Abstract
Neuroleptic malignant syndrome (NMS) is a rare, but severe adverse drug reaction of drugs with anti-dopaminergic properties. The main symptoms are fever and rigor. In addition, other symptoms such as creatine kinase elevation, alteration of consciousness and various neurological symptoms may occur. A total of 52 NMS cases have been documented in the drug safety program ‘Arzneimittelsicherheit in der Psychiatrie’ from 1993 to 2015. We calculated incidences and analyzed imputed substances and additional risk factors to study the impact of changing therapy regimes. The overall incidence was 0.16‰. High-potency first-generation antipsychotics (FGAs) had the highest incidences, e.g. flupentixol with 0.61‰. Second-generation antipsychotics (SGAs) had lower incidences. Low-potency FGAs had very low incidences, comparable to SGAs, but in contrast to SGAs, had not been imputed alone in any case of NMS. Preexisting organic pathologies of the central nervous system, lithium treatment, infection/exsiccosis and the withdrawal of medication with anticholinergic properties or alcohol were found to be additional risk factors. With the increasing use of SGAs, one should always be aware of the risk of NMS. Better suited diagnostic criteria for ‘atypical NMS’ would lead to a better understanding and, therefore, to improved treatment possibilities.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Neuroleptic malignant syndrome (NMS) is a severe idiosyncratic adverse reaction of anti-dopaminergic pharmacological interventions including dopamine receptor antagonism or withdrawal of dopaminergic medication. Thus, this adverse drug reaction (ADR) is reported to the database of the ongoing pharmacovigilance program ‘Arzneimittelsicherheit in der Psychiatrie’ (AMSP) by participating psychiatric centers.
NMS is characterized by a heterogeneous set of symptoms consisting not exclusively of muscle rigidity, hyperpyrexia without signs of infection, vegetative instability, metabolic changes, altered mental status, and elevated creatine kinase (CK) in laboratory tests. It may have serious sequelae or even be fatal in 5–15% of cases [1, 19], therefore its prompt recognition is mandatory. Although the incidence of NMS is low (< 0.4‰) and seen to be declining [24, 25, 33], there is a risk that it can easily be overlooked [26]. This is reinforced by a broader spectrum of indications especially for atypical antipsychotics [9, 27].
An NMS can occur at any time in the course of an antipsychotic treatment. Typically, the symptoms occur within the first 2 weeks after initiation or modification of the medication. In 79% of the patients, symptoms mostly develop within 3 days, but longer courses of up to 30 days have been described [1]. Altered consciousness and extrapyramidal motor symptoms (EPMS) like rigor and tremor are often at the beginning, and then fever, CK increase and disorders of the autonomic regulation follow the course [4].
Well-known risk factors for NMS are gender (males more than females) and age of the patient (less than 40 years) [14], as well as postpartum exposure to antipsychotics, organic central nervous system (CNS) pathology, extreme agitation, previous episodes of NMS and a recent episode of catatonia [1, 4, 7, 24, 25]. The type of antipsychotic, typical or atypical, and its potency are considered to be important, as well as the time course of therapy, rapid titration regimens, and the use of depot formulations [26]. Additionally, a hereditary component of the syndrome is discussed [15].
To diagnose NMS according to current international disease classification systems, there must have been an exposure to a medicinal product affecting the dopaminergic system in the described manner in an interval less than 72 h before onset of symptoms as well as exclusion of other causes.
After diagnosis of NMS in addition to the discontinuation of the culprit medication, which may lead to self-limiting of symptoms, other therapeutic interventions in a critical care unit are often immediately required. Therefore, early detection and treatment before the full clinical picture has evolved is crucial [7].
However, the current classification systems differ in some criteria for this possibly life-threatening diagnosis. In addition to the AMSP criteria for NMS, we used The International Classification of Diseases and Related Health Problems 10th Revision (ICD-10) and the Diagnostic and Statistical Manual of Mental Disorders (DSM)-IV /-IV TR and DSM-5 [2, 3, 39]. The respective diagnostic criteria for NMS are shown in Table 1.
It is to note that in the light of rising prescription rates for second-generation antipsychotics (SGAs) many clinicians are questioning the impact of atypical antipsychotics in comparison to typical remedies on NMS. We will show the contribution of the AMSP database to this question in terms of absolute numbers as well as relative risk rates. Additionally, the AMSP database was searched for risk factors.
Methods
AMSP
The ongoing AMSP drug surveillance program continually assesses severe ADRs of psychiatric inpatients in routine clinical treatment. Detailed information about the AMSP methodology has been described elsewhere [12, 13]. All relevant information—containing details of adverse events, patient demographics, psychiatric and somatic drug intake, alternative explanations, relevant risk factors, measures taken, previous exposure to the drug—are gathered from drug monitors (trained psychiatrists) in psychiatric hospitals (find further detailed information online at http://www.amsp.de) using standardized questionnaires to document cases. After re-examination by senior physicians at each participating hospital, the cases are discussed at regional and central case conferences—attended by the drug monitors of each participating institute, representatives of the national drug regulating authorities of the German Federal Institute for Drugs and Medicine Products (Bundesinstitut für Arzneimittel und Medizinprodukte, BfArM), the Drug Commission of the German Medical Association (Arzneimittelkommission der Ärzteschaft, AKdÄ) and drug safety experts from the pharmaceutical industry—held on a regular basis two times per year. Following discussions and analyses, ADR probability ratings are assigned. The probability of severe ADRs can be rated as follows: possible (the ADR is unknown or the probability of an alternative explanation is more than 50%), probable (the ADR, time course and dosage for a specific drug are known in accordance with previous experience and the likelihood of an alternative explanation is less than 50%) and definite (like probable with an additional re-appearance of the ADR after re-exposure to the drug in question). The completed cases are sent to the relevant authorities, pharmaceutical companies and are saved in the AMSP central database.
In addition to severe ADRs, the AMSP program also assesses drug use in all inpatients under surveillance. Thus, relative frequencies can be calculated for single drugs and drug groups [13, 33].
The program was approved by the leading boards of each participating institute prior to implementation. The Ethics Committee of the Ludwig-Maximilians-University Munich approved evaluations based on the AMSP database [1, 12, 20].
The AMSP database provides observational cross-sectional data of psychiatric inpatients treated with antipsychotics for any reason for the period from 01.10.1993 to 31.12.2015. We determined the overall frequency of NMS in the study cohort and listed the imputed drug or drugs administered or withdrawn < 48 h before the onset of symptoms [26]. Only cases rated as probable or definite were included.
Polypharmacy
Often combinations of drugs with known potential to cause NMS are prescribed in psychiatric inpatients. In the AMSP database, each drug of a drug combination is imputed, if additive effects are assumed. This was taken into account in the data analysis. In cases in which multiple drugs were imputed, only drugs rated as having probably or definitely caused the NMS in the AMSP consensus process were included in the analysis. “Imputed alone” means there is a probable or definite causal relationship between treatment and ADR for a single drug. However, this does not exclude that there is another drug “possibly” involved [33].
We present the absolute and relative numbers of NMS cases including the cases with abortive course related to different drugs in the observation period. Different administration forms of one drug are taken together in one prescription rate (i.e., flupentixol oral and i.m.).
For our analysis, FGAs were divided into high-potency FGAs and low-potency FGAs. High-potency FGAs involved in NMS cases in our study were benperidol, bromperidol, chlorpromazine, flupentixol, fluphenazine, haloperidol and zuclopenthixol; low-potency FGAs involved in NMS cases were chlorprothixene, levomepromazine, melperone, pipamperone and prothipendyl.
To avoid bias while calculating relative risks, we excluded drugs that were related to only one NMS case. Drugs with less than 5000 prescriptions from 1993 to 2015 were excluded in the analyses of each imputed substance. Rates obtained from smaller groups of exposed persons are not reliable for a very rare event such as NMS. Nevertheless, these drugs were included in the analyses of substance groups.
Assignment to diagnostic groups
In addition to the AMSP consensus process, NMS diagnoses were classified according to NMS criteria of ICD-10, DSM-IV /-IV TR, DSM-5 [2, 3, 39]:
We evaluated the case records provided by the AMSP database and arranged the most relevant clinical symptoms of the ICD- and DSM-classification systems in seven symptom groups and subsumed each case according to presence or absence of the symptom in the case history. Symptom groups were as follows:
-
1.
Fever, cutoff temperature with a minimum of 38 °C (100,4 °F) only strictly defined in DSM 5
-
2.
Rigor
-
3.
Tremor or other neurological symptoms, e.g., extrapyramidal motor symptoms (EPMS)
-
4.
Creatine kinase (CK) elevation > 4 times the normal value
-
5.
Alteration of consciousness/delirium-like mental status
-
6.
Vegetative instability (diaphoresis, hyper- or hypotensive deviation from normal blood pressure, tachy-/bradycardia, tachypnea, metabolic acidosis, incontinence, pallor)
-
7.
Other laboratory abnormalities (leukocytosis, C-reactive protein (CRP) elevation)
ICD-10, DSM-IV and IV-TR diagnostic criteria were considered to be fulfilled when the case included the symptoms’ fever and rigor as well as two or more of the other symptoms. In DSM-5, fever (> 38 °C) and rigor are counted as cardinal symptoms of NMS, while additional symptoms can occur.
To diagnose NMS within the AMSP consensus process, the presence of three or more of the following symptoms, fever, rigor, elevated CK and alteration of consciousness is obligatory. To note, in the AMSP consensus process cases fulfilling only two of the AMSP diagnostic criteria for complete NMS were assessed and rated as “NMS with an abortive course”.
Results
From 1st of October 1993 to 31st of December 2015, 443,966 psychiatric inpatients (195,537 male (44%) and 248,429 female (56%)) were monitored in the AMSP program. For any reason, 320,383 were treated with antipsychotics and 52 cases of NMS were reported out of which 23 cases (44.2%) were rated as NMS with abortive course. In 19 cases (36.5%), diagnostic criteria were not fulfilled according to ICD-10, DSM-IV/DSM-IV TR; however, five of these cases were diagnosed as NMS according to the AMSP consensus process. NMS symptoms documented in our cases are shown in Table 2, rigor was the most frequent symptom.
The incidence of NMS was 0.16‰ for all cases and 0.10‰ for the complete NMS cases. NMS patients were (arithmetic mean ± standard deviation) 51.02 ± 14.26 years old, thereof females were 36.5%.
Psychiatric diagnoses, gender and age
32 of 52 cases with NMS had a diagnosis of schizophrenia spectrum disorder (0.21‰ incidence inpatient admissions; Table 3). This is significantly more frequent than in patients with mood disorder (0.09‰; p ≤ 0.001) and other diagnoses (0.15‰). In the AMSP dataset, there was no significant difference in the incidence of NMS in patients under 60 years than in patients from 31 to 60 years or younger than 31 years (p ≤ 0.22). Male patients showed a higher incidence (0.23‰) than female patients (0.11‰; p ≤ 0.008).
Polypharmacy
In 29 out of 52 cases (55.8%), more than one drug was imputed to cause NMS (Table 4), and in 26 cases (50.0%) at least two antipsychotics were imputed. In 75.0% (n = 39) of cases, patients were prescribed more than one psychotropic medication not considering benzodiazepines.
Drug-related incidence of NMS
High-potency FGAs were imputed in 73.1% of all cases (n = 38), low-potency FGAs and SGAs in 19.2% (n = 10) and 61.5% (n = 32) of all cases. In cases with abortive course, high-potency FGAs were imputed in 65.2% of cases (n = 15), low-potency FGAs and SGAs in 26.1% (n = 6) and 69.6% (n = 16) of cases (Table 4). In comparison, in the AMSP dataset 28.6% of patients treated with antipsychotics received high-potency FGAs, 31.0% received low-potency FGAs and 67.1% received SGAs. The incidences of high-potency FGAs increased over time, while the incidences of low-potency FGAs and SGAs remained stable (see Fig. 1).
Additionally, benzodiazepines were prescribed in 57.7% (n = 30) of cases and in 32.1% (n = 142,301) of the monitored population on antipsychotics.
Regarding all cases, the highest incidences of NMS related to high-potency FGAs were: flupentixol 0.61‰, zuclopentixol 0.54‰ and haloperidol 0.48‰. Benperidol, bromperidol and fluphenazine were excluded due to low prescription rates. The highest incidences of NMS related to low-potency FGAs were: melperone 0.16‰, chlorprothixene 0.15‰ and pipamperone 0.09‰.
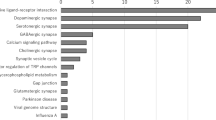
Considering SGAs, amisulpride (6 cases, 0.45‰) ranked first; however, it is to note that in 5 out of 6 cases, in which amisulpride was imputed, hyperpyrexia > 38 °C was not present. Thus, diagnostic criteria of ICD-10, DSM IV/IV TR and DSM 5 were not fulfilled. Aripiprazole (0.35‰) and risperidone (0.2‰) followed. Quetiapine had the lowest incidence (0.03‰). However, confidence intervals are wide and overlap (Fig. 2).
Incidence (95% CI) of reported NMS cases (n = 52) per drug class/single imputed drugs. Drugs with less than 5000 prescriptions were not included in the figure. The group of high-potency FGA imputed to cause a NMS includes the following drugs: benperidol, bromperidol, chlorpromazine, clupentixol, fluphenazine, haloperidol and zuclopenthixol. The group of low-potency FGA imputed to cause a NMS includes the following drugs: chlorprothixene, levomepromazine, melperone, pipamperone and prothipendyl
Regarding the cases with abortive course, the highest incidences were: flupentixol 0.41‰ (high-potency FGAs), melperone 0.16‰ (low-potency FGAs) and risperidone 0.16‰ (SGAs).
Lithium was co-imputed whenever it was present at the onset of NMS due to the data on increased neurotoxicity of the combination of antipsychotics and lithium. Thus, we found an incidence for lithium of 0.13‰. In three of the four lithium-related cases, there was a combination therapy of lithium and an SGA (i.e., amisulpride, aripiprazole (+ valproate), olanzapine). In the remaining case, lithium was combined with haloperidol, levomepromazine and risperidone. Three of these cases showed chronological coincidence of lithium dose escalation and the clinical onset of NMS, although NMS did not occur at the onset of lithium therapy.
In one case, citalopram was rated as a probable influence in a case of NMS in addition to amisulpride due to the given facts. Two other selective serotonin reuptake inhibitors (SSRIs), paroxetine and fluoxetine, were imputed as possible factors in one case each. In the paroxetine and fluoxetine cases, a pharmacokinetic interaction (CYP2D6 inhibition [13]) with potentially further increased haloperidol serum concentration was discussed but not controlled for.
Cases imputed to a single drug
In 23 cases, one drug was imputed alone (44.2%; shown in brackets in Table 4). Overall, high-potency FGAs had a higher proportion (60.9%, n = 14) than SGAs (39.1%, n = 9). No low-potency FGAs were imputed alone. Haloperidol ranked first with 34.8%, followed by risperidone with 17.4%. The SGAs amisulpride and olanzapine each were imputed alone in two cases (8.7%) for complete NMS. In the cases with abortive course, high-potency FGAs were imputed alone in 4 cases (17.4%), and SGAs in 5 cases (21.7%). Risperidone (17.4%; n = 4) and flupentixol (8.7%; n = 2) were imputed alone most often in cases with abortive course.
Risk factors
The following possible risk factors were mentioned in the documentation: preexisting organic CNS pathology (n = 7, 13.5%), infection/exsiccosis (n = 5), and alcohol withdrawal syndrome (= 2). We found in 16 cases (33.8%) a FGA and in 11 cases (21.2%) a SGA was newly prescribed or tapered up. A combination of FGA and SGA was newly prescribed or tapered up in 11 cases (21.2%). A change from one type of antipsychotic to another was found in 12 cases: from FGA to FGA (1 case), from FGA to SGA (5 cases), from SGA to FGA (2 cases), from SGA to SGA (2 cases). In two cases, the change of medication was more complex. Furthermore, in one case, the medication (one FGA and one SGA) was tapered down. There was no change of the dosage in one case. Overall, daily antipsychotics dosages of drugs that were imputed alone were in the middle range of the respective recommended daily dosages. Interestingly, the dosages of risperidone were relatively low (median: 1.5 mg). We did not calculate combination dosages. In three cases (5.8%), withdrawal of a strongly anticholinergic drug (clozapine twice, levomepromazine once) shortly prior to onset of NMS was assessed as a risk factor.
Treatment of NMS and outcome
The imputed antipsychotics were discontinued in all cases. Drug treatment for NMS (in 62% of all cases) most often included anti-parkinsonian drugs (n = 15 cases, 28.8%), benzodiazepines (n = 12 cases, 23.1%) and antibiotics due to complications (n = 10 cases, 19.2%). In one case, electroconvulsive therapy was necessary. 44.2% of all cases were admitted to an intensive care unit (n = 23). One case that occurred in the mid 1990s was fatal (1.9%): in this case, multiple high-potency FGAs had been used (fluphenazine depot 7 days before onset of NMS, flupentixol orally until 2 days before, benperidol i.v. 1 day before and on the day of NMS onset).
Missing data
All 52 cases were tabulated with 7 diagnostic criteria, thus yielding 364 items. However, for 27 items (7.41%) data was not specified. Vegetative instability was ranking first in 15 of 52 cases (28.9%, equal to 55.6% of all missing items; Table 3).
Discussion
Incidence
We analyzed 52 NMS cases documented in the AMSP database in the time range from 1993 to 2015. Incidences differ between 0.2 and 14‰ in the literature depending on the year of publication and diagnostic criteria used [16,17,18, 25, 29, 32, 36]. Thus, the overall incidence of 0.16‰ we found in our data is among the lowest. A recent study of comparable size [25] found an incidence of 0.4‰ using retrospective data of a 11-year observation period, and a preceding AMSP analysis reported 0.17‰ [33]. Older studies found larger incidences near 10‰ [16,17,18, 36]. This issue currently is seen as a result of decreasing prescription rates of high-potency FGAs [4, 36]. Correspondingly, in the present dataset we found lower NMS incidences for SGAs and low-potency FGAs [4, 8, 24, 25, 33, 35] than for high-potency FGAs in accordance with the literature. In this respect, incidences were not influenced by the diagnostic criteria used.
There is a growing tendency of prescribing SGAs rather than high-potency FGAs. However, the overall number of NMS cases are thought not to decrease due to the growing number of indications for SGAs (e.g., as mood stabilizer, augmentation strategies in depressive disorders) [9, 27], thus leading to increasing absolute prescription rates for these drugs. Interestingly, incidence rates of high-potency FGAs increased over time even though prescription rates are decreasing. This might be due to higher awareness and earlier recognition of beginning or less severe NMS.
In contrast to SGAs, no low-potency FGA was imputed alone.
Evaluation of diagnostic criteria for NMS
There is clinical need for the individual physician to decide, which NMS diagnostic criteria are useful for daily clinical practice. 19 out of 52 cases rated in the AMSP consensus process as complete or abortive NMS did not fulfill the ICD-10 and DSM-IV group diagnostic criteria. Similar to the procedure of AMSP, and due to the severity of untreated NMS, diagnostic criteria have been softened in the DSM-5 manual.
Moreover, with the shift from high-potency FGAs to SGAs, the early clinical picture of NMS might have changed, creating a clinical symptomatology which could be called ‘atypical NMS’ under SGAs [27]. This ‘atypical NMS’ is presenting clinically ‘softer’ and, thus is not fulfilling current diagnostic criteria of ICD-10 and DSM-IV/ -IV TR. The same showed true for the AMSP criteria since a total of 23 cases were diagnosed as abortive course. Therefore, the new DSM-5 criteria seem much more suitable to diagnose ‘atypical’ NMS and NMS in an early stage [4, 27, 37].
About half of the cases were NMS with abortive course. This also shows that the clinical picture of NMS can be less pronounced than the classical full symptomatology. On the other hand, it shows the increased attention of clinicians in regard to developing symptoms of NMS, which is crucial for a faster and more effective treatment.
With the information and the literature given above, we suggest the symptomatology of NMS to be regarded as a continuum from clinically less to more severe signs and symptoms analogous to Woodbury et al. for treatment of motoric symptoms, eventually resulting in the need for precise and individual diagnosis and therapy [38]. DSM-5, giving only two main criteria and not necessarily requiring two additional symptoms, is in better accordance with NMS having a continuous clinical picture.
Additional risk factors
Psychiatric disorders, gender and age
Bipolar disorder is a risk factor for developing NMS. At present, it is increasingly treated with SGAs, supporting our point of view concerning the influence of SGAs on the incidence of NMS by widening of indications [9, 34].
We found that about 60% of the NMS cases presented in our study are showing a higher risk for developing NMS in patients diagnosed with schizophrenia and in male patients, which fairly matches with data in the literature [4, 24]. Although the age, being under 40 years old, is a known risk factor [11] in the AMSP dataset there was no significant difference between the age groups.
Polypharmacy
Polypharmacy is a known risk factor for NMS due to greater pharmacodynamic and pharmacokinetic interaction risks in comparison to monotherapy [24]. This is confirmed in our analysis, 55.6% of all cases were caused by drug combinations, most of which included antipsychotics.
Lithium
Previous studies concerning the influence of lithium in the occurrence of NMS are conflicting [10, 24, 33, 34]. We report a coincidence of lithium dose escalation and occurrence of NMS in three out of four cases, thus indicating clinical relevance.
Lithium has been imputed as probably being responsible in the AMSP consensus process, because it is known to be possibly neurotoxic. Additionally, it influences the metabolism of serotonine in an agonistic manner and dopamine in an antagonistic manner [10, 33, 34]. To note, hypothyroidism is described as a risk factor for NMS [23] and occurs in some lithium-treated patients [5].
Benzodiazepines
Benzodiazepines are mentioned as a risk factor for NMS, but are possibly not a cause of NMS rather than being confounding by diagnosis [25]. In many cases, the onset of an antipsychotic therapy in psychiatric inpatients for treatment of psychomotor agitation is accompanied by the prescription of benzodiazepines. However, psychomotor agitation also is a risk factor for NMS, requiring the prescription of benzodiazepines and higher doses of antipsychotics [9, 24, 33].
Preexisting organic pathology of the central nervous system
In accordance with a preceding AMSP analysis on involuntary movement disorders [33], we found a high proportion of preexisting organic pathology of the central nervous system. However, we do not have corresponding data for the exposed patients on antipsychotics.
Withdrawal of anticholinergic medication
The withdrawal of anticholinergic medication as a risk factor for NMS has been described elsewhere [31].
SGAs
Amisulpride showed markedly less cases fulfilling ICD-10 and DSM-IV criteria compared with all registered cases (1 out of 6 cases). This ‘softer’ clinical presentation has been discussed in the literature as a less frequent appearance of high fever, other EPMS and autonomic symptoms in cases diagnosed as NMS caused by amisulpride [4, 21]. Also, high levels of CK increase, rigor and mental status alterations have been described [4]. We can confirm previous findings as in our results the main reason for not fulfilling diagnostic criteria was also missing temperature elevation > 38 °C in 5 of 6 cases. Two of the cases were counted as fulfilling all diagnostic criteria in the AMSP consensus process and three cases were counted as NMS with abortive course [4, 22, 28].
NMS cases during treatment with aripiprazole are described as clinically presenting at least in part ‘atypical’. There is a lower incidence of high fever and diaphoresis and a less severe and shorter lasting clinical duration [4, 37]. We found no case due to aripiprazole alone. For olanzapine, no ‘atypical’ clinical presentation is described [4]. In our data, two complete AMSP NMS cases were documented as due to olanzapine alone.
Among SGAs, risperidone was most frequently imputed alone. This supports the view that risperidone has a somewhat ‘typical’ pharmacodynamic profile [6]. Quetiapine on the other hand caused remarkably less cases considering the high prescription rates (Table 4).
Limitations
Limitations to consider concerning AMSP methodology, such as underreporting, were already discussed in preceding publications [11, 13, 30, 33].
NMS as a severe condition is probably assessed more comprehensively than other types of ADRs; however, it may have been misdiagnosed due to the lack of one of the cardinal symptoms. Drug usage and dose regimens change continually and therefore the ADR patterns also vary with time [33]. Although we only included drugs that were imputed probably and definitly having caused more than one case of NMS, there might be over- or underestimation of drug effects, particularly for drugs with low prescription rates.
It is important to take into account that more than one antipsychotic can be imputed for causing for NMS. This might have led to overestimation of overall incidences for some drugs with mainly additive effects. Low-potency FGAs were co-imputed for a significant proportion, whereas their pharmacodynamic profiles render them mostly improbable to cause NMS when given as a monotherapy. Our results with no low-potency FGA being imputed alone are further supporting this interpretation.
Additionally, missing data has to be mentioned as possibly influencing our results; we appreciate these as having a small impact.
Conclusions
Our data show that NMS remains a rare adverse drug effect that clinicians should be aware of. Considering the risk of a medication for severe adverse drug reactions may help to identify a case of NMS timely. In our study, the risk for high-potency FGAs was higher than for SGAs. However, the prescription rates for SGAs are rising.
Established diagnostic systems may lead to biased diagnoses and, therefore, should be applied with caution. In the AMSP consensus process atypical forms of NMS are also considered. Thus, as well as the AMSP consensus process, as in following DSM 5 criteria, more cases can be detected than by strictly applying criteria of the ICD-10 and DSM-IV/ -IV TR diagnostic systems.
Combining our results leads to the conclusion, that it is important, besides typical cases of NMS, to reasonably consider the clinical picture of ‘atypical’ NMS as well as developing NMS to avoid possibly severe sequelae.
References
Addonizio G, Susman VL, Roth SD (1987) Neuroleptic malignant syndrome: review and analysis of 115 cases. Biol Psychiatry 22(8):1004–1020
American Psychiatric Association (2000) Diagnostic and statistical manual of mental disorders DSM-IV-TR. Jaypee Medical Ltd, London
American Psychiatric Association (2013) Diagnostic and statistical manual of mental disorders DSM 5, 5 edn. American Psychiatric Association, London
Belvederi Murri M, Guaglianone A, Bugliani M, Calcagno P, Respino M, Serafini G et al (2015) Second-generation antipsychotics and neuroleptic malignant syndrome: systematic review and case report analysis. Drugs RD 15:45–62. https://doi.org/10.1007/s40268-014-0078-0
Bschor T, Bauer M (1998) Thyroid gland function in lithium treatment. Nervenarzt 69:189–195
Buzan B (1996) Risperidone-induced tardive dyskinesia. Am J Psychiatry 153(5):734–735
Byrd C (1993) Neuroleptic malignat syndrome: a dangerous complication of neuroleptic therapy. J Neurosci Nurs 25(1):62–65
Caroff SN, Mann SC, Campbell EC, Sullivan KA (2002) Movement disorders associated with atypical antipsychotic drugs. J Clin Psychiatry 63(Suppl 4):12–19
Chen Y, Guo JJ, Steinbuch M, Buckley PF, Patel NC (2009) Risk of neuroleptic malignant syndrome in patients with bipolar disorder: a retrospective, population-based case-control study. Int J Psychiatry Med 39:439–450
Fallgatter AJ, Strik WK (1997) Reversible neuropsychiatric side effects of lithium with normal serum levels. A case report. Nervenarzt 68:586–590
Friedrich ME, Akimova E, Huf W, Konstantinidis A, Papageorgiou K, Winkler D, Toto S, Greil W, Grohmann R, Kasper S (2016) Drug-induced liver injury during antidepressant treatment: results of AMSP, a drug surveillance program. Int J Neuropsychopharmacol 19(4):1–9. https://doi.org/10.1093/ijnp/pyv126
Grohmann R, Engel RR, Möller H-J, Rüther E, van der Velden JW, Stübner S (2013) Flupentixol use and adverse reactions in comparison with other common first- and second-generation antipsychotics: data from the AMSP study. Eur Arch Psychiatry Clin Neurosci 264(2):131–141. https://doi.org/10.1007/s00406-013-0419-y
Grohmann R, Engel RR, Ruther E, Hippius H (2004) The AMSP drug safety program: methods and global results. Pharmacopsychiatry 37(Suppl 1):S4–S11. https://doi.org/10.1055/s-2004-815505
Gurrera RJ (2017) A systematic review of sex and age factors in neuroleptic malignant syndrome diagnosis frequency. Acta Psychiatr Scand May 135(5):398–408
Kato D, Kawanishi C, Kishida I, Furuno T, Suzuki K, Onishi H, Hirayasu Y (2007) Effects of CYP2D6 polymorphisms on neuroleptic malignant syndrome. Eur J Clin Pharmacol 63(11):991–996
Keck PE, Pope HG, McElroy SL (1991) Declining frequency of neuroleptic malignant syndrome in a hospital population. Am J Psychiatry 148:880–882. https://doi.org/10.1176/ajp.148.7.880
Keck PE, Pope HG, McElroy SL (1987) Frequency and presentation of neuroleptic malignant syndrome: a prospective study. Am J Psychiatry 144:1344–1346. https://doi.org/10.1176/ajp.144.10.1344
Keck PE, Sebastianelli J, Pope HG, McElroy SL (1989) Frequency and presentation of neuroleptic malignant syndrome in a state psychiatric hospital. J Clin Psychiatry 50:352–355
Lannas PA, Pachar JV (1993) A fatal case of neuroleptic malignant syndrome. Med Sci Law 33(1):86–88
Letmaier M, Grohmann R, Kren C, Toto S, Bleich S, Engel R, Gary T, Papageorgiou K, Konstantinidis A, Holl KA, Painold A, Kasper S (2018) Venous thromboembolism during treatment with antipsychotics—results of a drug surveillance program. World J Biol Psychiatry 19(3):175–186. https://doi.org/10.1080/15622975.2017.1285048
Masi G, Milone A, Viglione V, Mancini A, Pisano S (2014) Massive asymptomatic creatine kinase elevation in youth during antipsychotic drug treatment: case reports and critical review of the literature. J Child Adolesc Psychopharmacol 24:536–542. https://doi.org/10.1089/cap.2014.0047
Meisenzahl EM, Schmitt G, Gründer G, Dresel S, Frodl T, la Fougère C et al (2008) Striatal D2/D3 receptor occupancy, clinical response and side effects with amisulpride: an iodine-123-iodobenzamide SPET study. Pharmacopsychiatry 41:169–175. https://doi.org/10.1055/s-2008-1076727
Moore AP, Macfarlane IA, Blumhardt LD (1990) Neuroleptic malignant syndrome and hypothyroidism. J Neurol Neurosurg Psychiatry 53:517–518
Nagel M, Freisberg S, Junghanns K, Moll CKE, Willenborg B (2015) The neuroleptic malignant syndrome]. Fortschr Neurol Psychiatr 83:373–380. https://doi.org/10.1055/s-0035-1553246
Nielsen RE, Wallenstein Jensen SO, Nielsen J (2012) Neuroleptic malignant syndrome-an 11-year longitudinal case-control study. Can J Psychiatry 57:512–518. https://doi.org/10.1177/070674371205700810
Oruch R, Pryme IF, Engelsen BA, Lund A (2017) Neuroleptic malignant syndrome: an easily overlooked neurologic emergency. Neuropsychiatr Dis Treat 13:161–175
Picard LS, Lindsay S, Strawn JR, Kaneria RM, Patel NC, Keck PE (2008) Atypical neuroleptic malignant syndrome: diagnostic controversies and considerations. Pharmacotherapy 28:530–535. https://doi.org/10.1592/phco.28.4.530
Perrault G, Depoortere R, Morel E, Sanger DJ, Scatton B (1997) Psychopharmacological profile of amisulpride: an antipsychotic drug with presynaptic D2/D3 dopamine receptor antagonist activity and limbic selectivity. J Pharmacol Exp Ther 280:73–82
Pileggi DJ, Cook AM (2016) Neuroleptic malignant syndrome. Ann Pharmacother 50:973–981. https://doi.org/10.1177/1060028016657553
Spindelegger CJ, Papageorgiou K, Grohmann R, Engel R, Greil W, Konstantinidis A et al (2014) Cardiovascular adverse reactions during antidepressant treatment: a drug surveillance report of German-speaking countries between 1993 and 2010. Int J Neuropsychopharmacol 18(4):1. https://doi.org/10.1093/ijnp/pyu080
Spivak B, Gonen N, Mester R, Averbuch E, Adlersberg S, Weizman A (1996) Neuroleptic malignant syndrome associated with abrupt withdrawal of anticholinergic agents. Int Clin Psychopharmacol 11(3):207–209
Spivak B, Maline DI, Kozyrev VN, Mester R, Neduva SA, Ravilov RS et al (2000) Frequency of neuroleptic malignant syndrome in a large psychiatric hospital in Moscow. Eur Psychiatry 15:330–333
Stübner S, Rustenbeck E, Grohmann R, Wagner G, Engel R, Neundörfer G et al (2004) Severe and uncommon involuntary movement disorders due to psychotropic drugs. Pharmacopsychiatry 37(Suppl 1):S54–S64. https://doi.org/10.1055/s-2004-815511
Susman VL, Addonizio G (1988) Recurrence of neuroleptic malignant syndrome. J Nerv Ment Dis 176:234–241
Su Y-P, Chang C-K, Hayes RD, Harrison S, Lee W, Broadbent M et al (2014) Retrospective chart review on exposure to psychotropic medications associated with neuroleptic malignant syndrome. Acta Psychiatr Scand 130:52–60. https://doi.org/10.1111/acps.12222
Tse L, Barr AM, Scarapicchia V, Vila-Rodriguez F (2015) Neuroleptic malignant syndrome: a review from a clinically oriented perspective. Curr Neuropharmacol 13:395–406
Tseng P-T, Chang Y-C, Chang C-H, Wang H-Y, Cheng Y-S, Wu C-K et al (2015) Atypical neuroleptic malignant syndrome in patients treated with aripiprazole and clozapine: a case-series study and short review. Int J Psychiatry Med 49:35–43. https://doi.org/10.2190/PM.49.1.c
Woodbury MM, Woodbury MA (1992) Neuroleptic-induced catatonia as a stage in the progression toward neuroleptic malignant syndrome. J Am Acad Child Adolesc Psychiatry 31(6):1161–1164
World Health Organisation (WHO) (2016) The international statistical classification of diseases and related health problems, ICD-10 2016 by World Health Organization (WHO) (2015-10-26)
Acknowledgements
This study was supported by the AMSP Drug Safety Programme, Hannover, Germany. The analysis of the NMSdata and this publication were made possible by a grant from Immanuel Klinik Rüdersdorf.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
T. Greiner, M. Schneider, S. Lensky, S. Stübner, J. Regente and M. Heinze declare no conflict of interests. The AMSP Drug Safety Program is based on non-profit associations in the German-speaking countries Germany, Austria and Switzerland. In recent years, financial support has been contributed by almost all the pharmaceutical companies involved in CNS research. Since 1993, educational and research grants have been awarded by the following pharmaceutical companies to the three national non-profit associations of the AMSP. Austrian companies: AstraZeneca Österreich GmbH, Boehringer Ingelheim Austria, Bristol–Myers Squibb GmbH, CSC Pharmaceuticals GmbH, Eli Lilly GmbH, Germania Pharma GmbH, GlaxoSmithKline Pharma GmbH, Janssen-Cilag Pharma GmbH, Lundbeck GmbH, Novartis Pharma GmbH, Pfizer Med Inform, Servier Pharma Austria, Wyeth Lederle Pharma GmbH. German companies: Abbott GmbH & Co. KG, AstraZeneca GmbH, Aventis Pharma Deutschland GmbH GE-O/R/N, Bayer Vital GmbH & Co. KG, Boehringer Mannheim GmbH, Bristol-Myers-Squibb, Ciba Geigy GmbH, Desitin Arzneimittel GmbH, Duphar Pharma GmbH & Co. KG, Eisai GmbH, esparma GmbH Arzneimittel, GlaxoSmithKline Pharma GmbH & Co. KG, Hoffmann-La Roche AG Medical Affairs, Janssen-Cilag GmbH, Janssen Research Foundation, Knoll Deutschland GmbH, Lilly Deutschland GmbH Niederlassung Bad Homburg, Lundbeck GmbH & Co. KG, Nordmark Arzneimittel GmbH, Novartis Pharma GmbH, Organon GmbH, Otsuka-Pharma Frankfurt, Pfizer GmbH, Pharmacia & Upjohn GmbH, Promonta Lundbeck Arzneimittel, Rhone-Poulenc Rohrer, Sanofi-Synthelabo GmbH, Sanofi-Aventis Deutschland, Schering AG, Servier Pharma, SmithKlineBeecham Pharma GmbH, Solvay Arzneimittel GmbH, Synthelabo Arzneimittel GmbH, Dr Wilmar Schwabe GmbH & Co., Thiemann Arzneimittel GmbH, Trommsdorff GmbH & Co. KG Arzneimittel, Troponwerke GmbH & Co. KG, Upjohn GmbH, Wander Pharma GmbH, Wyeth-Pharma GmbH. Swiss companies: AHP (Schweiz) AG, AstraZeneca AG, Bristol–Myers Squibb AG, Desitin Pharma GmbH, Eli Lilly (Suisse) S.A., Essex Chemie AG, GlaxoSmithKline AG, Janssen-Cilag AG, Lundbeck (Suisse) AG, Mepha Schweiz AG/Teva, MSD Merck Sharp & Dohme AG, Organon AG, Pfizer AG, Pharmacia, Sandoz Pharmaceuticals AG, Sanofi-Aventis (Suisse) S.A., Sanofi-Synthe´labo SA, Servier SA, SmithKlineBeecham AG, Solvay Pharma AG, Vifor SA, Wyeth AHP (Suisse) AG, Wyeth Pharmaceuticals AG. S. Bleich, R. Grohmann and S. Toto are project managers of the AMSP program. S. Toto has been a member of the advisory board for Otsouka and has received speaker’s honoria from Janssen-Cilag, Lundbeck, Otsouka and Servier.
Rights and permissions
About this article
Cite this article
Schneider, M., Regente, J., Greiner, T. et al. Neuroleptic malignant syndrome: evaluation of drug safety data from the AMSP program during 1993–2015. Eur Arch Psychiatry Clin Neurosci 270, 23–33 (2020). https://doi.org/10.1007/s00406-018-0959-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00406-018-0959-2