-6 and TNF-α were 1.5 pg/ml, 0.8 pg/ml and 0.8 pg/ml, respectively. All cases were above the detection limit.
Statistical analysis
Statistical analyses were performed using SPSS version 25.0. Continuous variables were initially assessed for normalization using the Kolmogorov-Smirnov one-sample test. Demographic and clinical characteristics, and cognitive function and inflammatory factors between groups was compared using the chi-square test and analysis of variance (ANOVA). Additionally, age, sex, education level and BMI were selected as covariates in the analysis of comparisons between healthy controls and TRS patients, and age, sex, education level, BMI, duration and onset age were selected as covariates in the analysis of comparisons between TRS patients with and without clozapine‑resistant symptoms. Bonferroni correction was performed for multiple tests. Categorical variables were described in terms of frequencies and percentages, while continuous variables are presented as means and standard values.
Sex stratification analysis was conducted for various inflammatory factors. Furthermore, multiple stepwise regression analysis was performed to assess potential influences of inflammatory factors on psychopathological symptoms and cognitive performance. Receiver operating characteristic (ROC) curves were utilized to explore the possibility of using inflammatory factors as potential biomarkers to differentiate between TRS and CTRS patients. Statistical significance was determined at p ≤ 0.05 (two-tailed).
Results
Sociodemographic and clinical characteristics
As shown in Table 1, the prevalence of clozapine‑resistant symptoms in TRS was 60%. Compared to healthy controls, TRS patients were older (F = 10.03, p = 0.002) and had less years of education (F = 10.79, p = 0.001) and higher BMI (F = 17.246, p < 0.001). In addition, compared to TRS patients without clozapine‑resistant symptoms, clozapine-resistant patients had more years of education (F = 18.063, p < 0.001), longer disease duration (F = 4.019, p = 0.047), an earlier onset age (F = 16.729, p < 0.001) and higher serum clozapine level (F = 331.090, p < 0.001).
Table 2 showed the PANSS and RBANS composite scores, as well as each domain score for all participants. Clozapine-resistant patients exhibited higher scores in PANSS total and each domain score compared to TRS patients without clozapine‑resistant symptoms (all ps < 0.05). These differences remained statistically significant even after controlling for age, sex, education level, BMI, duration and onset age (all ps < 0.05). Moreover, the RBANS composite and each domain scores of TRS patients were significantly lower than those of healthy controls (all ps < 0.01). These differences remained significant after adjusting for age, sex, education level and BMI (all ps < 0.05). Furthermore, clozapine-resistant patients had lower scores in visuospatial constructional compared to TRS patients without clozapine‑resistant symptoms (Fadjusted = 6.151, p = 0.015).
Inflammatory factors in CTRS, TRS patients, and HC groups.
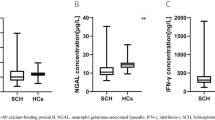
The levels of inflammatory factors in healthy controls and TRS patients with and without clozapine‑resistant symptoms are shown in Table 3. After controlling for age, sex, education level and BMI, TRS patients had significantly higher levels of IL-2 (Fadjusted = 106.554, p < 0.001), IL-6 (Fadjusted = 7.685, p = 0.006) and TNF-α (Fadjusted = 64.222, p < 0.001) compared to healthy controls. Furthermore, clozapine-resistant patients had higher IL-2 levels (Fadjusted = 32.781, p < 0.001) and lower TNF-α levels (Fadjusted = 13.758, p < 0.001) than TRS patients without clozapine‑resistant symptoms. These differences remained significant after Bonferroni corrections. Additionally, sex stratification analysis demonstrated that inflammatory factors, except for IL-6, showed significant differences between males and females in healthy controls, but there were no statistically significant differences in all inflammatory factors among TRS patients with and without clozapine‑resistant symptoms (Supplemental Table 1).
Relationships of inflammatory factors and clinical variables in TRS patients.
The correlations between PANSS scores and IL-2, IL-6, and TNF-α in all TRS patients are shown in Supplemental Tables 2 and Fig. 1. PANSS positive psychopathology score showed significant correlations with IL-2 (r = 0.516, p < 0.001) and TNF-α (r = − 0.284, p = 0.002) levels. PANSS negative psychopathology score was significantly correlated with IL-2 (r = 0.295, p = 0.001) and IL-6 (r = 0.189, p = 0.043) levels. PANSS general psychopathology score demonstrated significant correlations with IL-2 (r = 0.543, p < 0.001) and TNF-α (r = − 0.201, p = 0.031) levels. Additionally, there was a significant correlation between PANSS total score and the levels of IL-2 (r = 0.554, p < 0.001) and TNF-α (r = − 0.226, p = 0.015) in TRS patients.
Correlation analysis revealed significant associations between inflammatory factors and PANSS total scores in TRS patients with and without clozapine‑resistant symptoms. A, IL-2 and PANSS total scores in TRS patients with clozapine‑resistant symptoms (r = 0.272, p = 0.024). B, IL-6 and PANSS total scores in TRS patients without clozapine‑resistant symptoms (r = 0.020, p = 0.897)
In clozapine-resistant patients, significant correlations were found between PANSS positive psychopathology score and IL-6 levels (r = − 0.233, p = 0.018), PANSS general psychopathology score and IL-2 levels (r = 0.280, p = 0.020), as well as PANSS total score and IL-2 levels (r = 0.272, p = 0.024) (Supplemental Tables 2 and Fig. 1A). In TRS patients without clozapine‑resistant symptoms, PANSS negative psychopathology score was significantly correlated with IL-2 levels (r = 0.326, p = 0.027). Furthermore, stepwise multiple regression analyses were conducted in clozapine-resistant and non-resistant subgroups, respectively. In TRS patients with clozapine‑resistant symptom, IL-2 levels were independently associated with PANSS total score (Beta = 0.268, t = 2.075, p = 0.042). In TRS patients without clozapine‑resistant symptom, IL-6 levels were associated with PANSS total score (Beta = − 0.327, t = − 2.109, p = 0.042).
The correlations between inflammatory factors and clinical symptoms, after sex stratification, in TRS patients are shown in Supplemental Tables 4 and Supplemental Table 5. Finally, stepwise multiple regression analyses were conducted in CTRS patients, based on sex stratification. As shown in Supplemental Table 6, IL-2 levels were independently associated with PANSS total score (Beta = 0.436, t = 2.781, p = 0.009) and positive psychopathology score (Beta = 0.352, t = 2.161, p = 0.038) in females. In males, TNF-α levels were independently associated with PANSS positive psychopathology score (Beta = − 0.337, t = − 2.159, p = 0.039) (Supplemental Table 7).
Risk factors of refractory-SCZ patients.
ROC curves were analyzed to evaluate the diagnostic value of IL-2, IL-6 and TNF-α for distinguishing TRS patients and healthy controls, as well as clozapine-resistant patients from TRS patients without clozapine‑resistant symptoms. As shown in Supplemental Tables 8 and Fig. 2A, IL-2 and TNF-α levels provided an acceptable AUC for classifying TRS patients and healthy controls (both p < 0.05). Among them, IL-2 exhibited the best performance, yielding an AUC of 0.800 (95% CI 0.719–0.882). Furthermore, IL-2, IL-6 and TNF-α levels all displayed good performance in differentiating TRS patients with and without clozapine‑resistant symptoms (all p < 0.05). Notably, TNF-α showed the most outstanding performance, with an AUC of 0.981 (95% CI 0.958–1.000) (Supplemental Tables 9 and Fig. 2B).
Receiver operating characteristic (ROC) curves of the optimal sensitivity and specificity by using inflammatory factors levels to differentiate healthy controls, TRS patients with and without clozapine‑resistant symptoms. A The ROC curve of inflammatory factors of the optimal sensitivity and specificity by using inflammatory factors levels to differentiate healthy controls and TRS patients. B The ROC curve of inflammatory factors of the optimal sensitivity and specificity by using inflammatory factors levels to differentiate TRS patients with and without clozapine‑resistant symptoms
Discussion
The main findings of the present study were as follows: (1) TRS patients exhibited higher levels of IL-2, IL-6 and TNF-α compared to healthy controls. Additionally, clozapine-resistant patients had higher levels of IL-2 and lower levels of TNF-α when compared to TRS patients without clozapine‑resistant symptoms; (2) IL-2 levels were associated with psychopathology symptoms in CTRS patients, while IL-6 levels were associated with psychopathology symptoms in TRS patients without clozapine‑resistant symptoms; (3) there were sex differences in the inflammatory factors between TRS patients with and without clozapine‑resistant symptoms; (4) IL-2, IL-6 and TNF-α demonstrated good diagnostic value for recognizing TRS patients or CTRS patients.
The proportion of clozapine-resistant patients reported in our study (60%) aligned with previous research findings [31, 41, 45], indicating that a significant percentage of SCZ patients face clozapine-resistant symptoms, posing a challenge in terms of effective treatment. Cognitive impairment, a core symptom of SCZ, is typically present from the early onset and persists throughout the stages of the disorder. We observed that clozapine-resistant patients displayed greater cognitive deficits than TRS patients without clozapine‑resistant symptoms, particularly in the visuospatial constructional dimension of cognition assessed by RBANS. Furthermore, CTRS patients had more severe psychopathology symptoms compared with TRS patients without clozapine‑resistant symptoms. Some studies had found that CTRS patients might have distinct underlying pathophysiologies compared to those without clozapine‑resistant symptoms [4, 13]. A study comparing TRS patients and CTRS patients found that CTRS patients had significantly higher overall illness severity and greater cognitive impairments, along with significantly lower quality-of-life [34]. Nevertheless, the specific mechanisms underlying the poorer clinical and functional outcomes in CTRS patients require further investigation, as the shared brain and biological mechanisms contributing to psychopathology symptoms and cognitive deficits remain to be elucidated.
In our study, TRS patients exhibited significantly higher levels of IL-2, IL-6 and TNF-α compared to healthy controls. It is noteworthy that previous research has reported conflicting results regarding IL-2 levels in SCZ patients. One study demonstrated that clozapine reduced IL-2 and IL-6 levels in Microglial Cells [10], while a meta-analysis indicated that antipsychotic treatment decreased IL-2 levels in SCZ patients [7]. In contrast, another study focusing on first-episode psychosis patients showed no effect of antipsychotic medication on IL-2 levels [28]. The discrepancies between these studies may be attributed to variations in antipsychotic drugs used and treatment duration. Furthermore, Yuan et al. found that the IL-2 levels in TRS patients were positively correlated with the clozapine dose [51]. Similarly, several studies have reported elevated IL-6 levels in patients treated with clozapine [23, 25, 42, 43], which is consistent with our findings. However, there have also been reports of reduced IL-6 levels [26, 47] or no effect [16, 42] following clozapine treatment. Consistent with our findings, elevated levels of TNF-α had been reported in patients with chronic schizophrenia [11, 50] and deficit schizophrenia [12], and chronic treatment with clozapine upregulated IL-17, and TNF-α levels [51]. These inconsistent findings regarding the relationship between inflammatory factors and clozapine treatment may be attributed to the SCZ heterogeneity and the use of differing technological, experimental and analytical methods. Further research is needed to fully understand the underlying mechanisms contributing to these differences and to account for the heterogeneity of schizophrenia in future studies.
Importantly, to the best of our knowledge, our study is the first to report that CTRS patients exhibit higher levels of IL-2 and lower levels of TNF-α compared to TRS patients without clozapine‑resistant symptoms, indicating distinct inflammatory profiles between these two subgroups. Additionally, our findings reveal that IL-2 levels were independently associated with psychopathology symptoms in CTRS patients, and IL-6 levels were associated with psychopathology symptoms in TRS patients without clozapine‑resistant symptoms. These results align with previous studies suggesting that IL-2 is a key predictor of negative symptoms and cognitive impairment in SCZ outpatients [14]. Similarly, alterations in IL-6 and IL-1RA have been linked to the SCZ pathophysiology or specific phenotypic features [36]. It is worth noting that these previous studies were conducted in mixed-diagnostic and chronic patients, which may contribute to the observed discrepancies. Taken together, our findings support the notion that clozapine-resistant treatment-refractory SCZ may represent a distinct subtype with specific immune inflammatory mechanisms. However, due to the limited research on this particular SCZ subtype, further studies are essential to validate and extend the existing findings.
Intriguingly, our sex stratification analysis in CTRS patients revealed that a positive relationship between IL-2 levels and positive syndrome and total score in females, which aligns with previous findings [51]. Moreover, our study uncovered that in males, TNF-α was independently associated with positive syndrome. Consistent with our prior work, we previously reported that an interactive effect between TNF-α and MDA on the psychopathological symptoms solely in male SCZ patients [52]. These observations suggest that testosterone, known to influence the expression of inflammatory markers such as TNF-α, might play a role in the observed sex-specific correlations. Existing evidence has shown that testosterone use significantly reduced levels of inflammatory markers in males [27], and adult males with lower testosterone levels exhibit higher TNF-α levels [35]. These findings suggest that androgens may have complex interactions with the immune system, potentially explaining the independent relationship of TNF-α with psychopathology syndromes only in males with CTRS. As no prior studies have explored sex differences in immune characteristics in TRS patients with and without clozapine‑resistant symptoms, our present findings provide a starting point for further investigation. Future studies with larger, well-characterized samples are warranted to validate our results and shed light on the underlying biological mechanisms that may contribute to sex-specific immune responses in this population.
Several limitations were identified in our study. First, the cross-sectional design of the study limits our ability to establish causal relationship between variables. Second, incomplete information on the smoking and medications administered to the enrolled patients may have influenced the observed associations. The potential effects of these medications and smoking on inflammatory factors were not fully accounted for, necessitating further studies to address drug and smoking effects more comprehensively. Third, the single center sample and limited sample size may have restricted statistical power, particularly in the stratification analyses. Hence, future longitudinal investigations with larger studies are essential to validate and strengthen our findings. Fourth, the study involved only three inflammatory factors, IL-2, IL-6, and TNF-α. The roles of many other inflammatory factors remain unproven. Future studies should consider a broader spectrum of inflammatory factors for a more comprehensive understanding of immunoinflammatory dysregulation in TRS patients and CTRS patients.
Conclusion
In summary, our study sheds light on several key findings. CTRS patients displayed poorer cognitive function compared to TRS patients without clozapine‑resistant symptoms and healthy controls, and exhibited more severe psychopathology symptoms than the TRS patients without clozapine‑resistant symptoms. Moreover, CTRS patients exhibited distinct immune inflammatory characteristics, with higher levels of IL-2 and lower levels of TNF-α than TRS patients without clozapine‑resistant symptoms. In addition, IL-2 was independently associated with psychopathology symptoms in CTRS patients, particularly in females, while IL-6 was associated with psychopathology symptoms in TRS patients without clozapine‑resistant symptoms. These findings offer valuable insight into the pathophysiological mechanisms associated with inflammation of TRS patients with and without clozapine‑resistant symptoms. Nonetheless, further investigations involving larger and more diverse populations are imperative to validate and expand upon our results. Such efforts will advance our understanding of TRS and may pave the way for more personalized and targeted therapeutic approaches.
Data availability
The data supporting the findings of this study are available on request from the corresponding author.
References
Adami C, Sorci G, Blasi E, Agneletti AL, Bistoni F, Donato R (2001) S100b expression in and effects on microglia. Glia 33:131–142
Alberich S, Fernandez-Sevillano J, Gonzalez-Ortega I, Usall J, Saenz M, Gonzalez-Fraile E, Gonzalez-Pinto A (2019) A systematic review of sex-based differences in effectiveness and adverse effects of clozapine. Psychiatry Res 280:112506
Anderson SG, Livingston M, Couchman L, Smith DJ, Connolly M, Miller J, Flanagan RJ, Heald AH (2015) Sex differences in plasma clozapine and norclozapine concentrations in clinical practice and in relation to body mass index and plasma glucose concentrations: a retrospective survey. Ann Gen Psychiatry 14:39
Anderson VM, Goldstein ME, Kydd RR, Russell BR (2015) Extensive gray matter volume reduction in treatment-resistant schizophrenia. Int J Neuropsychopharmacol 18:pyv016
Bioque M, Parellada E, Garcia-Rizo C, Amoretti S, Fortea A, Oriolo G, Palau P, Boix-Quintana E, Safont G, Bernardo M (2020) Clozapine and paliperidone palmitate antipsychotic combination in treatment-resistant schizophrenia and other psychotic disorders: a retrospective 6-month mirror-image study. Eur Psychiatry 63:e71
Bozzatello P, Bellino S, Rocca P (2019) Predictive factors of treatment resistance in first episode of psychosis: a systematic review. Front Psychiatry 10:67
Capuzzi E, Bartoli F, Crocamo C, Clerici M, Carra G (2017) Acute variations of cytokine levels after antipsychotic treatment in drug-naive subjects with a first-episode psychosis: a meta-analysis. Neurosci Biobehav Rev 77:122–128
Fernandes BS, Steiner J, Bernstein HG, Dodd S, Pasco JA, Dean OM, Nardin P, Goncalves CA, Berk M (2016) C-reactive protein is increased in schizophrenia but is not altered by antipsychotics: meta-analysis and implications. Mol Psychiatry 21:554–564
Gillespie AL, Samanaite R, Mill J, Egerton A, MacCabe JH (2017) Is treatment-resistant schizophrenia categorically distinct from treatment-responsive schizophrenia? A systematic review. BMC Psychiatry 17:12
Giridharan VV, Scaini G, Colpo GD, Doifode T, Pinjari OF, Teixeira AL, Petronilho F, Macedo D, Quevedo J, Barichello T (2020) Clozapine prevents poly (i:C) induced inflammation by modulating nlrp3 pathway in microglial cells. Cells 9:577
Goldsmith DR, Rapaport MH, Miller BJ (2016) A meta-analysis of blood cytokine network alterations in psychiatric patients: comparisons between schizophrenia, bipolar disorder and depression. Mol Psychiatry 21:1696–1709
Goldsmith DR, Haroon E, Miller AH, Strauss GP, Buckley PF, Miller BJ (2018) Tnf-alpha and il-6 are associated with the deficit syndrome and negative symptoms in patients with chronic schizophrenia. Schizophr Res 199:281–284
Goldstein ME, Anderson VM, Pillai A, Kydd RR, Russell BR (2015) Glutamatergic neurometabolites in clozapine-responsive and -resistant schizophrenia. Int J Neuropsychopharmacol 18:pyu117
Gonzalez-Blanco L, Garcia-Portilla MP, Dal Santo F, Garcia-Alvarez L, de la Fuente-Tomas L, Menendez-Miranda I, Bobes-Bascaran T, Saiz PA, Bobes J (2019) Predicting real-world functioning in outpatients with schizophrenia: role of inflammation and psychopathology. Psychiatry Res 280:112509
Harwood HJ Jr. (2012) The adipocyte as an endocrine organ in the regulation of metabolic homeostasis. Neuropharmacology 63:57–75
Himmerich H, Schonherr J, Fulda S, Sheldrick AJ, Bauer K, Sack U (2011) Impact of antipsychotics on cytokine production in-vitro. J Psychiatr Res 45:1358–1365
Honer WG, Thornton AE, Chen EY, Chan RC, Wong JO, Bergmann A, Falkai P, Pomarol-Clotet E, McKenna PJ, Stip E, Williams R, MacEwan GW, Wasan K, Procyshyn R, Clozapine G (2006) Clozapine alone versus clozapine and risperidone with refractory schizophrenia. N Engl J Med 354:472–482
Howes OD, McCutcheon R, Agid O, de Bartolomeis A, van Beveren NJ, Birnbaum ML, Bloomfield MA, Bressan RA, Buchanan RW, Carpenter WT, Castle DJ, Citrome L, Daskalakis ZJ, Davidson M, Drake RJ, Dursun S, Ebdrup BH, Elkis H, Falkai P, Fleischacker WW, Gadelha A, Gaughran F, Glenthøj BY, Graff-Guerrero A, Hallak JE, Honer WG, Kennedy J, Kinon BJ, Lawrie SM, Lee J, Leweke FM, MacCabe JH, McNabb CB, Meltzer H, Möller HJ, Nakajima S, Pantelis C, Reis Marques T, Remington G, Rossell SL, Russell BR, Siu CO, Suzuki T, Sommer IE, Taylor D, Thomas N, Üçok A, Umbricht D, Walters JT, Kane J, Correll CU (2017) Treatment-resistant schizophrenia: treatment response and resistance in psychosis (trrip) working group consensus guidelines on diagnosis and terminology. Am J Psychiatry 174:216–229
Huang Y, Wang Y, Wang H, Liu Z, Yu X, Yan J, Yu Y, Kou C, Xu X, Lu J, Wang Z, He S, Xu Y, He Y, Li T, Guo W, Tian H, Xu G, Xu X, Ma Y, Wang L, Wang L, Yan Y, Wang B, Xiao S, Zhou L, Li L, Tan L, Zhang T, Ma C, Li Q, Ding H, Geng H, Jia F, Shi J, Wang S, Zhang N, Du X, Du X, Wu Y (2019) Prevalence of mental disorders in China: a cross-sectional epidemiological study. Lancet Psychiatry 6:211–224
Kane JM (1996) Treatment-resistant schizophrenic patients. J Clin Psychiatry 57:35–40
Kane JM, Agid O, Baldwin ML, Howes O, Lindenmayer JP, Marder S, Olfson M, Potkin SG, Correll CU (2019) Clinical guidance on the identification and management of treatment-resistant schizophrenia. J Clin Psychiatry 80:2783
Khandaker GM, Cousins L, Deakin J, Lennox BR, Yolken R, Jones PB (2015) Inflammation and immunity in schizophrenia: implications for pathophysiology and treatment. Lancet Psychiatry 2:258–270
Kluge M, Schuld A, Schacht A, Himmerich H, Dalal MA, Wehmeier PM, Hinze-Selch D, Kraus T, Dittmann RW, Pollmacher T (2009) Effects of clozapine and olanzapine on cytokine systems are closely linked to weight gain and drug-induced fever. Psychoneuroendocrinology 34:118–128
Li S, Gao Y, Lv H, Zhang M, Wang L, Jiang R, Xu C, Wang X, Gao M, He Y, Li J, Li WD (2018) T(4) and waist:hip ratio as biomarkers of antipsychotic-induced weight gain in Han Chinese inpatients with schizophrenia. Psychoneuroendocrinology 88:54–60
Loffler S, Loffler-Ensgraber M, Fehsel K, Klimke A (2010) Clozapine therapy raises serum concentrations of high sensitive c-reactive protein in schizophrenic patients. Int Clin Psychopharmacol 25:101–106
Lu LX, Guo SQ, Chen W, Li Q, Cheng J, Guo JH (2004) [effect of clozapine and risperidone on serum cytokine levels in patients with first-episode paranoid schizophrenia]. Di Yi Jun Yi Da Xue Xue Bao 24:1251–1254
Maggio M, Basaria S, Ceda GP, Ble A, Ling SM, Bandinelli S, Valenti G, Ferrucci L (2005) The relationship between testosterone and molecular markers of inflammation in older men. J Endocrinol Invest 28:116–119
Marcinowicz P, Wiedlocha M, Zborowska N, Debowska W, Podwalski P, Misiak B, Tyburski E, Szulc A (2021) A meta-analysis of the influence of antipsychotics on cytokines levels in first episode psychosis. J Clin Med 10:2488
Miller BJ, Goldsmith DR (2017) Towards an immunophenotype of schizophrenia: progress, potential mechanisms, and future directions. Neuropsychopharmacology 42:299–317
Miller BJ, Buckley P, Seabolt W, Mellor A, Kirkpatrick B (2011) Meta-analysis of cytokine alterations in schizophrenia: clinical status and antipsychotic effects. Biol Psychiatry 70:663–671
Muscatello MR, Bruno A, De Fazio P, Segura-Garcia C, Pandolfo G, Zoccali R (2014) Augmentation strategies in partial responder and/or treatment-resistant schizophrenia patients treated with clozapine. Expert Opin Pharmacother 15:2329–2345
Nucifora FC Jr., Mihaljevic M, Lee BJ, Sawa A (2017) Clozapine as a model for antipsychotic development. Neurotherapeutics 14:750–761
Nucifora FC Jr., Woznica E, Lee BJ, Cascella N, Sawa A (2019) Treatment resistant schizophrenia: clinical, biological, and therapeutic perspectives. Neurobiol Dis 131:104257
Nucifora FC Jr., Baker KK, Stricklin A, Cuerdo A, Parke KR, DuBois S, Nucifora LG, Margolis RL, Sawa A, Harvey PD (2021) Better functional capacity and cognitive performance in clozapine responders compared to non-responders: a cross-sectional study. Schizophr Res 229:134–136
Olmos-Ortiz A, Garcia-Quiroz J, Halhali A, Avila E, Zaga-Clavellina V, Chavira-Ramirez R, Garcia-Becerra R, Caldino-Soto F, Larrea F, Diaz L (2019) Negative correlation between testosterone and tnf-alpha in umbilical cord serum favors a weakened immune milieu in the human male fetoplacental unit. J Steroid Biochem Mol Biol 186:154–160
Potvin S, Stip E, Sepehry AA, Gendron A, Bah R, Kouassi E (2008) Inflammatory cytokine alterations in schizophrenia: a systematic quantitative review. Biol Psychiatry 63:801–808
Randolph C, Tierney MC, Mohr E, Chase TN (1998) The repeatable battery for the assessment of neuropsychological status (rbans): preliminary clinical validity. J Clin Exp Neuropsychol 20:310–319
Remington G, Lee J, Agid O, Takeuchi H, Foussias G, Hahn M, Fervaha G, Burton L, Powell V (2016) Clozapine’s critical role in treatment resistant schizophrenia: ensuring both safety and use. Expert Opin Drug Saf 15:1193–1203
Rodrigues-Silva C, Semedo AT, Neri H, Vianello RP, Galaviz-Hernandez C, Sosa-Macias M, de Brito RB, Ghedini PC (2020) The cyp2c19*2 and cyp2c19*17 polymorphisms influence responses to clozapine for the treatment of schizophrenia. Neuropsychiatr Dis Treat 16:427–432
Rodriguez-Jimenez R, Bagney A, Mezquita L, Martinez-Gras I, Sanchez-Morla EM, Mesa N, Ibanez MI, Diez-Martin J, Jimenez-Arriero MA, Lobo A, Santos JL, Palomo T, Parg (2013) Cognition and the five-factor model of the positive and negative syndrome scale in schizophrenia. Schizophr Res 143:77–83
Roerig JL (2019) Clozapine augmentation strategies. Ment Health Clin 9:336–348
Roge R, Moller BK, Andersen CR, Correll CU, Nielsen J (2012) Immunomodulatory effects of clozapine and their clinical implications: what have we learned so far? Schizophr Res 140:204–213
Schmitt A, Bertsch T, Tost H, Bergmann A, Henning U, Klimke A, Falkai P (2005) Increased serum interleukin-1beta and interleukin-6 in elderly, chronic schizophrenic patients on stable antipsychotic medication. Neuropsychiatr Dis Treat 1:171–177
Shlomi Polachek I, Manor A, Baumfeld Y, Bagadia A, Polachek A, Strous RD, Dolev Z (2017) Sex differences in psychiatric hospitalizations of individuals with psychotic disorders. J Nerv Ment Dis 205:313–317
Siskind D, Siskind V, Kisely S (2017) Clozapine response rates among people with treatment-resistant schizophrenia: data from a systematic review and meta-analysis. Can J Psychiatry 62:772–777
Sommer IE, Tiihonen J, van Mourik A, Tanskanen A, Taipale H (2020) The clinical course of schizophrenia in women and men-a nation-wide cohort study. NPJ Schizophr 6:12
Sugino H, Futamura T, Mitsumoto Y, Maeda K, Marunaka Y (2009) Atypical antipsychotics suppress production of proinflammatory cytokines and up-regulate interleukin-10 in lipopolysaccharide-treated mice. Prog Neuropsychopharmacol Biol Psychiatry 33:303–307
Wallwork RS, Fortgang R, Hashimoto R, Weinberger DR, Dickinson D (2012) Searching for a consensus five-factor model of the positive and negative syndrome scale for schizophrenia. Schizophr Res 137:246–250
Wimberley T, Støvring H, Sørensen HJ, Horsdal HT, MacCabe JH, Gasse C (2016) Predictors of treatment resistance in patients with schizophrenia: a population-based cohort study. Lancet Psychiatry 3:358–366
Yeh TC, Chu HT, Tsai CK, Chang HA, Yang FC, Huang SY, Liang CS (2019) Distinct inflammation biomarkers in healthy individuals and patients with schizophrenia: a reliability testing of multiplex cytokine immunoassay by bland-altman analysis. Psychiatry Investig 16:607–614
Yuan X, Wang S, Shi Y, Yang Y, Zhang Y, Xia L, Zhang K, Liu H (2022) Pro-inflammatory cytokine levels are elevated in female patients with schizophrenia treated with clozapine. Psychopharmacology 239:765–771
Zhu M, Liu Z, Guo Y, Sultana MS, Wu K, Lang X, Lv Q, Huang X, Yi Z, Li Z (2021) Sex difference in the interrelationship between tnf-alpha and oxidative stress status in first-episode drug-naive schizophrenia. J Neuroinflammation 18:202
Acknowledgements
We thank all the participants in the study.
Funding
This work was supported by National Natural Science Foundation of China (82371512), Guangzhou Municiple Health Commission (2023 C-TS26), Tianjin Key Medical Discipline (Specialty) Construction Project (TJYXZDXK-033 A), Opening Foundation of Jiangsu Key Laboratory of Neurodegeneration, Nanjing Medical University (KF202202), Open Project Program of State Key Laboratory of Virtual Reality Technology and Systems, Beihang University (VRLAB2022 B02), Clinical Research Project of Shanghai Municipal Health Commission (20204Y0173), Shanghai Key Laboratory of Psychotic Disorders Open Grant (21-K03), Guangzhou Key R&D Program Agriculture and Social Development Science and Technology Project (202206010034), Tertiary Education Scientific research project of Guangzhou Municipal Education Bureau (202234641), Guangzhou Municipal Key Discipline in Medicine (2021–2023), Tianjin Municipal Education Commission Scientific Research Program Project (2022KJ265), Foundation of Tianjin Health Commission for Young Scholars (TJWJ2021QN064), Guangzhou High-level Clinical Key Specialty, and Guangzhou Research-oriented Hospital. All funding had no role in study design, data analysis, paper submission and publication.
Author information
Authors and Affiliations
Contributions
Shen Li and Zezhi Li was responsible for study concept and design, revising and editing the manuscript. Yanzhe Li and Minghuan Zhu collected data and prepared the initial draft of the manuscript. Yeqing Dong and Xinxu Wang processed the blood sample and performed the experiments. Nannan Liu collected and interpreted data. All authors have contributed to and have approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare they have no conflict of interest.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, Y., Zhu, M., Dong, Y. et al. Immunoinflammatory features and cognitive function in treatment-resistant schizophrenia: unraveling distinct patterns in clozapine-resistant patients. Eur Arch Psychiatry Clin Neurosci (2024). https://doi.org/10.1007/s00406-024-01885-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00406-024-01885-x