Abstract
Introduction
While physical activity (PA) can play an important role in the treatment of mental disorders (MD), large proportions of patients with MD do not meet PA recommendations. The aim of this trial was to evaluate whether structured psychological intervention (MoVo-LISA) is effective in helping outpatients with MD to increase their level of PA. As active control group (CG) we modified MoVo-LISA to target healthy diet behavior.
Methods
N = 83 outpatients with MD (F1–F4) were randomized to the two conditions. PA (self-report and accelerometry), dietary behavior, social-cognitive determinants of health behavior change, psychiatric symptoms and health-related quality of life were assessed at baseline, 1 and 12 weeks after the intervention.
Results
Significant time*group interaction effects for objectively measured PA, dietary behavior and fruit and vegetable consumption indicated differential effects of the interventions on these outcomes. PA increased in the MoVo-LISA group (IG) from baseline to follow-up while it decreased in CG. IG showed a significant higher level of objectively measured PA at follow-up compared to CG. Dietary behavior and fruit and vegetable consumption significantly increased from baseline to follow-up in CG, but not IG. IG showed a significant increase in some, but not all social cognitive determinants of health behavior change.
Conclusions
MoVo-LISA is effective in helping outpatients with MD to increase their level of PA in short- and mid-term. The used intervention strategies are effective for the promotion of healthy diet in patients with MD as well.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
While regular physical activity (PA) engagement has a large positive impact on general health [1], physical inactivity represents a major modifiable risk factor for the development of non-communicable diseases and global mortality [2]. Besides the effect on physical diseases, there is increasing evidence for positive effects of PA on mental health and mental diseases as well [3]. Epidemiologic and prospective studies show a negative relationship between regular PA and prevalence and incidence of several mental disorders (MD) [4]. Therefore, promotion of regular PA in the general population could help to reduce the prevalence and incidence of MD [5]. Besides this, there is also evidence for the efficacy of PA in the treatment of MD. Several studies show general and disorder-specific positive effects on MD like depression, anxiety disorders and psychotic disorders (e.g. [6,7,8,9]). For a detailed overview see Zschucke et al. [10].
For a healthy lifestyle, the World Health Organization (WHO; [2]) recommends for adults to engage in at least 150 min of moderate or 75 min of vigorous PA per week that is performed in bouts of at least 10 min. Similarly, the American College of Sports Medicine (ACSM) recommends for adults to engage in at least 5 days per week of at least 30 min of moderate PA or at least 3 days of at least 20 min of vigorous PA or a combination of both achieving a minimum energy expenditure of > 500–1000 MET minutes (metabolic equivalent of task minutes, a measure that expresses the energy expenditure of PA) per week [11].
In patients with MD, levels of PA are low [12,13,14] and their level of PA is lower than in populations without MD [15,16,17]. As life expectancy is reduced in patients with MD [18] and physical inactivity is an important factor that contributes to premature mortality [19], promotion of regular PA might be able to play an important role in reducing burden of disease and excess mortality in patients with MD.
Several reviews and meta-analyses show that interventions to promote PA using different interventional strategies (e.g. counseling, planning, exercise logs, addressing self-efficacy, information of benefits of PA etc.) are able to help participants of different populations to increase their level of PA on short-, mid- and long-term [20,21,22]. There are several specific barriers concerning the performance of PA that arise either directly from the symptoms of MD (e.g. avolition, lack of motivation and initiative, cognitive limitations, low self-efficacy and self-esteem) or are the result of social, physiological, medical or psychological consequences of MD (e.g. decreased social interaction, side effects of medication and resulting weight gain, fear of discrimination), [23,24,25]. Therefore, it is unclear whether the promising results of interventions in other populations can directly be generalized to populations with MD.
Some small intervention studies report positive effects of PA interventions in patients with MD (e.g. [26,27,28,29]). More extensive RCT studies on lifestyle interventions in larger samples show mixed results [30,31,32,33]. Reviews on health behavior interventions in patients with MD [34,35,36,37,38] show some evidence that PA interventions might be effective, but all demonstrate a lack of RCT studies.
For the use in clinical practice, there is a need for intervention programs that are ideally theory guided, manualized and evaluated as effective for the target population.
The intervention MoVo-LISA (Motivation-Volition: Lifestyle Integrated Sport Activity) by Göhner, Mahler and Fuchs [39] represents such an intervention program. It is based on MoVo-Model, a social cognitive model of health behavior change that represents a specific advancement of the much wider known HAPA model [40] (for a more detailed description of the model and its properties in our sample see Petzold et al. [41]). It contains theory-derived intervention strategies and was developed as a group intervention for in-patients in an orthopedic rehabilitation setting [42] and has been shown to be effective in helping these patients to increase their level of PA [43]. Göhner, Dietsche and Fuchs [44] adapted the program to a rehabilitation setting for in-patients with MD and found it to be effective in helping patients to increase their level of PA compared to a treatment as usual group at 6-month follow-up.
The aim of the present study was to evaluate the feasibility and effectiveness of MoVo-LISA as a PA intervention in outpatients with MD. We decided to use an active CG and, therefore, adapted the program MoVo-LISA so that it focused on a healthy diet as targeted outcome. We decided for healthy diet as this represents an important health behavior as well. Healthy diet shows protective effects for mental health and improving diet quality might have mental health benefits for patients with MD [45].
Meanwhile the authors of MoVo-LISA already evaluated their program with 112 in-patients with MD in a rehabilitation setting that received either MoVo-LISA plus treatment as usual (TAU) or TAU only. In this study, IG showed a significant higher level of PA compared to CG at 6-month follow-up [44]. Important differences to our study are (a) the inclusion of in-patients, whereas our sample consisted of out-patients, (b) the focus of patients with disorders in the range of F32–39, F40–42 and F45–48, whereas we included patients from F1–F4, (c) inclusion only of patients that reported PA under 60 min/week, whereas we included all interested patients that wanted to improve their health behavior, (d) measurement of PA solely by self-report, whereas we measured PA by self-report and objective measurement, (d) the use of TAU as CG while we used an active CG.
Our hypotheses were as follows: (1) There is a differential effect of the interventions on PA, indicating an increase in PA in IG at follow-up while PA remains unchanged in CG; (2) there is a differential effect of the interventions on dietary behavior, indicating an increase in healthy diet behavior in CG at follow-up while dietary behavior in IG remains unchanged; (3) there is a differential effect of the interventions on social cognitive determinants of health behavior change regarding PA, indicating that patients in IG show an increase in social cognitive determinants of health behavior at post- and follow-up measurement while patients in CG do not; (4) explore if there are differential effects on mental health outcomes between the groups.
Method
Design
This was a randomized controlled parallel-group trial with an allocation ratio of 1:1 conducted in Germany. The study was registered (ClinicalTrials.gov Identifier: NCT02569619) and approved by the local ethics committee of Charité Universitätsmedizin Berlin (EA1/371/13). Formal written informed consent was given by the participants.
Recruitment
This trial was carried out at the psychiatric outpatient clinics at three hospitals: Charité Universitätsmedizin Berlin, Department of Psychiatry and Psychotherapy, St. Hedwig Hospital Berlin, Department of Psychiatry and Oberhavel Hospital Henningsdorf, Brandenburg, Department of Psychiatry. Participants were recruited using flyers and posters in the waiting areas or by their doctors at the three psychiatric outpatient clinics of the participating hospitals and at local psychiatrists in Berlin. They could contact the research team and, if found eligible, were invited to an information event at the study center. At the information event, the study was described in detail and written consent of patients that wanted to participate was obtained. Participants filled out a confidentiality release form and information about diagnosis, course of treatment, medication and global assessment of functioning; (GAF) was obtained from their psychiatrists by mail (to protect the identity of the patients, this information was transmitted only connected to the participant code). Recruitment took place from April 2014 to October 2015.
Eligibility criteria
Eligible participants were woman and men of an age of at least 18 years with a diagnosis of a MD [ICD-10: mental and behavioral disorders due to psychoactive substance abuse (F1); Schizophrenia, schizotypal and delusional disorders (F2); mood disorders (F3) and neurotic, stress-related and somatoform disorders (F4)], that were currently in outpatient treatment at one of the clinics or at a local psychiatrist in Berlin, did not have any contra-indications for PA, did not have a legal custodian, were able to understand German and willing to improve their health behavior. Diagnoses beyond ICD-10: F1–F4 were allowed if the main diagnosis was within this spectrum.
Interventions
The interventions where delivered by three trained and supervised psychologists. All of them did cover both intervention arms in equal portions.
Physical activity intervention (MoVo-LISA)
MoVo-LISA [46] is a theory-based, manualized and successfully evaluated psychological group intervention. It is based on MoVo-model [40] and aims at helping the participants to increase their level of PA by addressing motivational and volitional strategies of behavior change. It consists of two group sessions of around 90 min and one single session of around 15 min. The group sessions took place 1 and 2 weeks after the information event. The single session took place in between the two group sessions. If required by the patients, the single session was delivered by phone. In the first group session, benefits of PA and individual goals that the participants want to achieve by regular PA are discussed, individual ideas for PA performance are set up and the participants are instructed to formulate first plans how they want to integrate regular PA in their everyday routine. In the single session, the individual PA plans are discussed with the participants. In the second group session, individual barriers for PA are discussed, and plans, how to overcome these, are formulated. At the end, every participant formulates an individual plan, how to integrate PA into his everyday life. Used behavior change techniques are: clarification of personal health objectives, contemplation of different actions to achieve these objectives and formation of goal intentions, checking the self-concordance of these and reflections on outcome experiences, generating implementation intentions, anticipation of personal barriers, development of barrier management strategies and self-monitoring by behavior protocols ([42, 47]). For detailed information about MoVo-LISA see Göhner, Mahler and Fuchs [46].
Healthy diet intervention
As active comparator, we developed a healthy diet intervention which was in structure and used behavior change strategies exactly like the PA intervention (MoVo-LISA) but targeted a healthy diet instead of PA (Benefits of healthy diet are discussed, individuals dietary goals set, barriers to a healthy diet discussed…). Therefore, we used the healthy diet intervention parts from the manualized PA and healthy diet intervention MoVo-LIFE, developed by Göhner and Fuchs [39].
Measures
Physical activity (primary outcome)
PA was measured by self-report with the short past 7 days self-administered format of the International Physical Activity Questionnaire (IPAQ; [48]). This questionnaire asks for the time spent in different activity domains (vigorous activity, moderate activity, walking and sitting) during the past 7 days. The IPAQ has been frequently used in international research. In previous studies it shows an adequate test–retest reliability (Spearmans correlation coefficients of about ρ = 0.76) as well as an acceptable criterion validity compared to accelerometry (around ρ = 0.30; [48]).
PA was measured objectively using ActiGraph GT1M accelerometers (ActiGraph, Pensacola, FL, USA), a small, wearable device that is designed to monitor human activity and calculate energy expenditure from acceleration [49]. It measures the amount of changes in acceleration during a sampling period (epoch) of 60 s. These are summarized as activity counts and quantify the amount of activity during that sampling period. The GT1M shows good reliability in laboratory [50] and free-living conditions [51] and has successfully been used in clinical studies with people with diagnosis of MD [52]. The patients were instructed to wear the accelerometer for 7 consecutive days of measurement during wake time around their hip. Measured activity counts were categorized into three activity levels (moderate: between 1953 and 5724 counts/min, equals 3.0–5.99 METs; hard : between 5725 and 9498 counts/min, equals 6.0–8.99 METs; very hard: > 9498 counts/min, equals over 9 METs) using the ActiLife Software [49]. Wearing period was defined as the number of hours per day where at least one count was measured. Days were counted as valid if the wearing period was ≥ 10 h; days in which the accelerometer was worn less than 10 h were set to missing. For the calculation of accelerometer measures only datasets with at least three valid days were used (see Mâsse et al. [53] for an overview on this and other criteria in accelerometer data reduction).
Dietary behavior
Dietary behavior was assessed using a Food-Frequency Questionnaire developed by Winkler and Döring [54]. This questionnaire is designed as a short measure to assess the overall dietary pattern of participants in epidemiologic studies and for the use in nutrition counseling. The questionnaire asks for the frequency of the consumption of 24 different food groups (e.g. meat, rice, salad, vegetables with answers on a 6-point Likert scale ranging from “nearly every day” to “never”). Based on the recommendations of the German Association for Nutrition the questionnaire allows to calculate a dietary pattern index (DPI) that reflects the degree to that the dietary behavior complies with the recommendations (DPI ≥ 16: favorable dietary pattern; DPI 14–15: a regular dietary pattern; DPI ≤ 13: unfavorable dietary pattern) [54]. The authors reported a good external validity of the Food-Frequency Questionnaire compared with detailed 7-day nutrition protocols in a study with 899 men.
Fruit and vegetable consumption was assessed by a questionnaire from Steptoe et al. [55] that consists of two items asking for the portions of fruit and vegetable that the participant consumes on an average day. Portions are defined as a handful of fruit/vegetable.
Social-cognitive determinants of health behavior change
The items measuring self-efficacy, outcome expectancies, intention, action planning and coping planning were to be answered on 4-point Likert scales.
Self-efficacy for physical activity was assessed by two items by Parschau et al. [56] who reported inter-item correlations between 0.81 and 0.84.
Outcome expectancies for physical activity were assessed by six items by Lipple, Ziegelmann and Schwarzer [57] who reported a reliability between α = 0.67 and α = 0.73.
Intention for physical activity was assessed by using two items by Schüz et al. [58], who reported an inter-item-correlation of 0.9.
Action planning for physical activity was assessed by using four items from Sniehotta et al. [59] who reported internal consistencies between α = 0.92 and α = 0.95.
Coping planning for physical activity was assessed by using four items from Renner et al. [60], who reported an internal consistency of α = 0.94.
Self-concordance was assessed with the self-concordance of sport- and exercise-related goals scale (SSK-Scale) by Seelig and Fuchs [61] which contains 12 items and measures an index value (SSK-Index) for the self-concordance of the intention for PA as well as four different modes of motivation for PA (intrinsic, extrinsic, introjected and identified). According to the authors the subscales show internal consistencies between α = 0.70 and α = 0.82.
Clinical measures
Psychiatric Symptoms were assessed using the Symptom-Checklist-27 (SCL-27; [62]), a short form of the widely used Symptom-Checklist-90 Revised (SCL-90-R). It is designed as a screening instrument for psychiatric complaints from which the global severity index (GSI-27) can be calculated. In previous studies, the index GSI-27 showed an internal consistency of α = 0.93 and all subscales showed internal consistencies of α ≥ 0.7 [62].
Health-related quality of life was assessed using the 12-Item Short-Form Health Survey (SF-12; [63]) that measures a person’s perceived health status using two summary scores: one for physical (Physical Component Summary, PCS) and one for mental health (Mental Component Summary, MCS). In previous studies it showed high correlations with the scores of the SF-36 and shows a 2-week retest-reliability of 0.76 for the mental and 0.89 for the physical component [63].
Points of measurement
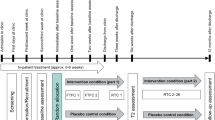
The participants were instructed to fill out the questionnaires and wear the accelerometer for 7 consecutive days at the information event 1 week before the start of the intervention (T0), 1 week (T1) and 3 months (T2) after the last intervention session.
Sample size calculation
For repeated-measurements ANCOVA interaction effects time*group we expected medium effects based on previous research (e.g. [30]). A statistical power analysis using G*Power 3.1.9.2 [64] with f(U) = 0.25, α = 0.05 and β = 0.8 indicated that 82 patients would be needed.
Randomization
Eighty-three patients were randomized, which was carried out by a study employee using urn-design (adaptive coin biased randomization). Allocation ratio was 1:1. The urn always contained more randomization codes than patients that had to be randomized so it was never possible to predict the allocation of a patient. Obviously, patients could not be blinded to the intervention but they were blind to the hypotheses.
Data analysis
Including dropouts, the average percentage of missing data on item basis was 17.60% for the questionnaire items (maximum 32.52%) and 22.30% for the accelerometer data (maximum 36.14%). Missing data were replaced by multiple imputation on scale basis, which represents one of the state-of-the-art procedures to handle missing data [65]. Multiple imputation of the follow-up data was carried out separately for IG and CG to preserve interaction effects. All analyses were conducted using SPSS 23.0 and reported values are pooled results from a set of five imputations (n = 83 if not stated elsewhere).
For all outcome measures, repeated-measurements ANCOVAs with time (T0, T1, T2) as within-subject factor and group (IG, CG) as between-subject factor were calculated. Due to baseline differences in age and walking minutes per week, these variables were included as covariates. Sphericity was analyzed using the Mauchly test. In case of violation of sphericity Greenhouse-Geisser correction was used. Bonferroni corrected post-hoc tests based on EM-Means were calculated for time and group. Significance level was set to 0.05 (two-tailed). Besides statistical significance, clinical significance was calculated for PA and dietary behavior outcome measures. For PA as measured by self-report (IPAQ), the performance of at least 150 min of MVPA per week was defined as a sufficient level of PA. For PA as measured by accelerometer, the performance of at least 30 min of MVPA on an average day was defined as sufficient level of PA. For fruit and vegetable consumption, a sufficient level of intake was defined as at least five portions per day (following the recommendation of many international dietary guidelines; [66]). For dietary behavior, unfavorable dietary pattern was defined as DPI ≤ 13 following the manual of the questionnaire.
Results
Participants
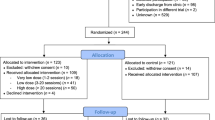
Figure 1 shows flow of participants through the study. Attrition rate was 31.33% (n = 26) with no differences between the groups (χ2 = 0.06, p = .80).
Table 1 shows baseline characteristics of our sample and results of t tests for independent samples and χ2-tests for baseline differences.
PA and dietary behavior—statistical significance
Figure 2 shows means of the PA and dietary behavior outcomes for the two groups and three different points of measurement with post-hoc comparisons based on repeated-measurements ANCOVAs.
Means for PA and dietary behavior outcomes for the two groups and three points of measurement with post-hoc comparisonsa. *significant at .05 level (two-tailed), **significant at .01 level (two-tailed), apost-hoc comparisons based on repeated measurements ANCOVAs using Bonferroni-correction, counts per day as measured by accelerometry (see “Methods” section for interpretation of accelerometer counts), METs per week are metabolic equivalent of task minutes per week, a measure that expresses the energy expenditure of PA (see “Methods” section for calculation and interpretation of this measure), DPI is dietary pattern index (DPI ≥ 16: favorable dietary pattern; DPI 14–15: a regular dietary pattern; DPI ≤ 13: unfavorable dietary pattern)
For PA as measured by average accelerometer counts per day, a repeated-measurements ANCOVA showed a significant group × time interaction effect, F(1.71, 158) = 6.00, p < .01, ηp2 = 0.07, indicating an increase in average accelerometer counts per day in the IG compared to a decrease in the CG. Post hoc tests showed a significant higher level of average accelerometer counts per day in the IG compared to the CG at T2 and significant reductions of average accelerometer counts per day in the CG from T0 to T1 and T0 to T2.
For PA as measured by total METs per week from IPAQ, a repeated-measurements ANCOVA showed a trend towards but not significant group × time interaction effect, F(1.74, 137.19) = 2.75, p = .075, ηp2 = 0.03, post-hoc tests revealed no significant differences.
For dietary behavior as measured by DPI, a repeated measurements ANCOVA showed a significant group × time interaction effect, F(2, 158) = 3.93, p = .03, ηp2 = 0.05. Post hoc tests showed a significant increase in DPI in CG from T0 to T1 and T0 to T2 as well as a significant difference between IG and CG at T1.
For fruit and vegetable consumption as measured by average portions per day, a repeated measurements ANCOVA showed a significant group × time interaction effect, F(24.01, 135.74) = 5.20, p = .01, ηp2 = 0.06. Post hoc tests showed a significant increase in fruit and vegetable consumption from T0 to T1 and T0 to T2 in CG.
PA and dietary behavior outcomes—clinical significance
See Table 2 for changes in clinical significance criteria regarding PA and healthy diet from baseline to follow-up measurement.
Social cognitive determinants of health behavior change and clinical outcome measures
Table 3 shows results of repeated-measurements ANCOVAs for social-cognitive determinants of health behavior change and clinical variables.
Discussion
Effects of the PA intervention on PA
Our first hypothesis was confirmed by a significant group*time interaction effect in objective measured PA and a trend towards a significant interaction in self-reported PA that indicates differential effects of the interventions in the PA outcome variables. Levels of PA measured by objective measurement and self-report increased from baseline to post and follow-up in IG while there was a decrease in CG. Post-hoc tests showed a significant higher level of objective measured level of PA at follow-up. This indicates that, as expected, the PA intervention helped the participants to increase their level of PA. PA levels in IG were stable from post to follow-up measurement or even increased further, so the intervention is, despite its short duration over 2 weeks with only three sessions, able to produce somewhat stable effects. Increase in PA seems larger in self-report (about + 100% in METs per week from T0 to T2) than in objective measurement (about + 10% from T0 to T2). This might partly be explained by the fact that self-report focuses on explicit PA (moderate, vigorous and walking), while the counts from accelerometer include all forms of body movements over the day, which are likely to remain constant throughout the intervention. Therefore, the increase, which should mainly consist of planned, explicit forms of PA, might seem smaller. However, the differences in the results between self-report and objective-measurement might also be accounted for by methodical problems of the self-report in samples with mental disorders. Despite its wide-spread use in interventions in samples with mental disorders, recent studies show several methodical problems of the IPAQ (e.g. difficulties of the participants to differentiate in moderate and vigorous activity) and recommend the use of objective measurement for intervention studies [67, 68].
We observed a decrease in level of PA in both self-report and objective measurement in CG, which we did not expect. Possible explanations for this could be that the participants overestimated their amount of PA in self-report at baseline measurement as some participants reported that this would have happened. However, this would not explain the decrease in objective measured PA. This decrease might be explained by a positive effect of the measurement by accelerometry on the level of PA. The fact that the participants knew that their level of PA was measured might have motivated them to perform more PA and this effect might be reduced with repeated measurement. This interpretation is supported by a study by van Sluijs et al. [69], while other studies consider accelerometer as not very sensitive to reactivity [70]. Other possible explanations are uncontrolled effects (weather, season, holidays…) as PA does vary with seasonality [71] or that the focus on dietary behavior in the dietary intervention did lead participants in CG to neglect other forms of health behavior as PA.
While at baseline the majority of the participants in IG did not meet our criterion of clinical significance for a health beneficial level of PA, this value increased by 30–40% at follow-up. Therefore, at the 3-month follow-up, three quarters of the participants in IG met our criterion for a health beneficial level of PA as measured by self-report as well as objective measurement. In CG there were nearly no changes. This indicates that the intervention is effective in helping patients with MD to increase their level of PA in a clinical significant extent.
These results are in line with the study by Göhner, Dietsche and Fuchs [44] with patients with MD in psychosomatic rehabilitation, where stable positive effects of the intervention on level of PA were found at 3- and 6-month follow-up. The participants in the present study were initially more active than the participants in the study by Göhner, Dietsche and Fuchs [44], where in contrast to the present study, the participants were inpatients. Therefore, while the percentage of participants that did not reach this recommendation at baseline but did reach it at 3-month follow-up was higher than in our study (59% vs. 29.23% objective and 39.54% self-reported), the percentage of participants in IG that did reach the recommendation at follow-up was higher in our study (76.67% objective and 76.75% self-reported vs. 59%). This indicates the intervention is suitable not only for populations with very low levels of PA but also for those who perform an average level of PA.
Effect of the healthy diet intervention on dietary behavior
Our second hypothesis was confirmed by significant time*group interaction effects in dietary behavior (as measured by DPI) and fruit and vegetable consumption that indicate a differential effect of the interventions on these outcomes. Post-hoc tests revealed significant increases in CG in DPI and fruit and vegetable consumption from baseline to post measurement and these effects remained stable at follow-up. At the same time, there were no significant changes in these variables in IG. This indicates that, as expected, the dietary intervention helped the participants to change their dietary behavior towards a healthier diet. The decrease in percentage of participants that met the defined criterion of an unfavorable dietary pattern was about nearly double the size in CG compared to IG (21.50% vs. 11.63%) and percentage of participants that met the defined criterion for fruit and vegetable consumption increased in CG (+ 22.00%) while it decreased in IG (− 15.14%).
This shows that the dietary intervention can be effective in helping patients with MD to change their dietary behavior towards a healthier behavior (see Naslund et al. [72] to compare these results with other lifestyle interventions in patients with MD).
Effects of the PA intervention on social cognitive determinants of health behavior change
Our third hypothesis was partly confirmed by significant time*group interaction effects for self-efficacy and action planning that indicate a differential influence of the interventions on these variables. Post-hoc tests show a significant increase in self-efficacy, self-concordance of the motivation, action planning and coping planning in IG, but not CG. This shows that the intervention was successful in changing these variables as proposed by the model that it is built of. These results are basically in line with the findings by Göhner, Seelig and Fuchs [42] and Göhner, Dietsche and Fuchs [44]. As the participants show higher values on action planning than coping planning at baseline and the increase in action planning is higher than in coping planning from T0 to T2, a possible modification of the intervention for patients with MD would be a stronger focus on coping planning (e.g. more explicit targeting of how to overcome avolition).
Effect of the interventions on mental health outcomes
ANCOVAs revealed no significant main or interaction effects for GSI, MCS or PCS. Post-hoc tests showed a significant improvement in MCS in both IG and CG. These results cannot be clearly interpreted as positive results of both interventions on the mental component of health-related quality of life as there was no control group that did not receive an intervention.
Strengths and limitations
This study evaluated the feasibility and effectiveness of a published, manualized, theory-guided and positively evaluated psychological group intervention that aims to help participants to increase their everyday PA. We used an active CG that was in form, structure and used behavioral change techniques exactly as the IG targeting a healthy diet behavior. Therefore, differential effects between the groups can be directly attributed to the content of the interventions and the probability that general effects are accountable for the differences are low. Our sample was heterogeneous regarding age, type and severity of MD what speaks for a good generalizability to natural outpatient settings with patients with MD. Another strength represents the pragmatic approach of the intervention MoVo-LISA, which due to its short duration and high level of standardization, can be easily integrated into standard treatment settings. We assessed PA by self-report as well as by objective measurement. This combination allows a more detailed investigation of PA effects as both forms of measurement come with different forms of bias [73].
Nevertheless, there are some limitations. Our sample was large enough to evaluate the effectiveness of the intervention overall but too small to investigate differential effects in participants with different characteristics (e.g. diagnosis, or baseline level of PA). Due to the heterogenous sample with different groups of diagnoses of MD, we were not able to further evaluate the potential influence of specific symptoms (e.g. avolition) on the results. Further research on the program in more homogenous populations, e.g. for patients with different groups of diagnoses of MD, would be needed. A large proportion of our sample received psychopharmacotherapy, which could have influenced PA (e.g. by sedation). We were not able to further evaluate potential influences by psychotropic medication due to the sample size. Our study focused primarily on the evaluation of the PA intervention MoVo-LISA, so the results of the healthy diet intervention which served as comparison group would have to be replicated in a trial with a more detailed assessment of dietary behavior as we only used retrospective self-assessment of which comes with a number of biases [74]. Incorporation of more elaborated forms of assessment (e.g. dietary records) would be needed. As we incorporated follow-ups at 1 week and 3 months after the end of the intervention period, we were only able to evaluate short- and mid-term effects of the intervention. A further evaluation of long-term effects of the intervention would be needed. Our population only consisted of patients that were already motivated to increase their health behavior; therefore, the results might not be representative of patients in different motivational stages. The interventions consist of a combination of different behavior change strategies, so it is not possible to say which of them are accountable for the observed changes in health behavior. Dismantling studies might be useful for a better understanding of effective mechanisms. Another limitation is the fact that our design did not include a non-treatment control group. Therefore, from our data solely conclusions on the differential effects of the two interventions can be drawn but no conclusions about the effect of the interventions compared to treatment-as-usual. Furthermore, we did not assess objective measures of physical health like, e.g. BMI, fasting blood glucose levels or waist circumference, which might be interesting for future research. As in previous research on exercise interventions in patients with MD, the supervision by sports therapists and the availability of continuous contact persons have been considered as important factors to increase motivation for aerobic exercise [75]; the incorporation of continuous supervision by sports therapists into the intervention might be a potential advancement to fit the needs of patients with MD.
Conclusion
The results of the present study indicate that the psychological group intervention MoVo-LISA can be effective in helping patients with MD to increase their level of PA in short- and mid-term. Further research in larger and more specific samples, and with longer follow-up periods to determine long time effects are strongly encouraged. This example illustrates that psychological theories of health behavior change from sports and exercise psychology can be successfully used to design interventions in populations with MD. Results on the CG show that the structure and used behavior change techniques of MoVo-LISA might be effective in helping patients with MD to improve their dietary behavior as well. To fit the need of patients with MD, modification of the intervention with a stronger focus on coping planning, the incorporation of continuous supervision by sports therapists and the addition of one or two booster sessions after the end of the intervention seem reasonable.
References
Colditz GA, Nguyen N, Dart H (2017) Physical activity and health. In: Quah SR (ed) International encyclopedia of public health, 2nd edn. Elsevier, Heidelberg, pp 463–472
World Health Organization (2009) Global health risks—mortality and burden of disease attributable to selected major risks, Geneva
Ströhle A (2018) Sports psychiatry: mental health and mental disorders in athletes and exercise treatment of mental disorders. Eur Arch Psychiatry Clin Neurosci. https://doi.org/10.1007/s00406-018-0891-5
Ströhle A, Höfler M, Pfister H et al (2007) Physical activity and prevalence and incidence of mental disorders in adolescents and young adults. Psychol Med 37(11):1657–1666. https://doi.org/10.1017/S003329170700089X
Meng X, D’Arcy C (2013) The projected effect of increasing physical activity on reducing the prevalence of common mental disorders among Canadian men and women: a national population-based community study. Prev Med 56(1):59–63. https://doi.org/10.1016/j.ypmed.2012.11.014
Martin H, Beard S, Clissold N et al (2017) Combined aerobic and resistance exercise interventions for individuals with schizophrenia. A systematic review. Ment Health Phys Act 12:147–155. https://doi.org/10.1016/j.mhpa.2017.04.003
Firth J, Cotter J, Elliott R et al (2015) A systematic review and meta-analysis of exercise interventions in schizophrenia patients. Psychol Med 45(7):1343–1361. https://doi.org/10.1017/S0033291714003110
Stubbs B, Vancampfort D, Rosenbaum S et al (2017) An examination of the anxiolytic effects of exercise for people with anxiety and stress-related disorders: a meta-analysis. Psychiatry Res 249:102–108. https://doi.org/10.1016/j.psychres.2016.12.020
Schuch FB, Vancampfort D, Richards J et al (2016) Exercise as a treatment for depression: a meta-analysis adjusting for publication bias. J Psychiatr Res 77:42–51. https://doi.org/10.1016/j.jpsychires.2016.02.023
Zschucke E, Gaudlitz K, Ströhle A (2013) Exercise and physical activity in mental disorders: clinical and experimental evidence. J Prev Med Public Health 46(Suppl 1):12–21. https://doi.org/10.3961/jpmph.2013.46.S.S12
Garber CE, Blissmer B, Deschenes MR et al (2011) American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc 43(7):1334–1359. https://doi.org/10.1249/MSS.0b013e318213fefb
Stubbs B, Vancampfort D, Firth J et al (2017) Physical activity correlates among people with psychosis: data from 47 low- and middle-income countries. Schizophr Res. https://doi.org/10.1016/j.schres.2017.06.025
Vancampfort D, Stubbs B, Firth J et al (2017) Physical activity correlates among 24,230 people with depression across 46 low- and middle-income countries. J Affect Disord 221:81–88. https://doi.org/10.1016/j.jad.2017.06.012
Jerome GJ, Young DR, Dalcin A et al (2009) Physical activity levels of persons with mental illness attending psychiatric rehabilitation programs. Schizophr Res 108(1–3):252–257. https://doi.org/10.1016/j.schres.2008.12.006
Kruisdijk F, Deenik J, Tenback D et al (2017) Accelerometer-measured sedentary behaviour and physical activity of inpatients with severe mental illness. Psychiatry Res 254:67–74. https://doi.org/10.1016/j.psychres.2017.04.035
Mangerud WL, Bjerkeset O, Lydersen S et al (2014) Physical activity in adolescents with psychiatric disorders and in the general population. Child Adolesc Psychiatry Ment Health 8(1):2. https://doi.org/10.1186/1753-2000-8-2
Scott D, Happell B (2011) The high prevalence of poor physical health and unhealthy lifestyle behaviours in individuals with severe mental illness. Issues in mental health nursing 32(9):589–597. https://doi.org/10.3109/01612840.2011.569846
Walker ER, McGee RE, Druss BG (2015) Mortality in mental disorders and global disease burden implications: a systematic review and meta-analysis. JAMA Psychiatry 72(4):334–341. https://doi.org/10.1001/jamapsychiatry.2014.2502
Lee I-M, Shiroma EJ, Lobelo F et al (2012) Effect of physical inactivity on major non-communicable diseases worldwide. An analysis of burden of disease and life expectancy. Lancet 380(9838):219–229. https://doi.org/10.1016/S0140-6736(12)61031-9
Hillsdon M, Foster C, Thorogood M (2005) Interventions for promoting physical activity. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD003180.pub2
Kahn EB, Ramsey LT, Brownson RC et al (2002) The effectiveness of interventions to increase physical activity. A systematic review. Am J Prev Med 22(4 Suppl):73–107
Müller-Riemenschneider F, Reinhold T, Nocon M et al (2008) Long-term effectiveness of interventions promoting physical activity: a systematic review. Prev Med 47(4):354–368. https://doi.org/10.1016/j.ypmed.2008.07.006
McDevitt J, Snyder M, Miller A et al (2006) Perceptions of barriers and benefits to physical activity among outpatients in psychiatric rehabilitation. J Nurs Scholarsh 38(1):50–55. https://doi.org/10.1111/j.1547-5069.2006.00077.x
Roberts SH, Bailey JE (2011) Incentives and barriers to lifestyle interventions for people with severe mental illness: a narrative synthesis of quantitative, qualitative and mixed methods studies. J Adv Nurs 67(4):690–708. https://doi.org/10.1111/j.1365-2648.2010.05546.x
Ussher M (2007) Physical activity preferences and perceived barriers to activity among persons with severe mental illness in the United Kingdom. Psychiatr Serv 58(3):405. https://doi.org/10.1176/appi.ps.58.3.405
Bradshaw T, Lovell K, Bee P et al (2010) The development and evaluation of a complex health education intervention for adults with a diagnosis of schizophrenia. J Psychiatr Ment Health Nurs 17(6):473–486. https://doi.org/10.1111/j.1365-2850.2009.01543.x
Brown S, Chan K (2009) A randomized controlled trial of a brief health promotion intervention in a population with serious mental illness. J Ment Health 15(5):543–549. https://doi.org/10.1080/09638230600902609
Kerr J, Calfas KJ, Caparosa S et al (2008) A pilot study to assess the feasibility and acceptability of a community based physical activity intervention (involving internet, telephone, and pedometer support), integrated with medication and mood management for depressed patients. Ment Health Phys Act 1(1):40–45. https://doi.org/10.1016/j.mhpa.2008.06.002
Vickers KS, Patten CA, Lewis BA et al (2009) Feasibility of an exercise counseling intervention for depressed women smokers. Nicotine Tob Res 11(8):985–995. https://doi.org/10.1093/ntr/ntp101
Bartels SJ, Pratt SI, Aschbrenner KA et al (2013) Clinically significant improved fitness and weight loss among overweight persons with serious mental illness. Psychiatr Serv 64(8):729–736. https://doi.org/10.1176/appi.ps.003622012 (Washington DC)
Speyer H, Christian Brix Nørgaard H, Birk M et al (2016) The CHANGE trial: no superiority of lifestyle coaching plus care coordination plus treatment as usual compared to treatment as usual alone in reducing risk of cardiovascular disease in adults with schizophrenia spectrum disorders and abdominal obesity. World Psychiatry 15(2):155–165. https://doi.org/10.1002/wps.20318
Vanroy J, Seghers J, Bogaerts A et al (2017) ‘Join The Walk?’: short-term and follow-up effects of a 10-week walking intervention in patients with a mental disorder. Ment Health Phys Act 12:73–82. https://doi.org/10.1016/j.mhpa.2017.02.005
Verhaeghe N, Clays E, Vereecken C et al (2013) Health promotion in individuals with mental disorders: a cluster preference randomized controlled trial. BMC Public Health 13:657. https://doi.org/10.1186/1471-2458-13-657
Bonfioli E, Berti L, Goss C et al (2012) Health promotion lifestyle interventions for weight management in psychosis: a systematic review and meta-analysis of randomised controlled trials. BMC Psychiatry 12:78. https://doi.org/10.1186/1471-244X-12-78
Cabassa LJ, Ezell JM, Lewis-Fernández R (2010) Lifestyle interventions for adults with serious mental illness: a systematic literature review. Psychiatr Serv 61(8):774–782. https://doi.org/10.1176/ps.2010.61.8.774
Happell B, Davies C, Scott D (2012) Health behaviour interventions to improve physical health in individuals diagnosed with a mental illness: a systematic review. Int J Ment Health Nurs 21(3):236–247. https://doi.org/10.1111/j.1447-0349.2012.00816.x
Rosenbaum S, Newby JM, Steel Z et al (2015) Online physical activity interventions for mental disorders. A systematic review. Internet Interv 2(2):214–220. https://doi.org/10.1016/j.invent.2015.04.001
Verhaeghe N, Maeseneer J de, Maes L et al (2011) Effectiveness and cost-effectiveness of lifestyle interventions on physical activity and eating habits in persons with severe mental disorders: a systematic review. Int J Behav Nutr Phys Act 8:28. https://doi.org/10.1186/1479-5868-8-28
Göhner W, Fuchs R (eds) (2007) Aufbau eines körperlich-aktiven Lebensstils: Theorie, Empirie und Praxis. Hogrefe, Göttingen
Fuchs R (2013) Das Motivations-Volitions-Konzept. Public Health Forum 21(2):179. https://doi.org/10.1016/j.phf.2013.03.004
Petzold MB, Bischoff S, Rogoll J et al (2017) Physical activity in outpatients with mental disorders: status, measurement and social cognitive determinants of health behavior change. Eur Arch Psychiatry Clin Neurosci 267(7):639–650. https://doi.org/10.1007/s00406-017-0772-3
Göhner W, Seelig H, Fuchs R (2009) Intervention effects on cognitive antecedents of physical exercise. A 1-year follow-up study. Appl Psychol Health Well-Being 1(2):233–256. https://doi.org/10.1111/j.1758-0854.2009.01014.x
Fuchs R, Goehner W, Seelig H (2011) Long-term effects of a Psychological Group intervention on physical exercise and health: the MoVo concept. J Phys Act Health 8:794–803
Göhner W, Dietsche C, Fuchs R (2015) Increasing physical activity in patients with mental illness—a randomized controlled trial. Patient Educ Couns 98(11):1385–1392. https://doi.org/10.1016/j.pec.2015.06.006
Dawson SL, Dash SR, Jacka FN (2016) The importance of diet and gut health to the treatment and prevention of mental disorders. Int Rev Neurobiol 131:325–346. https://doi.org/10.1016/bs.irn.2016.08.009
Göhner W, Mahler C, Fuchs R (2007) MoVo-LISA: Ein Kleingruppenprogramm zur Änderung des Bewegungsverhaltens. In: Göhner W, Fuchs R (eds) Aufbau eines körperlich-aktiven Lebensstils: Theorie, Empirie und Praxis. Hogrefe, Göttingen, pp 340–353
Fuchs R, Seelig H, Göhner W et al (2012) Cognitive mediation of intervention effects on physical exercise: causal models for the adoption and maintenance stage. Psychol Health 27(12):1480–1499. https://doi.org/10.1080/08870446.2012.695020
Craig CL, Marshall AL, Sjöström M et al (2003) International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc 35(8):1381–1395. https://doi.org/10.1249/01.MSS.0000078924.61453.FB
ActiGraph (2008) ActiLife users manual. ActiGraph, LLC Engineering, Pensacola, Fl
Silva P, Mota J, Esliger D et al (2010) Technical reliability assessment of the actigraph GT1M accelerometer. Measurement 14(2):79–91. https://doi.org/10.1080/10913671003715524
Kaminsky LA, Ozemek C (2012) A comparison of the Actigraph GT1M and GT3X accelerometers under standardized and free-living conditions. Physiol Meas 33(11):1869–1876. https://doi.org/10.1088/0967-3334/33/11/1869
Mota-Pereira J, Ribeiro JC, Fonte D et al (2012) P-506–12 Weeks of moderate intensity exercise improves treatment-resistant major depressive disorder (MDD). daily use of accelerometers contributes to 97% adherence. Eur Psychiatry 27:1. https://doi.org/10.1016/S0924-9338(12)74673-4
Mâsse LC, Fuemmeler BF, Anderson CB et al (2005) Accelerometer data reduction: a comparison of four reduction algorithms on select outcome variables. Med Sci Sports Exerc 37(11 Suppl):S544–S554
Winkler G, Döring A (1995) Kurzmethoden zur Charakterisierung des Ernaehrungsmusters. Einsatz und Auswertung eines Food-Frequency-Fragebogens. Ernährungs-Umschau 42(8):289–291
Steptoe A, Perkins-Porras L, McKay C et al (2003) Psychological factors associated with fruit and vegetable intake and with biomarkers in adults from a low-income neighborhood. Health Psychol 22(2):148–155
Parschau L, Fleig L, Warner LM et al (2014) Positive exercise experience facilitates behavior change via self-efficacy. Health Educ Behav 41(4):414–422. https://doi.org/10.1177/1090198114529132
Lippke S, Ziegelmann JP, Schwarzer R (2005) Stage-specific adoption and maintenance of physical activity. Testing a three-stage model. Psychol Sport Exerc 6(5):585–603. https://doi.org/10.1016/j.psychsport.2004.11.002
Schüz B, Wurm S, Warner LM et al (2014) Health motives and health behaviour self-regulation in older adults. J Behav Med 37(3):491–500. https://doi.org/10.1007/s10865-013-9504-y
Sniehotta FF, Schwarzer R, Scholz U et al (2005) Action planning and coping planning for long-term lifestyle change. Theory and assessment. Eur J Soc Psychol 35(4):565–576. https://doi.org/10.1037/0882-7974.22.3.482
Renner B, Spivak Y, Kwon S et al (2007) Does age make a difference? Predicting physical activity of South Koreans. Psychol Aging 22(3):482–493. https://doi.org/10.1037/0882-7974.22.3.482
Seelig H, Fuchs R (2006) Messung der sport- und bewegungsbezogenen Selbstkonkordanz. Zeitschrift für Sportpsychologie 13(4):121–139. https://doi.org/10.1026/1612-5010.13.4.121
Hardt J, Gerbershagen HU (2001) Cross-validation of the SCL-27: a short psychometric screening instrument for chronic pain patients. Eur J pain 5(2):187–197. https://doi.org/10.1053/eujp.2001.0231
Ware J, Kosinski M, Keller SD (1996) A 12-Item short-form health survey: construction of scales and preliminary tests of reliability and validity. Med Care 34(3):220–233
Faul F, Erdfelder E, Lang A-G et al (2007) G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 39(2):175–191
Enders CK (2010) Applied missing data analysis. The Guilford Press, New York
Painter J, Rah J-H, Lee Y-K (2002) Comparison of international food guide pictorial representations. J Am Diet Assoc 102(4):483–489. https://doi.org/10.1016/S0002-8223(02)90113-6
Firth J, Stubbs B, Vancampfort D et al (2017) The validity and value of self-reported physical activity and accelerometry in people with schizophrenia: a population-scale study of the UK Biobank. Schizophr Bull. https://doi.org/10.1093/schbul/sbx149
Duncan MJ, Arbour-Nicitopoulos K, Subramanieapillai M et al (2017) Revisiting the International Physical Activity Questionnaire (IPAQ): assessing physical activity among individuals with schizophrenia. Schizophr Res 179:2–7. https://doi.org/10.1016/j.schres.2016.09.010
van Sluijs EM, van Poppel MN, Twisk JW et al (2006) Physical activity measurements affected participants’ behavior in a randomized controlled trial. J Clin Epidemiol 59(4):404–411. https://doi.org/10.1016/j.jclinepi.2005.08.016
Trost SG (2016) State of the art reviews. Measurement of physical activity in children and adolescents. Am J Lifestyle Med 1(4):299–314. https://doi.org/10.1177/1559827607301686
Tucker P, Gilliland J (2007) The effect of season and weather on physical activity: a systematic review. Public Health 121(12):909–922. https://doi.org/10.1016/j.puhe.2007.04.009
Naslund JA, Whiteman KL, McHugo GJ et al (2017) Lifestyle interventions for weight loss among overweight and obese adults with serious mental illness. A systematic review and meta-analysis. Gen Hosp Psychiatry 47:83–102. https://doi.org/10.1016/j.genhosppsych.2017.04.003
Strath SJ, Kaminsky LA, Ainsworth BE et al (2013) Guide to the assessment of physical activity. Clin Res Appl Circ 128(20):2259–2279. https://doi.org/10.1161/01.cir.0000435708.67487.da
Thompson FE, Subar AF (2008) Dietary assessment methodology. Nutr Prev Treat Dis 2: 5–46. https://doi.org/10.1016/B978-0-12-391884-0.00001-9
Keller-Varady K, Hasan A, Schneider-Axmann T et al (2016) Endurance training in patients with schizophrenia and healthy controls: differences and similarities. Eur Arch Psychiatry Clin Neurosci 266(5):461–473. https://doi.org/10.1007/s00406-015-0651-8
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This study has been approved by the local ethics committee and has, therefore been, performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Informed consent
All participants gave formal written informed consent prior to their inclusion in the study.
Rights and permissions
About this article
Cite this article
Petzold, M.B., Mumm, J.L.M., Bischoff, S. et al. Increasing physical activity and healthy diet in outpatients with mental disorders: a randomized-controlled evaluation of two psychological interventions. Eur Arch Psychiatry Clin Neurosci 269, 529–542 (2019). https://doi.org/10.1007/s00406-018-0941-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00406-018-0941-z