Abstract
The purpose of this study was to investigate the relationship between specific symptom severity and D2/3 receptor availability in extrastriatal regions in outpatients with schizophrenia to shed light on the role of extrastriatal dopaminergic neurotransmission in the pathophysiology of symptoms of schizophrenia. Sixteen schizophrenia patients receiving relatively low-dose maintenance atypical antipsychotics and seventeen healthy controls underwent 3-Tesla magnetic resonance imaging and high-resolution positron emission tomography with [18F]fallypride. For D2/3 receptor availability, the binding potential with respect to non-displaceable compartment (BPND) was derived using the simplified reference tissue model. The BPND values were lower in patients on antipsychotic treatment than in controls across all regions with large effect sizes (1.03–1.42). The regions with the largest effect size were the substantia nigra, amygdala, and insula. Symptoms of schizophrenia were assessed using a five-factor model of the Positive and Negative Syndrome Scale (PANSS). The region of interest-based analysis showed that PANSS excitement factor score had a significant positive correlation with the [18F]fallypride BPND in the insula. The equivalent dose of antipsychotics was not significantly correlated with PANSS factor scores or regional BPND values. The voxel-based analysis also revealed a significant positive association between the PANSS excitement factor and the [18F]fallypride BPND in the insula. The present study revealed a significant association between excitement symptom severity and D2/3 receptor availability in the insula in schizophrenia, suggesting a possible important role of D2/3 receptor-mediated neurotransmission in the insula and related limbic system in the pathophysiology of this specific symptom cluster.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Since the introduction of second generation antipsychotics, extrastriatal dopamine receptors have received much attention in schizophrenia research [1]. The development of high-affinity D2-like receptor radiotracers has also allowed the exploration of D2-like receptors in relatively low density regions including thalamus, temporal cortex, and limbic regions in recent years [2]. In particular, dopamine D2-like receptors in extrastriatal regions have been implicated as the possible site of antipsychotic action, although a double-blind positron emission tomography (PET) study found no relationship between extrastriatal D2-like receptor binding and treatment response but observed a significant relationship between striatal D2-like receptor binding and antipsychotic response [3].
The investigation into extrastriatal dopamine receptors using high-affinity D2/3 radiotracers such as [18F]fallypride, a very high-affinity, specific radioligand for extrastriatal D2/3 receptors [4, 5], is still an emerging field and more research is clearly required to better understand the role of extrastriatal D2/3 receptor-mediated neurotransmission in schizophrenia. Previous studies in drug-naïve or drug-free patients with schizophrenia reported reduced D2/3 receptor availability measured by [18F]fallypride in the thalamus [4, 6, 7], temporal cortex [6, 7], prefrontal cortex [6], amygdala [7], uncus [8], and cingulate region [7]. Decreased thalamic binding was also reported in studies using [11C]FLB, another PET tracer for extrastriatal D2/3 receptor [9, 10]. In contrast, increased D2/3 receptor availability assessed by [18F]fallypride was also observed in the substantia nigra [4] and thalamus [8]. Although more research is clearly needed to reach conclusive evidence, the literature on extrastriatal D2/3 receptors in schizophrenia shows a tendency toward reduced D2/3 receptor availability in antipsychotic-free or antipsychotic-naïve patients with schizophrenia, which was reported in a recent meta-analysis [11]. The putative extrastriatal selectivity in the occupancy rate achieved by second generation antipsychotics has also been investigated using [18F]fallypride [12,13,14,15,16,17]. However, previous studies were performed on antipsychotic-naïve or antipsychotic-free patients who were mainly admitted to research units. Few studies have been conducted to investigate D2/3 receptor availability in clinically stable outpatients receiving maintenance antipsychotics and to quantify the level of extrastriatal D2/3 receptors in those with maintenance treatment in a naturalistic clinical setting.
Moreover, while striatal D2/3 receptor density in patients with schizophrenia has been extensively studied with molecular PET imaging and the clinical significance of striatal dopaminergic transmission is well documented [18,19,20], the clinical correlates of extrastriatal D2/3 receptors are largely unknown [11]. In particular, only a few PET studies have reported the relationship between extrastriatal D2/3 receptor availability and the psychopathology of schizophrenia, with the overall results being inconclusive [4, 6, 7, 10, 21]. Kessler et al. [4] found a significant positive correlation of positive symptom score with D2/3 receptor availability in the right temporal cortex, while Lehrer et al. [6] and Buchsbaum et al. [7] reported significant negative correlations between the D2/3 receptor availability in the thalamus and the psychotic symptom severity. Talvik et al. [21] reported a significant negative correlation between [11C]raclopride binding potential in the right thalamus and grandiosity symptom score. In a single-photon emission computerized tomography (SPECT) study with [123I]epidepride, a significant positive correlation between positive symptoms and cortical D2/3 receptor binding was also reported [22]. Thus, further investigation into the relationship between extrastriatal D2/3 receptor availability and the specific symptoms of schizophrenia is clearly warranted, considering that dopaminergic dysfunction may exist across the wide range of cortico-subcortical circuits in schizophrenia [2]. In addition, schizophrenia is a complex illness that is characterized by heterogeneous and multiple symptom domains beyond the dichotomous positive and negative symptoms [23,24,25].
Therefore, the purpose of the present study was to quantify D2/3 receptor availability in extrastriatal and striatal regions in stable outpatients with schizophrenia receiving relatively low-dose maintenance treatment and to investigate the relationship between specific symptom severity and D2/3 receptor availability to further shed light on the role of extrastriatal dopaminergic neurotransmission in the pathophysiology of symptoms of schizophrenia. In the present study, we used 3-Tesla magnetic resonance imaging (MRI) and high-resolution PET with [18F]fallypride.
Materials and methods
Subjects
The study protocol was approved by the Institutional Review Board of the Gachon University Gil Medical Center, and all procedures used in the study were conducted in accordance with international ethical standard, Declaration of Helsinki. Patients were recruited from the outpatient clinic. Informed consent was obtained from all subjects after a full explanation of the study procedure. Sixteen patients (six men and ten women) were enrolled in the study (Table 1). All patients were diagnosed with schizophrenia by Diagnostic and Statistical Manual of Mental Disorders Fourth Edition (DSM-IV) [26], which was established by the Structured Clinical Interview for DSM-IV (SCID) [27], and they did not meet the diagnostic criteria for a psychiatric diagnosis other than schizophrenia. They also did not have a concurrent diagnosis of substance abuse/dependence or medical/neurological disorders. Patients had a mean [standard deviation (SD)] age of 36.9 (11.4) years and a mean (SD) duration of illness of 6.5 (3.7) years. The mean (SD) years of education were 10.4 (1.9) years. All patients were receiving at least 4 weeks of maintenance antipsychotic monotherapy without changes in the dosage of antipsychotics over the same period at the time of enrollment. Medication compliance was confirmed in weekly visits by reports from the patients and caregivers, i.e., family members, who were considered highly reliable by the investigator in providing support to the patient to ensure compliance with the medication. The antipsychotics that patients were taking at the time of enrollment were paliperidone [n = 5, mean (SD) dose: 3.6 (1.3) mg/day], aripiprazole [n = 3, mean (SD) dose: 4.7 (4.6) mg/day], quetiapine [n = 3, mean (SD) dose: 410.9 (43.2) mg/day], olanzapine [n = 2, mean (SD) dose: 5.0 (3.5) mg/day], risperidone [n = 2, mean (SD) dose: 1.5 (0.7) mg/day], and ziprasidone (n = 1, dose: 100.0 mg/day). The chlorpromazine equivalent doses of antipsychotics were calculated based on the method proposed by Gardner et al. [28]. The mean (SD) chlorpromazine equivalent dose was 170.0 (102.8) mg/day. None of the patients were taking antidepressants or mood stabilizers. Seventeen healthy control subjects (eight men and nine women), who met the criteria of no current or past psychiatric, neurological, or medical illness and no current use of medications, were also recruited, provided written informed consent, and underwent the same MRI and PET protocols. All patients and control subjects performed urine tests for opiates and methamphetamines prior to PET scans to exclude substance abuse. For female participants, pregnancy was excluded using urine pregnancy tests before the PET scans. None of the participants showed any gross structural abnormalities on brain MRI, which was confirmed by a board-certified radiologist.
Clinical assessments
Symptoms of schizophrenia were assessed using the Positive and Negative Syndrome Scale (PANSS) [29]. The PANSS is a 30-item instrument that measures positive, negative, and general psychiatric symptoms. A five-factor model of the PANSS was used based on evidence from factor analysis studies [23,24,25]. The factors were positive, negative, cognitive/disorganization, excitement, and depression/anxiety. The global severity of schizophrenia was assessed using the Clinical Global.
Impression Scale of Severity (CGI-S) [30]. Antipsychotic-induced parkinsonism and akathisia were assessed using the Simpson-Angus Scale (SAS) [31] and the Barnes Akathisia Rating Scale (BARS) [32], respectively.
Scan protocol for high-resolution imaging
All participants were scanned using 3-Tesla MRI and High Resolution Research Tomograph (HRRT)-PET with [18F]fallypride. The HRRT-PET is the high-resolution brain-dedicated PET scanner [33, 34]. The tracer [18F]fallypride was synthesized, as previously described [35]. After a mean (SD) bolus injection of 198.5 (22.3) MBq [18F]fallypride with a mean (SD) specific activity of 106.1 (55.3) GBq/μmol, an emission scan was conducted in a dynamic scan mode for 120 min. After the PET scan, 3-Tesla MRI scans were performed using a three-dimensional T1-weighted magnetization-prepared rapid gradient echo (3-D T1MPRAGE) sequence for structural brain imaging. The 3-D T1MPRAGE images were acquired with the following parameters: repetition time = 1900 ms, echo time = 3.3 ms, inversion time = 900 ms, flip angle = 9°, voxel size = 1.0 × 1.0 × 1.0 mm3, and number of slices = 160.
The [18F]fallypride PET images were reconstructed using the 3-dimensional ordinary Poisson ordered-subset expectation maximization (OP-OSEM) algorithm based on the symmetry and single-instruction multiple-data-based projection and backprojection [36]. The reconstructed PET images had a matrix of 256 × 256 × 207 and an iso-voxel resolution of 1.22 × 1.22 × 1.22 mm3.
Image analysis
To calculate the [18F]fallypride binding potential with respect to non-displaceable compartment (BPND), the emission data of [18F]fallypride PET were reconstructed as 22 frames of increasing duration as follows: 4 × 30, 3 × 60, 2 × 150, 4 × 300, and 9 × 600 s (total 120 min). Attenuation, scatter, and decay time correction were estimated and applied for each frame.
The MRI scan of each subject was coregistered to his or her PET scan using statistical parameter mapping 8 (SPM8; Wellcome Trust Center for Neuroimaging, UK). The spatial normalization of the coregistered MRI images of each subject was performed on the Montreal Neurological Institute template using SPM8, and the estimated transform was applied to the corresponding PET images. Time-activity curves of [18F]fallypride PET were generated from the dynamic PET images by averaging all the voxels within each region of interest (ROI) (Fig. 1), which were coregistered to the corresponding MRI images. For the estimation of D2/3 receptor availability, [18F]fallypride BPND was derived from each ROI using the simplified reference tissue model 2 (SRTM2) [37] with the cerebellar cortex devoid of D2/3 receptors [38] as the reference region, based on the parameter estimation implemented in the PMOD software v3.2 (PMOD Technologies Ltd., Zürich, Switzerland). Representative examples of [18F]fallypride BPND, HRRT-PET, and 3-Tesla MR images are shown (Fig. 2). The BPND values were obtained in the 18 predefined ROIs using the automated anatomical labeling (AAL) program [39]. The ROIs were the superior temporal cortex, thalamus, hippocampus, amygdala, insula, substantia nigra, caudate, putamen, and ventral striatum, and left and right regions were analyzed separately.
Time activity curve of the [18F]fallypride was extracted from the each left (a) and right (b) region of interest (ROI) of the PET image. The [18F]fallypride BPND of each ROI was obtained from the time activity curve with the cerebellum as the reference region. lt left, rt right, BP ND binding potential with respect to non-displaceable compartment
Statistical analysis
The mean [18F]fallypride BPND values were compared between the groups using two-tailed t tests. Effect sizes (Cohen’s d) were also calculated. Relationships between demographic variables and [18F]fallypride BPND values were analyzed using Pearson’s correlation analysis. The two-tailed t tests were performed to examine gender differences in regional [18F]fallypride BPND values. To examine the possible effects of antipsychotic dosage on PANSS scores and D2/3 receptor availability, the relationship of chlorpromazine equivalents with PANSS factor scores and [18F]fallypride BPND was evaluated using Pearson’s correlation analysis.
The relationship between the [18F]fallypride BPND values and the severity of clinical symptoms as measured by the PANSS factor scores was analyzed using ROI-based and voxel-based approaches. In the ROI-based analysis, the relationship was analyzed using Pearson’s product–moment correlations. Age-adjusted correlation coefficients were also obtained using partial correlation analysis. We performed the Bonferroni correction for multiple correlations in the ROI-based analysis. The Bonferroni-corrected p value for multiple correlations (18 regions and 5 PANSS factors) is 0.00055 (0.05/90). Therefore, statistical significance was determined by a two-tailed p < 0.0005. The level of p < 0.005 was considered a statistical tendency.
To supplement and confirm the ROI-based results, a voxel-based analysis was also conducted for the same data. The voxelwise linear regression analysis was conducted using spatially normalized BPND images with the PANSS factor scores as regressors. In the voxel-based analysis, the significance was set at p < 0.05, with a false discovery rate (FDR) correction for multiple correlations. However, in view of the supplementary and exploratory nature of the voxel-based analysis in our study, when no significant associations were found at the FDR-corrected threshold, the correlations were further examined using less restrictive criteria and regions surviving an uncorrected p < 0.0001 with an extent threshold of 20 voxels were considered to be significant. The height threshold was set to as low as 0.0001 to reduce the chance of false-positive findings and the extent threshold was set to 20 voxels, which was reported to be acceptable in previous PET imaging studies [40,41,42,43].
Results
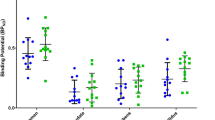
The mean values of [18F]fallypride BPND for ROIs are shown in Table 2. As expected, the [18F]fallypride BPND values were significantly lower in the patient group than in the control group across all regions with large effect sizes (Cohen’s d = 1.03–1.42, p < 0.01), reflecting D2/3 receptor antagonism by antipsychotics in the patient group. The regions with the largest effect size were the left substantia nigra, both amygdala, and right insula. The percentage difference of BPND values between the groups across all measured regions was 32.0–53.6%. Regarding the relationship between demographic variables and D2/3 receptor availability, Pearson’s correlation analysis revealed that age was negatively correlated with the [18F]fallypride BPND values in the superior temporal cortex (left: r = −0.61, p = 0.01; right: r = −0.60, p = 0.02), thalamus (left: r = −0.53, p = 0.04; right: r = −0.54, p = 0.03), hippocampus (left: r = −0.57, p = 0.02; right: r = −0.59, p = 0.02), amygdala (left: r = −0.55, p = 0.03; right: r = −0.60, p = 0.01), and insula (left: r = −0.56, p = 0.03; right: r = −0.61, p = 0.01) in the patient group. In healthy control subjects, a decline of [18F]fallypride BPND values with age was observed in the left amygdala (r = −0.49, p < 0.05), left insula (r = −0.49, p = 0.04), and left caudate (r = −0.48, p < 0.05). No significant gender differences were found in regional [18F]fallypride BPND values in either the patient group (t = 0.19–1.58, p > 0.01) or the control group (t = −1.41 to 0.54, p > 0.01). The [18F]fallypride BPND values had no significant correlations with years of education (patients: r = −0.15 to 0.22, p > 0.05; controls: r = −0.32 to 0.27, p > 0.05). In addition, no significant correlation was found between illness duration and regional D2/3 receptor availability (r = −0.12 to 0.16, p > 0.05). There were no significant correlations between chlorpromazine equivalent doses and [18F]fallypride BPND values in any region (r = −0.41 to −0.20, p > 0.05).
The mean (SD) PANSS scores of the patient group were as follows: PANSS total score, 58.6 (17.8) (median 59.5, range 31–88); PANSS positive symptom score, 8.0 (2.9) (median 8.0, range 4–14); PANSS negative symptom, 15.1 (5.3) (median 15.5, range 7–25); PANSS cognitive/disorganization symptom score, 13.3 (4.1) (median 15.0, range 7–19); PANSS excitement symptom score, 8.8 (3.7) (median 7.5, range 5–18); and PANSS depression/anxiety symptom score, 7.9 (3.2) (median 7.5, range 4–15). The mean (SD) CGI-S score of the patients was 3.0 (0.7) (median 3.0, range 2–4). The mean (SD) SAS and BARS scores of the patients were 1.0 (3.0) (median 0, range 0–10) and 0.2 (0.5) (median 0, range 0–1), respectively. One patient presented with antipsychotic-induced parkinsonism, and none of the patients had akathisia. There were no significant correlations between chlorpromazine equivalent doses and PANSS factor scores (r = −0.35 to −0.03, p > 0.05). In addition, no significant correlation was found between equivalent dose and CGI-S score (r = 0.11, p = 0.70).
Regarding the associations between D2/3 receptor availability and clinical symptoms, the ROI-based analysis showed that the PANSS excitement factor score had significant positive correlations with the [18F]fallypride BPND in the right insula (r = 0.78, p = 0.0004, age-adjusted r = 0.72, p = 0.002) and left insula (r = 0.81, p = 0.0001, age-adjusted r = 0.77, p = 0.001) at the level of p < 0.0005 (Fig. 3). There was a positive correlation with a statistical tendency between the PANSS excitement factor score and the [18F]fallypride BPND in the right amygdala at the level of p < 0.005 (r = 0.68, p = 0.004, age-adjusted r = 0.59, p = 0.021) (Fig. 3). No other significant or trend-level correlations were observed between D2/3 receptor availability and other symptoms measured by the PANSS at the threshold of p < 0.005 (positive factor score: r = 0.42–0.62; negative factor score: r = 0.35–0.61; cognitive/disorganization factor score: r = 0.27–0.42; depression/anxiety factor score: r = 0.39–0.62) (Supplementary Table). No significant or trend-level correlations were found between the BPND values in the striatal regions and the PANSS factor scores (r = 0.31–0.61, p > 0.005) (Supplementary Table). There were no significant correlations between D2/3 receptor availability and CGI-S score (r = 0.18–0.42, p > 0.05). In addition, no significant correlations were found between D2/3 receptor availability and side effect scores (SAS: r = −0.13 to 0.30, p > 0.05; BARS: r = −0.10 to 0.09, p > 0.05).
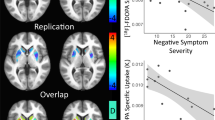
Excitement factor score had significant or trend-level positive correlations with the [18F]fallypride BPND in the right insula (r = 0.78, p = 0.0004) (a), left insula (r = 0.81, p = 0.0001) (b) and right amygdala (r = 0.68, p = 0.004) (c). The asterisk, solid line, and dotted line indicate the superposition of different subjects, regression line, and 95% confidence line, respectively. BP ND binding potential with respect to non-displaceable compartment
To confirm ROI-based findings, a voxel-based analysis was also conducted for the same data. The voxel-based analysis using SPM8 revealed a significant positive correlation between the PANSS excitement factor score and the [18F]fallypride BPND in the right insula (MNI coordinate: x = 38, y = 0, z = −2; cluster size: 96; z equivalent: 4.47, p < 0.0001 uncorrected) (Fig. 4), which did not survive FDR correction for multiple correlations (FDR-corrected p = 0.357).
Voxel-based analysis using SPM8 showed a significant positive correlation between the excitement factor score and the [18F]fallypride BPND in the right insula (MNI coordinate: x = 38, y = 0, z = −2; cluster size 96; z equivalent 4.47, p < 0.0001 uncorrected at a cluster level of 20 voxels). SPM8 statistical parameter mapping 8, BP ND binding potential with respect to non-displaceable compartment
Discussion
In the present study, we have quantitatively analyzed D2/3 receptor availability in extrastriatal as well as striatal regions using [18F]fallypride PET and investigated the relationship between the specific symptom severity and the D2/3 receptor availability in patients with schizophrenia. We found that the PANSS excitement factor score had significant positive correlations with the D2/3 receptor availability as measured by the [18F]fallypride BPND in the right and left insula. The voxel-based analysis confirmed a significant positive correlation between the PANSS excitement factor score and the [18F]fallypride BPND in the right insula. To our knowledge, this is the first report on the association of excitement symptom severity with dopamine D2/3 receptor availability in extrastriatal regions in clinically stable patients with schizophrenia receiving maintenance treatment.
A previous study using fluorodeoxyglucose (FDG) PET reported that patients with a history of violence showed decreased FDG uptake in the anterior inferior temporal regions [44]. Our high-resolution PET study extends the earlier investigation by showing that dopaminergic dysfunction within the limbic system, particularly the insula, underlies excitement symptoms. Our results are in line with previous suggestion that the neural circuit involving the insula and amygdala mediates impulsive aggression [45]. It has also been suggested that the insula and its associated neural circuit orchestrates necessary changes in behavioral response and that the functional integrity of the structures is associated with behavioral inhibition [45]. The insula is closely connected with the amygdala, hypothalamus, and periaqueductal gray matter, which together form a network that serves to appraise external stimuli and initiate autonomic responses [46]. In addition, the insula has been implicated as an important component of the neural circuits mediating impulsivity in schizophrenia [47]. A lack of cortico-subcortical limbic regulation may result in affective and behavioral dysregulation characterized by hostility, excitation, poor impulse control, and uncooperativeness.
The amygdala and insula were also suggested to be the main limbic regions of bottom-up drives associated with aggression [48]. Our results may support the notion that the imbalance between the top-down control system subserved by the frontal cortex and the bottom-up drives triggered by limbic regions including the insula and amygdala is implicated in the neurobiology of aggression in schizophrenia [48]. Coexisting cognitive impairments in patients with schizophrenia may further aggravate this imbalance [49]. The results of the present study along with previous findings on the significant relationship between striatal D2/3 binding and antipsychotic response suggest that dopaminergic dysfunction in extrastriatal as well as striatal regions may underlie multiple symptom domains of schizophrenia. The present study also extends the findings of earlier studies on the importance of altered extrastriatal dopaminergic neurotransmission in schizophrenia [50, 51]. Interestingly, the right insula was one of the regions with the largest effect size, where the mean BPND was 53.6% lower in patients on antipsychotic treatment than in healthy controls. The result may further emphasize the role of the insula in the pathophysiology and treatment of symptoms of schizophrenia. However, due to the absence of antipsychotic-free baseline data, it is difficult to interpret this finding in relation to the significant correlation with the PANSS excitement factor. Prospective PET scans before and after treatment with antipsychotics in antipsychotic-free patients, as well as analysis of the correlations between changes in symptom severity and changes in BPND values are required to better understand the role of the insula in schizophrenia. It is possible that different antipsychotics may have differential effects on the excitement symptom dimension.
In addition, the excitement symptom dimension is not exclusively found in schizophrenia, but is frequently observed in various psychiatric disorders including affective disorders, anxiety disorders, and personality disorders. The excitement symptom domain is significantly associated with affective symptoms and a disinhibition of motor and language domains [52]. Therefore, it may well be that dopamine D2/3 receptor availability in the insula and related limbic system may also contribute to excited or agitated behavior in healthy subjects or patients with non-psychotic psychiatric disorders, and may have no direct association with pathophysiology of schizophrenia. Future studies should employ various measures of excited behavior, impulsivity, and aggression in non-psychotic populations and in medication-naïve patients with schizophrenia to clarify the disease-specific, physiological and pharmacological causes of the relationship that was observed in the present study.
It should be noted that although previous studies have shown the utility of quantifying D2/3 receptor availability in patients receiving stable antipsychotic treatment in evaluating the relationship with clinical characteristics [53,54,55], the present study has the limitation of being a cross-sectional study without a drug-free time point. Therefore, we could not calculate D2/3 receptor occupancy by antipsychotics due to the absence of baseline (medication-free) PET data. The observed relationship between psychopathology and D2/3 receptor availability might have been influenced by the antipsychotics that patients were taking at the time of study enrollment. In our study, the daily chlorpromazine equivalent dose was not significantly correlated with the PANSS factor scores or the regional [18F]fallypride BPND values. However, the chlorpromazine equivalent doses do not adequately represent differential bindings of various antipsychotics to D2/3 receptors in extrastriatal and striatal regions. In addition, previous studies have shown that long-term antipsychotic treatment leads to differential changes in dopamine receptor availability depending on receptor subtypes, brain regions, and the types of antipsychotic medications [56, 57]. Moreover, the antipsychotics that patients were taking at the time of enrollment were not equal in terms of sedative effects, and excited patients may have received medications different from those that patients without excitement or agitation received. Therefore, the confounding effect of antipsychotic treatment can neither be corrected nor be ignored in the experimental design of the present study.
Previous in vivo SPECT study showed that the correlation between psychopathology and striatal dopamine parameters such as D2/3 receptor availability and dopamine transporter availability is different between drug-naïve patients and haloperidol-treated patients [58]. In addition, in the present study, the antipsychotics that patients were taking at the time of the study were heterogeneous. Previous studies have shown that D2/3 occupancy by aripiprazole is high in extrastriatal and striatal regions [13, 14] and that quetiapine yields a low occupancy and has a preferential binding to extrastriatal D2/3 receptors [17]. Therefore, we cannot rule out the possibility that the variation of BPND values might be ascribed to antipsychotic treatment rather than psychopathology itself. BPND represents a combined parameter of the concentration of receptors available to bind with radioligand and the affinity of the radioligand for the receptor. The higher D2/3 receptor availability could result from either an increase in D2/3 receptor density or greater affinity of the radiotracer for the D2/3 receptor [2]. Further study is warranted to clarify the relationship between specific symptom severity and extrastriatal D2/3 receptor availability in schizophrenia, such as longitudinal studies including drug-naïve or drug-free time points.
The results of our study are in line with those of the study by Mizrahi et al. [59], where a significant correlation was found between the extrastriatal D2/3 BPND measured with [11C]FLB 457 in the insula and the subjective well-being score in patients with schizophrenia after 2 weeks of continuous antipsychotic treatment. In the study by Mizrahi et al. [59], the higher the BPND value (i.e., lower occupancy by antipsychotics) in the insula, the higher the Subjective Well-Being Under Neuroleptics Scale score. Since the functional activity of the insula has been implicated in subjective awareness of inner feelings and emotionality [60], it may be speculated that the feeling of detachment from one’s emotions that could be induced by dopamine receptor blockade in the insula is a common cause of lower subjective well-being and reduced excitement scores in antipsychotic-treated patients with schizophrenia.
It should be noted that the [18F]fallypride scan duration in our study was shorter than that in previous studies, which might have led to an underestimation of BPND values. Although the optimal [18F]fallypride scan duration is a matter of debate, Vernaleken et al. [61] reported that [18F]fallypride scan durations of 180 min reliably reached equilibrium and that moderate reductions in scan durations only caused small changes to SRTM-derived BPND results even in D2/3 receptor-rich regions. Vernaleken et al. [61] suggested that 180-min duration is adequate for [18F]fallypride PET scanning.
In the present study, the mean BPND value of the left insula was substantially higher than that of the right insula. Previous studies have suggested the possibility of lateralization of the striatal dopaminergic system in the human brain [62]. A study using [18F]desmethoxyfallypride PET by Vernaleken et al. [63] revealed a significant rightward lateralization in D2/3 receptor availability in the caudate of healthy men, suggesting a functional asymmetry in the striatal dopamine neurotransmission system. However, the functional asymmetries of D2/3 receptors in extrastriatal regions are largely unknown, although asymmetrical involvement of mesolimbic dopaminergic neurons in affective-perceptual processes was suggested [64]. Therefore, further in vivo imaging studies with a large sample size using high-affinity ligands, e.g., [123I]epidepride, are required to examine possible asymmetries of D2/3 receptor availability in extrastriatal regions with low receptor density such as the limbic and cortical regions.
The strengths of the present study include the use of high-resolution PET imaging techniques. Compared with conventional scanners, the HRRT-PET system that was used in the present study has been reported to improve the quantification of monoamine neurotransmission parameters owing to reduced partial volume effects [65, 66].
The interpretation of the results of the present study should be considered in light of limitations. The relatively small sample size may not have provided adequate power to detect small-to-moderate effect sizes of the correlation coefficients. The symptom dimensions might be differentially affected by different antipsychotic drugs that were used in the present study. The cross-sectional measures of D2/3 receptor availability do not necessarily account for occupancy of the receptor by endogenous dopamine. The D2/3 receptors are configured in interconvertible states of high- or low-affinity for agonists [67], and antagonists, such as [18F]fallypride, bind with equal affinity to both states. In addition, most antipsychotic drugs exert their effects on D2 and D3 receptors. However, [18F]fallypride has nearly equal affinity for both D2 and D3 receptors [68, 69]. Therefore, further studies using both [18F]fallypride and an agonist radiotracer such as [11C]PHNO [70] are required to elucidate whether the relationship between specific symptom severity and dopamine receptor availability is selectively associated with the high- or low-affinity state of D2/3 receptors and whether the relationship is specifically related to D3 receptors.
In conclusion, the present study, which used high-resolution imaging techniques and [18F]fallypride, revealed a significant association between excitement symptom severity and D2/3 receptor availability in the insula in patients with schizophrenia. This suggests that D2/3 receptor-mediated neurotransmission in the insula and its related limbic system may play an important role in the pathophysiology of this specific cluster of symptoms. Further studies including drug-naïve or drug-free patients are warranted to confirm the relationship and to elucidate the extent to which antipsychotic treatment affects it.
References
Takahashi H, Higuchi M, Suhara T (2006) The role of extrastriatal dopamine D2 receptors in schizophrenia. Biol Psychiatry 59:919–928
Kim JH, Abi-Dargham A (2009) Psychiatric Disorders. In: Van Heertum RL, Tikofsky RS, Ichise M (eds) Functional cerebral SPECT and PET imaging, 4th edn. Lippincott Williams & Wilkins, Philadelphia, pp 187–200
Agid O, Mamo D, Ginovart N, Vitcu I, Wilson AA, Zipursky RB, Kapur S (2007) Striatal vs extrastriatal dopamine D2 receptors in antipsychotic response—a double-blind PET study in schizophrenia. Neuropsychopharmacology 32:1209–1215
Kessler RM, Woodward ND, Riccardi P, Li R, Ansari MS, Anderson S, Dawant B, Zald D, Meltzer HY (2009) Dopamine D2 receptor levels in striatum, thalamus, substantia nigra, limbic regions, and cortex in schizophrenic subjects. Biol Psychiatry 65:1024–1031
Slifstein M, Kegeles LS, Xu X, Thompson JL, Urban N, Castrillon J, Hackett E, Bae SA, Laruelle M, Abi-Dargham A (2010) Striatal and extrastriatal dopamine release measured with PET and [(18)F] fallypride. Synapse 64:350–362
Lehrer DS, Christian BT, Kirbas C, Chiang M, Sidhu S, Short H, Wang B, Shi B, Chu KW, Merrill B, Buchsbaum MS (2010) 18F-fallypride binding potential in patients with schizophrenia compared to healthy controls. Schizophr Res 122:43–52
Buchsbaum MS, Christian BT, Lehrer DS, Narayanan TK, Shi B, Mantil J, Kemether E, Oakes TR, Mukherjee J (2006) D2/D3 dopamine receptor binding with [F-18]fallypride in thalamus and cortex of patients with schizophrenia. Schizophr Res 85:232–244
Kegeles LS, Slifstein M, Xu X, Urban N, Thompson JL, Moadel T, Harkavy-Friedman JM, Gil R, Laruelle M, Abi-Dargham A (2010) Striatal and extrastriatal dopamine D2/D3 receptors in schizophrenia evaluated with [18F]fallypride positron emission tomography. Biol Psychiatry 68:634–641
Talvik M, Nordström AL, Olsson H, Halldin C, Farde L (2003) Decreased thalamic D2/D3 receptor binding in drug-naive patients with schizophrenia: a PET study with [11C]FLB 457. Int J Neuropsychopharmacol 6:361–370
Yasuno F, Suhara T, Okubo Y, Sudo Y, Inoue M, Ichimiya T, Takano A, Nakayama K, Halldin C, Farde L (2004) Low dopamine d(2) receptor binding in subregions of the thalamus in schizophrenia. Am J Psychiatry 161:1016–1022
Kambeitz J, Abi-Dargham A, Kapur S, Howes OD (2014) Alterations in cortical and extrastriatal subcortical dopamine function in schizophrenia: systematic review and meta-analysis of imaging studies. Br J Psychiatry 204:420–429
Gründer G, Landvogt C, Vernaleken I, Buchholz HG, Ondracek J, Siessmeier T, Härtter S, Schreckenberger M, Stoeter P, Hiemke C, Rösch F, Wong DF, Bartenstein P (2006) The striatal and extrastriatal D2/D3 receptor-binding profile of clozapine in patients with schizophrenia. Neuropsychopharmacology 31:1027–1035
Gründer G, Fellows C, Janouschek H, Veselinovic T, Boy C, Bröcheler A, Kirschbaum KM, Hellmann S, Spreckelmeyer KM, Hiemke C, Rösch F, Schaefer WM, Vernaleken I (2008) Brain and plasma pharmacokinetics of aripiprazole in patients with schizophrenia: an [18F]fallypride PET study. Am J Psychiatry 165:988–995
Kegeles LS, Slifstein M, Frankle WG, Xu X, Hackett E, Bae SA, Gonzales R, Kim JH, Alvarez B, Gil R, Laruelle M, Abi-Dargham A (2008) Dose-occupancy study of striatal and extrastriatal dopamine D2 receptors by aripiprazole in schizophrenia with PET and [18F]fallypride. Neuropsychopharmacology 33:3111–3125
Kessler RM, Ansari MS, Riccardi P, Li R, Jayathilake K, Dawant B, Meltzer HY (2005) Occupancy of striatal and extrastriatal dopamine D2/D3 receptors by olanzapine and haloperidol. Neuropsychopharmacology 30:2283–2289
Kessler RM, Ansari MS, Riccardi P, Li R, Jayathilake K, Dawant B, Meltzer HY (2006) Occupancy of striatal and extrastriatal dopamine D2 receptors by clozapine and quetiapine. Neuropsychopharmacology 31:1991–2001
Vernaleken I, Janouschek H, Raptis M, Hellmann S, Veselinovic T, Bröcheler A, Boy C, Cumming P, Hiemke C, Rösch F, Schäfer WM, Gründer G (2010) Dopamine D2/3 receptor occupancy by quetiapine in striatal and extrastriatal areas. Int J Neuropsychopharmacol 13:951–960
Abi-Dargham A, Gil R, Krystal J, Baldwin RM, Seibyl JP, Bowers M, van Dyck CH, Charney DS, Innis RB, Laruelle M (1998) Increased striatal dopamine transmission in schizophrenia: confirmation in a second cohort. Am J Psychiatry 155:761–767
Laruelle M, Abi-Dargham A, van Dyck CH, Gil R, D’Souza CD, Erdos J, McCance E, Rosenblatt W, Fingado C, Zoghbi SS, Baldwin RM, Seibyl JP, Krystal JH, Charney DS, Innis RB (1996) Single photon emission computerized tomography imaging of amphetamine-induced dopamine release in drug-free schizophrenic subjects. Proc Natl Acad Sci USA 93:9235–9240
Howes OD, Bose SK, Turkheimer F, Valli I, Egerton A, Valmaggia LR, Murray RM, McGuire P (2011) Dopamine synthesis capacity before onset of psychosis: a prospective [18F]-DOPA PET imaging study. Am J Psychiatry 168:1311–1317
Talvik M, Nordström AL, Okubo Y, Olsson H, Borg J, Halldin C, Farde L (2006) Dopamine D2 receptor binding in drug-naïve patients with schizophrenia examined with raclopride-C11 and positron emission tomography. Psychiatry Res 148:165–173
Glenthoj BY, Mackeprang T, Svarer C, Rasmussen H, Pinborg LH, Friberg L, Baaré W, Hemmingsen R, Videbaek C (2006) Frontal dopamine D(2/3) receptor binding in drug-naive first-episode schizophrenic patients correlates with positive psychotic symptoms and gender. Biol Psychiatry 60:621–629
Kim JH, Kim SY, Lee J, Oh KJ, Kim YB, Cho ZH (2012) Evaluation of the factor structure of symptoms in patients with schizophrenia. Psychiatry Res 197:285–289
Levine SZ, Rabinowitz J (2007) Revisiting the 5 dimensions of the Positive and Negative Syndrome scale. J Clin Psychopharmacol 27:431–436
Marder SR, Davis JM, Chouinard G (1997) The effects of risperidone on the five dimensions of schizophrenia derived by factor analysis: combined results of the North American trials. J Clin Psychiatry 58:538–546
American Psychiatric Association (1994) Diagnostic and statistical manual of mental disorders, 4th edn. American Psychiatric Press, Washington, DC
First MB, Spitzer RL, Gibbon M, Williams JBW (1996) Structured clinical interview for DSM-IV axis I disorders research version (SCID-I). New York State Psychiatric Institute Biometrics Research, New York
Gardner DM, Murphy AL, O’Donnell H, Centorrino F, Baldessarini RJ (2010) International consensus study of antipsychotic dosing. Am J Psychiatry 167:686–693
Kay SR, Fiszbein A, Opler LA (1987) The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull 13:261–276
Guy W (1976) ECDEU assessment manual for psychopharmacology. U.S. Department of Health, Education, and Welfare, Bethesda, MD
Simpson GM, Angus JW (1970) A rating scale for extrapyramidal side effects. Acta Psychiatr Scand Suppl 212:11–19
Barnes TR (1989) A rating scale for drug-induced akathisia. Br J Psychiatry 154:672–676
Cho ZH, Son YD, Kim HK, Kim KN, Oh SH, Han JY, Hong IK, Kim YB (2008) A fusion PET-MRI system with a high-resolution research tomograph-PET and ultra-high field 7.0 T-MRI for the molecular-genetic imaging of the brain. Proteomics 8:1302–1323
Wienhard K, Schmand M, Casey ME, Baker K, Bao J, Eriksson L, Jones WF, Knoess C, Lenox M, Lercher M, Luk P, Michel C, Reed JH, Richerzhagen N, Treffert J, Vollmar S, Young JW, Heiss WD, Nutt R (2002) The ECAT HRRT: performance and first clinical application of the new high resolution research tomography. IEEE Trans Nucl Sci 49:104–110
Mukherjee J, Yang ZY, Das MK, Brown T (1995) Fluorinated benzamide neuroleptics-III. Development of (S)-N-[(1-allyl-2-pyrrolidinyl)methyl]-5-(3-[18F]fluoropropyl)-2, 3-dimethoxybenzamide as an improved dopamine D-2 receptor tracer. Nucl Med Biol 22:283–296
Hong IK, Chung ST, Kim HK, Kim YB, Son YD, Cho ZH (2007) Ultra fast symmetry and SIMD-based projection-backprojection (SSP) algorithm for 3-D PET image reconstruction. IEEE Trans Med Imaging 26:789–803
Wu Y, Carson RE (2002) Noise reduction in the simplified reference tissue model for neuroreceptor functional imaging. J Cereb Blood Flow Metab 22:1440–1452
Hurley MJ, Mash DC, Jenner P (2003) Markers for dopaminergic neurotransmission in the cerebellum in normal individuals and patients with Parkinson’s disease examined by RT-PCR. Eur J Neurosci 18:2668–2672
Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M (2002) Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 15:273–289
Friston KJ, Holmes A, Poline JB, Price CJ, Frith CD (1996) Detecting activations in PET and fMRI: levels of inference and power. Neuroimage 4:223–235
Farrer C, Franck N, Georgieff N, Frith CD, Decety J, Jeannerod M (2003) Modulating the experience of agency: a positron emission tomography study. Neuroimage 18:324–333
Gaura V, Bachoud-Lévi AC, Ribeiro MJ, Nguyen JP, Frouin V, Baudic S, Brugières P, Mangin JF, Boissé MF, Palfi S, Cesaro P, Samson Y, Hantraye P, Peschanski M, Remy P (2004) Striatal neural grafting improves cortical metabolism in Huntington’s disease patients. Brain 127:65–72
Mikhno A, Devanand D, Pelton G, Cuasay K, Gunn R, Upton N, Lai RY, Libri V, Mann JJ, Parsey RV (2008) Voxel-based analysis of 11C-PIB scans for diagnosing Alzheimer’s disease. J Nucl Med 49:1262–1269
Wong MT, Fenwick PB, Lumsden J, Fenton GW, Maisey MN, Lewis P, Badawi R (1997) Positron emission tomography in male violent offenders with schizophrenia. Psychiatry Res 68:111–123
Blair RJ (2016) The neurobiology of impulsive aggression. J Child Adolesc Psychopharmacol 26:4–9
Williamson P, Allman J (2011) The human illnesses: neuropsychiatric disorders and the nature of the human brain. Oxford University Press, New York
Giakoumatos CI, Tandon N, Shah J, Mathew IT, Brady RO, Clementz BA, Pearlson GD, Thaker GK, Tamminga CA, Sweeney JA, Keshavan MS (2013) Are structural brain abnormalities associated with suicidal behavior in patients with psychotic disorders? J Psychiatr Res 47:1389–1395
Siever LJ (2008) Neurobiology of aggression and violence. Am J Psychiatry 165:429–442
Soyka M (2011) Neurobiology of aggression and violence in schizophrenia. Schizophr Bull 37:913–920
Hernaus D, Mehta MA (2016) Prefrontal cortex dopamine release measured in vivo with positron emission tomography: implications for the stimulant paradigm. Neuroimage 142:663–667
Slifstein M, van de Giessen E, Van Snellenberg J, Thompson JL, Narendran R, Gil R, Hackett E, Girgis R, Ojeil N, Moore H, D’Souza D, Malison RT, Huang Y, Lim K, Nabulsi N, Carson RE, Lieberman JA, Abi-Dargham A (2015) Deficits in prefrontal cortical and extrastriatal dopamine release in schizophrenia: a positron emission tomographic functional magnetic resonance imaging study. JAMA Psychiatry 72:316–324
Lang FU, Müller-Stierlin AS, Walther S, Stegmayer K, Becker T, Jäger M (2016) Dimensional approaches to schizophrenia: a comparison of the Bern Psychopathology scale and the five-factor model of the Positive and Negative Syndrome Scale. Psychiatry Res 239:284–290
Yang YK, Yeh TL, Chiu NT, Lee IH, Chen PS, Lee LC, Jeffries KJ (2004) Association between cognitive performance and striatal dopamine binding is higher in timing and motor tasks in patients with schizophrenia. Psychiatry Res 131:209–216
Heinz A, Knable MB, Coppola R, Gorey JG, Jones DW, Lee KS, Weinberger DR (1998) Psychomotor slowing, negative symptoms and dopamine receptor availability-an IBZM SPECT study in neuroleptic-treated and drug-free schizophrenic patients. Schizophr Res 31:19–26
Klemm E, Grünwald F, Kasper S, Menzel C, Broich K, Danos P, Reichmann K, Krappel C, Rieker O, Briele B, Hotze AL, Möller HJ, Biersack HJ (1996) [123I]IBZM SPECT for imaging of striatal D2 dopamine receptors in 56 schizophrenic patients taking various neuroleptics. Am J Psychiatry 153:183–190
Hurley MJ, Stubbs CM, Jenner P, Marsden CD (1996) Effect of chronic treatment with typical and atypical neuroleptics on the expression of dopamine D2 and D3 receptors in rat brain. Psychopharmacology 128:362–370
Hurley MJ, Stubbs CM, Jenner P, Marsden CD (1996) Dopamine D3 receptors are not involved in the induction of c-fos mRNA by neuroleptic drugs: comparison of the dopamine D3 receptor antagonist GR103691 with typical and atypical neuroleptics. Eur J Pharmacol 318:283–293
Schmitt GJ, Dresel S, Frodl T, la Fougère C, Boerner R, Hahn K, Möller HJ, Meisenzahl EM (2012) Dual-isotope SPECT imaging of striatal dopamine: a comparative study between never-treated and haloperidol-treated first-episode schizophrenic patients. Eur Arch Psychiatry Clin Neurosci 262:183–191
Mizrahi R, Rusjan P, Agid O, Graff A, Mamo DC, Zipursky RB, Kapur S (2007) Adverse subjective experience with antipsychotics and its relationship to striatal and extrastriatal D2 receptors: a PET study in schizophrenia. Am J Psychiatry 164:630–637
Craig AD (2004) Human feelings: why are some more aware than others? Trends Cogn Sci 8:239–241
Vernaleken I, Peters L, Raptis M, Lin R, Buchholz HG, Zhou Y, Winz O, Rösch F, Bartenstein P, Wong DF, Schäfer WM, Gründer G (2011) The applicability of SRTM in [(18)F]fallypride PET investigations: impact of scan durations. J Cereb Blood Flow Metab 31:1958–1966
Larisch R, Meyer W, Klimke A, Kehren F, Vosberg H, Müller-Gärtner HW (1998) Left-right asymmetry of striatal dopamine D2 receptors. Nucl Med Commun 19:781–787
Vernaleken I, Weibrich C, Siessmeier T, Buchholz HG, Rösch F, Heinz A, Cumming P, Stoeter P, Bartenstein P, Gründer G (2007) Asymmetry in dopamine D(2/3) receptors of caudate nucleus is lost with age. Neuroimage 34:870–878
Besson C, Louilot A (1995) Asymmetrical involvement of mesolimbic dopaminergic neurons in affective perception. Neuroscience 68:963–968
Leroy C, Comtat C, Trébossen R, Syrota A, Martinot JL, Ribeiro MJ (2007) Assessment of 11C-PE2I binding to the neuronal dopamine transporter in humans with the high-spatial-resolution PET scanner HRRT. J Nucl Med 48:538–546
Varrone A, Sjöholm N, Eriksson L, Gulyás B, Halldin C, Farde L (2009) Advancement in PET quantification using 3D-OP-OSEM point spread function reconstruction with the HRRT. Eur J Nucl Med Mol Imaging 36:1639–1650
Seeman P (2013) Schizophrenia and dopamine receptors. Eur Neuropsychopharmacol 23:999–1009
Slifstein M, Hwang DR, Huang Y, Guo N, Sudo Y, Narendran R, Talbot P, Laruelle M (2004) In vivo affinity of [18F]fallypride for striatal and extrastriatal dopamine D2 receptors in nonhuman primates. Psychopharmacology 175:274–286
Mukherjee J, Yang ZY, Brown T, Lew R, Wernick M, Ouyang X, Yasillo N, Chen CT, Mintzer R, Cooper M (1999) Preliminary assessment of extrastriatal dopamine D-2 receptor binding in the rodent and nonhuman primate brains using the high affinity radioligand, 18F-fallypride. Nucl Med Biol 26:519–527
Graff-Guerrero A, Mamo D, Shammi CM, Mizrahi R, Marcon H, Barsoum P, Rusjan P, Houle S, Wilson AA, Kapur S (2009) The effect of antipsychotics on the high-affinity state of D2 and D3 receptors: a positron emission tomography study with [11C]-(+)-PHNO. Arch Gen Psychiatry 66:606–615
Acknowledgements
This research was supported by the Brain Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and Future Planning (2016M3C7A1914451) and by a grant of the Korean Health Technology R&D Project, Ministry of Health and Welfare, Republic of Korea (number: HI14C2750). This research was also supported by the Gachon University Research Fund of 2015 (number: GCU-2015-5031).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Informed consent
The study protocol was approved by the institutional review board of the Gachon University Gil Medical Center, and all procedures used in the study were conducted in accordance with international ethical standard, Declaration of Helsinki. All participants gave their written informed consent prior to their inclusion in the study.
Conflict of interest
The authors declare that they have no conflicts of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Joo, YH., Kim, JH., Son, YD. et al. The relationship between excitement symptom severity and extrastriatal dopamine D2/3 receptor availability in patients with schizophrenia: a high-resolution PET study with [18F]fallypride. Eur Arch Psychiatry Clin Neurosci 268, 529–540 (2018). https://doi.org/10.1007/s00406-017-0821-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00406-017-0821-y