Abstract
The vast majority of smokers are unable or unwilling to quit, but many are open to reducing smoking. No treatment options exist for these smokers besides medication-based therapies. Thus, this study investigated the efficacy of a cognitive behavioral therapy (CBT) smoking reduction program, Smoke_less. A sample of 155 outpatient smokers aged 18–70 years was recruited at the Tobacco Dependence Outpatient Clinic of the Medical Center of the University of Munich, Germany, and randomly assigned to the experimental group (Smoke_less: four weekly CBT group sessions and two telephone calls over 5 weeks, n = 51), active comparator group (one 15-minute counseling session, n = 49), or waiting control group (no intervention during the study, n = 55). The primary endpoint was a ≥50% smoking reduction in the intention-to-treat group 1 week and 6 months after the intervention. We evaluated also abstinence rates at follow-up. Significantly more participants in the Smoke_less group had reduced smoking ≥50% compared to the waiting group at 1 week [OR 7.59 (2.59–22.19)] and 6 months [OR 5.00 (1.68–14.84)] and compared to the active comparison group at 1 week [OR 8.58 (2.67–27.31)] but not at 6 months [OR 1.73 (0.71–4.20)]. We found no significant effects on abstinence rates. The CBT smoking reduction program Smoke_less is effective for smoking reduction but is superior to brief counseling only in the short term. Further research is required to improve its efficacy in long-term smoking reduction to provide a valid, non-medication-based alternative to smokers unable or unwilling to quit.
Trial Registration
Clinicaltrials.gov Identifier: NCT02337400.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tobacco dependence is a worldwide problem that has severe negative consequences for individuals and society as a whole. In 2012, 31.1% of all men and 6.2% of all women were daily smokers [1], and in Germany 25.2% of people aged 15 years or above smoke [2]. Smoking is the single most preventable cause of death worldwide and is linked to approximately 6 million deaths per year [1]. Successful smoking cessation is very effective in reducing these risks and can greatly improve the quality of life and life expectancy. However, research shows that more than 90% of all smokers are not interested in quitting smoking in the near future [3]. Smoking reduction may represent an opportunity to broaden the options for smokers and, thus, provide treatment to a larger group of them. As a treatment for smokers unable or unwilling to quit, smoking reduction is seen as having two primary benefits: first, reduced cigarette consumption is accompanied by a reduced health risk (harm reduction); and second, smoking reduction can be an intermediate step towards quitting smoking. Glasgow et al. [4] estimate that an additional 22–39% of smokers could be reached by offering assistance in reducing the amount smoked. Thus, treatment options are needed for smokers unable or unwilling to quit smoking.
The strength of the health benefits of smoking reduction is currently a topic of controversial discussion [5, 6]. While there is a clear dose–response relationship between the amount smoked and disease risk for the three major smoking-associated diseases, ischemic heart disease, lung cancer, and chronic obstructive pulmonary disease (COPD) [7,8,9], evidence whether the opposite is true is inconsistent, i.e., whether reducing the number of cigarettes smoked also reduces the damage associated with smoking [6]. For example, a reduction in consumption of at least 50% was associated with a reduction of risk for lung cancer [10] and a decreased rate of developing cardiovascular diseases [11]. At the same time, no positive effects of smoking reduction on COPD, the birth weight of infants in smoking pregnant women, or postoperative complications are reported [10, 12, 13]. The results of studies are inconsistent also with regard to the effect of smoking reduction on premature mortality. For example, Gerber et al. [11] sum up the results of their studies in Israel by stating that a reduction in tobacco consumption decreases the premature mortality risk, whereas in a Scottish study Hart et al. [14] documented such a decrease only for the subsample of heavy smokers (>21 cigarettes/day).
The above findings indicate that the health benefits of smoking reduction might not be as great as we might assume on the basis of the known dose–response relationship [6]. Nevertheless, one can argue that any improvement in health is better than none at all, i.e., any reduction in smoking is better than none. In addition, studies have provided significant evidence that smoking reduction measures increase the likelihood of quitting smoking [12, 15]. Hughes and Carpenter [12] showed that even smokers who at first cannot imagine quitting smoking have a significantly higher cessation rate after smoking reduction than smokers who do not participate in reduction measures. These results were confirmed by a recent Cochrane review [16]. In a review on smokers not ready to attempt quitting, Asfar et al. [17] also reported a significantly higher abstinence rate both for pharmacological (nicotine replacement therapy, NRT) and combined (NRT + behavior therapy) interventions to reduce smoking. These reduction measures double the likelihood of quitting smoking [15]. This finding was confirmed by Moore and colleagues [18], who made the additional observation that NRT significantly promotes a 50% reduction rate. In addition to results concerning NRT, findings in smokers who do not currently want to stop smoking show that, compared to placebo, varenicline also significantly increases the number of attempts to stop, probability of abstinence, and the number of people who halve their consumption [19].
Besides the numerous studies on pharmacological (NRT, varenicline) and combined (NRT + behavior therapy or consultation) reduction measures (cf. [20]), however, research into effective, purely behavior-based interventions on smoking reduction is still rare [21]. The 6-month program for hospital outpatients “smoke less, live more,” which consists of 4 telephone consultations, individually tailored letters, and an additional information letter, had a positive effect on the abstinence rate, although the effect was not significant [22, 23]. Gelkopf et al. [24] studied patients with schizophrenia and compared a 5-week behavior therapy smoking reduction program (n = 35), which included one 1-h session per week, with a waiting list (n = 18). They found a significantly greater smoking reduction in the experimental group (p < 0.05), but their report does not provide any data on the abstinence rate in the study groups. The single-group study of the “oxygen group” program for schizophrenia patients (5 × 75 min smoking reduction consultations in a group and 3 × 90 min physical training over a period of 8 weeks) did not provide proof of efficacy [25]. Tang et al. [26] compared “Integrative Body-Mind Training,” a form of mindfulness meditation, and progressive muscle relaxation training in smoking and non-smoking healthy college students. After 10 daily, 30-min sessions, the smokers in the meditation group achieved a 60% reduction in smoking, but no reduction was found in the relaxation group. Against this background, further research is required into purely behavioral interventions for smoking reduction [20] to evaluate whether an effective behavioral intervention for smoking reduction could benefit the subgroup of those smokers who do not want to either quit smoking or receive drug treatment for smoking reduction. In accordance with international guidelines on the treatment of tobacco dependence [27,28,29,30], the Tobacco Dependence Outpatient Clinic at the Medical Center of the University of Munich developed Smoke_less, a scientifically founded, manualized cognitive-behavioral therapy group program for the reduction of tobacco consumption in outpatients. The randomized controlled trial (RCT) presented here aimed to evaluate whether Smoke_less is effective in reducing smoking. The primary hypothesis of our study was that the intervention would increase the reduction rate per se and also the rate of a ≥ 50% reduction in daily smoked cigarettes. Furthermore, we wanted to explore whether the cognitive behavioral smoking reduction program can affect abstinence rates and promote abstinence in smokers currently unable or unwilling to quit.
Methods
Study design
This randomized, three-armed, parallel group study was conducted at the Tobacco Dependence Outpatient Clinic of the Department of Psychiatry and Psychotherapy at the Medical Center of the University of Munich, Germany, from August 2014 to July 2016. The aim of the study was to investigate the efficacy of the cognitive behavioral smoking reduction program Smoke_less in reducing smoking in the short and long term by comparing it to an active comparator and a waiting control group. Data were collected at baseline (1 week before the intervention phase, t 0) and at follow-ups 1 week (t 1) and 3 (t 2) and 6 months (t 3) after the intervention phase.
The study was registered at clinicaltrials.gov (Identifier: NCT02337400) and approved by the ethics committee of the LMU Munich. It was performed in accordance with GCP-ICH and the Declaration of Helsinki.
Participants
Inclusion criteria were as follows: age 18–70 years; tobacco dependence according to the ICD-10 (F17.2) criteria; Fagerström Test of Nicotine Dependence (FTND) score >3 [31]; ≥10 cigarettes/day smoked in the last year; ≥8 ppm carbon monoxide (CO) in exhaled air, measured by a Mikro+ Smokerlyzer; unable or unwilling to stop smoking at the start of the study; agreement not to use e-cigarettes, smoke-free tobacco, or NRT or any other smoking cessation aid during the study; and written informed consent. Exclusion criteria were a medication-based quit attempt in the 3 months before the start of the study; having a legal guardian; pregnancy; any history of drug, medication, or alcohol abuse; severe medical, psychiatric, or neurological disorders; and consumption of psychotropic medication (e.g., bupropion) that could interfere with the study protocol. A clinically trained psychologist assessed inclusion and exclusion criteria at the baseline visit and also conducted the German version of the standardized, fully structured Mini International Neuropsychiatric Interview (M.I.N.I.) [32].
Participants were recruited via announcements in the local press and on the department homepage, posters in university buildings, and flyers in the university hospital and medical practices. Potential participants were screened for basic inclusion criteria and availability in a telephone interview or during information evenings before they were invited to the baseline visit. All participants received €100 financial compensation for taking part in the follow-up assessment which was paid by the Tobacco Dependence Outpatient Clinic.
A computer-generated list of random numbers was used to randomly assign participants in a 1:1:1 ratio to the experimental group (Smoke_less program, i.e., cognitive behavioral group therapy for smoking reduction, n = 51), active comparator group (standardized, brief intervention for smoking reduction consisting of one 15-min counseling session, n = 49), or waiting control group (no intervention during the intervention phase of the study, n = 55). Participants allocated to the active comparator and waiting control groups were given the opportunity to attend the Smoke_less smoking reduction program free of charge after the follow-up assessments were completed.
Interventions
Smoke_less smoking reduction program- experimental group
In the course of the 5-week intervention phase, the experimental group participated in the behavioral smoking reduction program Smoke_less. The program consisted of 2.5-h therapy groups once a week for 4 weeks and two individual 15-min telephone consultations in weeks 2 and 5 of the program. The two telephone consultations were conducted by the smoke_free trainer. The group program Smoke_less is a behavior therapy smoking reduction program that was developed in 2014 by one of the authors (KE) in collaboration with the Tobacco Dependence Outpatient Clinic at the Medical Center of the University of Munich (under the direction of TR) and with the director (CK) of the IFT (Institut für Therapieforschung) in Munich. In accordance with the recommendations of the German and international guidelines for the treatment of harmful and dependent tobacco consumption [27, 29, 30], the Smoke_less program applies the following established behavior therapy components: psychoeducation, motivation reinforcement, behavior observations, behavior experiments, problem-solving and skills training, establishment of alternative behavior, contingency management, and prevention of a relapse to the former amount of smoking. Details of the respective content and methods of the individual program sessions are provided in the Supplementary Material. During the study, participants in the Smoke_less program attended four group sessions, each with a mean of seven participants (range 5–9), that were conducted by 1 of 3 certified smoke-free trainers. Before the study, all smoke-free trainers were trained in administering the Smoke_less program.
Active control group
The active control group received one 15-min, standardized brief consultation on smoking reduction. The guide for the brief intervention included the following elements: a 3-min section with an introduction and questions on smoking history and current smoking behavior; a 5-min motivational discussion in which the participants were interviewed on their motivation and goals for smoking reduction; a psychoeducation section that presented the four reduction strategies “smoking according to a schedule,” “omitting superfluous cigarettes,” “extending smoke-free islands,” and “delaying the first cigarette;” and finally clear advice to reduce and ultimately quit smoking.
Waiting control group
Participants who were randomly assigned to the waiting control group did not receive any intervention or consultation during the study.
Clinical assessments and ratings
Sociodemographic data and medical history were recorded. Smoking history, habits, and status and number of cigarettes smoked daily were evaluated at baseline by the Nicotine Use Inventory [33], the carbon monoxide (CO) concentration in expired air (Micro Smokerlyzer (Bedfont Scientific Ltd., Maidstone, England), and the Fagerström Test of Nicotine Dependence (FTND) [31]. Smoking status, number of cigarettes smoked daily, and CO in expired air were re-evaluated at follow-up visits. The primary endpoints were the rate of smoking reduction and the rate of halving the amount smoked, and the secondary endpoint was the 7-day abstinence rate. This was measured with the questionnaire NUI (Nicotine-Use-Inventory) as described by Koegelenberg et al. [33].
We studied also participants’ readiness for change, self-efficacy, and level of knowledge and also the acceptance of the course manual among course instructors and participants. For reasons of space limitations, these findings will be published separately.
Participants of all three study groups answered always the same questionnaires.
Sample size
Sample size was calculated with G*Power [34]. On the basis of an average effect size of the intervention (f (V) = 0.25), an α-level of 5% (α = 0.05), and a power of 80% (1 − β = 0.80), we calculated a sample size of 159. Due to the exhausted resources, we were not able to run a further course so we ultimately recruited 155 participants for the study.
Statistical analysis
Data were analyzed according to the intention-to-treat (ITT) principle. Missing data were completely random (χ 2 = 2521.63, df = 2684, p = 0.988) and estimated by means of multiple imputation. Besides descriptive data analysis, the cross-sectional comparison of the nominal data between the study groups was performed by the χ 2 test. Metric data were analyzed cross sectionally by a one-way analysis of variance (ANOVA). To calculate longitudinal intervention effects, we applied a repeated measures ANOVA for metric variables (e.g., number of cigarettes/day) and a binary logistic regression model for dichotomous outcome variables (e.g., 50% smoking reduction). For all analyses, the alpha level was set at 0.05 (two tailed). Statistical analyses were performed with the Statistical Package for the Social Sciences (SPSS) Release 23.0 [35].
Results
Demographic data
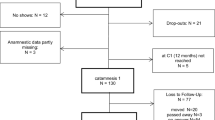
From December 2014 to July 2015, a total of 161 volunteers were screened; 155 people met all criteria and were randomized to one of the study arms. A total of 51 participants were randomized to the experimental group; 49, to the active control group; and 55, to the waiting control group. Participants attended a baseline visit; the respective interventions of their study group; and 3 follow-up visits 1 week (t 1), 3 months (t 2), and 6 months (t 3) after the intervention phase. The drop-out rate at t 3 was 7.7%. Figure 1 shows the CONSORT diagram of the study [36].
The majority of participants were women (61%) with a mean age of 51.87 years (SD 11.05). The mean duration of smoking was 31.14 years (SD 11.16), and the mean number of cigarettes smoked per day was 19.96 (SD 7.16). Table 1 shows the sociodemographic variables and smoking characteristics at baseline. No significant differences between the study groups were found at that time (p > 0.05).
Primary outcome measures: reduction rate and ≥50% reduction rate
Figure 2 shows the number of cigarettes smoked daily in the different study groups during the course of the study. Participants in the Smoke_less study group reduced their mean consumption from initially 19.51 cigarettes/day (SD 7.43) to 10.78 cigarettes/day (SD 5.13) 1 week after the end of the intervention phase (t 1), corresponding to a mean reduction rate of 44.69% (SD 16.20). In comparison, the active control group reduced its mean daily cigarette consumption from baseline to the first follow-up visit by 19.28% (SD 17.42); and the waiting control group, by 9.88% (SD 23.86). In both control groups, the reduction was significantly less than in the experimental group (t 1: F(2,151) = 42.57, p < 0.001; mean difference between experimental and active control group = 25.60, 95% CI (16.05, 35.15), p < 0.001; mean difference between experimental and waiting control group = 34.86, 95% CI (25.57, 44.14), p < 0.001).
Change in number of cigarettes smoked per day in the experimental group (Smoke_less program, n = 51), active control group (brief counseling, n = 49) and waiting control group (no intervention, n = 55) from baseline (t 0) to the follow-ups 1 week (t 1) and 3 (t 2) and 6 (t 3) months after the intervention phase
Smoking rates were different at the 6-month follow-up, t 3: the mean reduction was 34.53% (SD 26.06) in the experimental group, 25.14% (SD 26.41) in the active comparator group, and 13.02% (SD 26.42) in the waiting control group. The change differed significantly between the experimental and waiting control groups [t 3: F(2,151) = 8.99, p < 0.001; mean difference = 21.63, 95% CI (9.23, 34.02), p < 0.001] but not between the experimental and active control groups [mean difference = 9.76, 95% CI (−2.99, 22.52), p = 0.197] and active control and waiting control groups [mean difference = 12.07, 95% CI (−0.39, 24.53), p = 0.061].
Results were similar for the stricter primary outcome variable of ≥50% reduction in cigarette consumption. At t 1, more participants in the Smoke_less group had reduced the number of cigarettes smoked daily by at least half than in the active comparator group [odds ratio (OR) 8.58, 95% CI (2.67–27.31), p < 0.001) and in the waiting control group [OR 7.59, (2.59–22.19), p < 0.001]. At the 3- (t2) and 6-month (t 3) follow-ups, the difference remained significant compared to the waiting control group but not compared to the active comparator group, although it was still numerically higher. Figure 3 shows the results in detail.
Rates of at least 50% reduction of daily smoked cigarettes in the experimental group (Smoke_less program, n = 51), active control group (brief counseling, n = 49), and waiting control group (no intervention, n = 55) from baseline (t 0) to the follow-ups 1 week (t 1) and 3 (t 2) and 6 (t 3) months after the intervention phase; OR odds ratio, 95% CI 95% confidence interval
Secondary outcome measures: smoking cessation rate
At (t 2) two participants of the waiting control group and one of each of the active control group and the experimental group had stopped smoking. At (t 3), two participants in the experimental group and one in the waiting control group had stopped smoking (difference not significant).
Discussion
This study provides first evidence in a randomized controlled trial design for superior efficacy of a cognitive behavioral smoking reduction program that is in full accordance with specific guideline recommendations in the field of treating tobacco consumption. The Smoke_less cognitive behavioral smoking reduction program showed superior efficacy to a waiting control group in all primary outcome variables, i.e., in the rate of smoking reduction per se and the rate of a ≥50% reduction after 1 week and 3 and 6 months. Compared to the active control group, the Smoke_less program showed superior efficacy after 1 week; at the 3- and 6-month follow-ups the difference between the two groups was no longer significant, although the rates of smoking reduction and a reduction of smoking by ≥50% still were numerically higher in the experimental group. When considering the OR [1.73, (0.71–4.20), p = 0.228] in terms of effect size, according to the convention of Cohen, the Smoke_less program shows a noteworthy small effect of d = 0.30 after 6 months [37]. Abstinence rates were not higher in the smoking reduction program group.
A potential explanation why the smoking reduction rates in the experimental group were no longer significantly higher than those in the active control group at the 3- and 6-month follow-ups may be that the sample size was too small. The sample size was chosen to demonstrate a potential mean intervention effect, and a larger sample size calculated to detect smaller intervention effects might demonstrate superiority of the experimental intervention compared to the active control group also at the 3- and 6-month follow-ups. The frequently proven efficacy of brief interventions in smoking cessation is also relevant here [38, 39] because it means that the newly conceptualized Smoke_less intervention had to have even stronger effects to show significant superiority over the brief intervention active control.
We can see a decrease of tobacco consumption in all three study groups. Even in the waiting control group that did not receive any intervention, we found a reduction rate of 13%. This implicates that not only tailored interventions (in this case the “smoke_less” programme or a brief intervention) do have any effect on the smoking behavior, but also the simple act of participating in a study and thereby taking a conscious look at one’s cigarette consumption [40, 41].
Contrary to previous studies on purely drug-based (NRT, varenicline) or combined smoking reduction interventions (NRT and behavioral intervention), our study found no effect of the program on smoking abstinence [12, 16, 17, 19, 20]. This is in line with the findings for other behavioral smoking reduction interventions [22, 24,25,26] and leads us to the hypothesis that purely behavioral therapy programs can primarily lead to abstinence only if the participating smokers can conceive from the start that they will quit smoking. Drug treatment options may also help smokers to quit who do not initially want to do so by reducing craving and withdrawal symptoms. Wu and colleagues even express the assumption that behavioral support of drug treatment is not absolutely necessary [20]. Similarly, the duration of the follow-up period is a potential reason for a lack of an intervention effect on the abstinence rate among the participants. Although the follow-up of 6 months is significantly longer than that of other studies on behavioral reduction interventions [24,25,26], the data of Falba et al. [42], Broms et al. [43], and Klemperer and colleagues [44] indicate that a behavior change from smoking reduction to quitting smoking could take longer than 6 months.
With regard to efficacy in terms of consumption reduction, our findings are in line with those from previous studies in other countries in which behavioral interventions proved to be effective in promoting smoking reduction [24,25,26]. The present results add substantially to those of previous studies by evaluating the efficacy of a behavioral smoking reduction intervention with a rigorous experimental design and examining the target group of outpatient smokers. Nevertheless, several limitations have to be taken into account. First, we included outpatient smokers without acute or severe medical, psychiatric, or neurological disorders. Thus, our results might not generalize to patients with acute comorbid severe medical, psychiatric, or neurological illness. Second, the inclusion criteria limited participants to those with a moderate nicotine dependence who were smoking ten or more cigarettes a day. Thus, the findings of the present study might not generalize to less severely dependent or occasional smokers. Third, the 24-week post-intervention phase of the study with its three follow-up study visits might not mirror a real-world smoking reduction attempt.
With these limitations in mind, the study has practical implications in that the cognitive behavioral smoking reduction program Smoke_less can be a valuable alternative intervention for smokers who are neither willing to quit nor ready to use medication-based reduction interventions.
The findings of our study indicate ways to improve the Smoke_less smoking reduction program. Our first suggestion would be to present smoking reduction less as an endpoint of the intervention and rather as a step towards smoking cessation and to integrate medical advice on stopping smoking and promote smoking cessation more strongly at the end of the program. Second, booster sessions 4 and 8 weeks after the last session could help to better maintain the excellent short-term intervention effects in the longer term. And third, in light of the consistent positive effects of medication-based interventions in smoking reduction, a combination of first-line medication (i.e., NRT, varenicline) with the Smoke_less reduction program could improve long-term reduction and abstinence rates. Further studies should take these aspects into account and also include a larger number of participants and a longer follow-up period to further evaluate this approach in treating tobacco dependence.
References
WHO (2014) Global status report on non-communicable dieseases 2014. http://apps.who.int/iris/bitstream/10665/148114/1/9789241564854_eng.pdf?ua=1. Accessed 21 Nov 2016
Statistisches Bundesamt (2013) Mikrozensus - Fragen zur Gesundheit - Rauchgewohnheiten der Bevölkerung 2013. Im Internet: https://www.destatis.de/DE/Publikationen/Thematisch/Gesundheit/Gesundheitszustand/Rauchgewohnheiten5239004139004.pdf?__blob=publicationFile. Accessed 19 Nov, 2016
Wewers ME, Stillman FA, Hartman AM, Shopland DR (2003) Distribution of daily smokers by stage of change: Current Population Survey results. Prev Med 36:710–720
Glasgow RE, Gaglio B, France EK, Marcus A, Riley KM, Levinson A, Bischoff K (2006) Do behavioral smoking reduction approaches reach more or different smokers? Two studies; similar answers. Addict Behav 31:509–518. doi:10.1016/j.addbeh.2005.05.039
Rüther T, Eberhardt K, Kiss A, Pogarell O (2014) Reduziertes Rauchen: Was können Interventionen erreichen und wie sollten sie gestaltet sein? Suchttherapie 15:179–186. doi:10.1055/s-0034-1390490
Begh R, Lindson-Hawley N, Aveyard P (2015) Does reduced smoking if you can’t stop make any difference? BMC Med 13:1–5. doi:10.1186/s12916-015-0505-2
Lee PN, Forey BA, Coombs KJ (2012) Systematic review with meta-analysis of the epidemiological evidence in the 1900s relating smoking to lung cancer. BMC Cancer 12:385. doi:10.1186/1471-2407-12-385
Forey BA, Thornton AJ, Lee PN (2011) Systematic review with meta-analysis of the epidemiological evidence relating smoking to COPD, chronic bronchitis and emphysema. BMC Pulm Med 11:36. doi:10.1186/1471-2466-11-36
Keil U, Liese AD, Hense HW, Filipiak B, Doring A, Stieber J, Lowel H (1998) Classical risk factors and their impact on incident non-fatal and fatal myocardial infarction and all-cause mortality in southern Germany. Results from the MONICA Augsburg cohort study 1984-1992. Monitoring trends and determinants in cardiovascular diseases. Eur Heart J 19:1197–1207
Pisinger C, Godtfredsen NS (2007) Is there a health benefit of reduced tobacco consumption? A systematic review. Nicotine Tob Res 9:631–646. doi:10.1080/14622200701365327
Gerber Y, Myers V, Goldbourt U (2012) Smoking reduction at midlife and lifetime mortality risk in men: a prospective cohort study. Am J Epidemiol 175:1006–1012. doi:10.1093/aje/kwr466
Hughes JR, Carpenter MJ (2006) Does smoking reduction increase future cessation and decrease disease risk? A qualitative review. Nicotine Tob Res 8:739–749. doi:10.1080/14622200600789726
Tverdal A, Bjartveit K (2006) Health consequences of reduced daily cigarette consumption. Tob Control 15:472–480. doi:10.1136/tc.2006.016246
Hart C, Gruer L, Bauld L (2013) Does smoking reduction in midlife reduce mortality risk? Results of 2 long-term prospective cohort studies of men and women in Scotland. Am J Epidemiol 178:770–779. doi:10.1093/aje/kwt038
Stead LF, Lancaster T (2007) Interventions to reduce harm from continued tobacco use. Cochrane Database Syst Rev. doi:10.1002/14651858.CD005231.pub2
Lindson-Hawley N, Hartmann-Boyce J, Fanshawe TR, Begh R, Farley A, Lancaster T (2016) Interventions to reduce harm from continued tobacco use. Cochrane Database Syst Rev 10:CD005231. doi:10.1002/14651858.CD005231.pub3
Asfar T, Ebbert JO, Klesges RC, Relyea GE (2011) Do smoking reduction interventions promote cessation in smokers not ready to quit? Addict Behav 36:764–768. doi:10.1016/j.addbeh.2011.02.003
Moore D, Aveyard P, Connock M, Wang D, Fry-Smith A, Barton P (2009) Effectiveness and safety of nicotine replacement therapy assisted reduction to stop smoking: systematic review and meta-analysis. BMJ 338:b1024. doi:10.1136/bmj.b1024
Hughes JR, Rennard SI, Fingar JR, Talbot SK, Callas PW, Fagerstrom KO (2011) Efficacy of varenicline to prompt quit attempts in smokers not currently trying to quit: a randomized placebo-controlled trial. Nicotine Tob Res 13:955–964. doi:10.1093/ntr/ntr103
Wu L, Sun S, He Y, Zeng J (2015) Effect of smoking reduction therapy on smoking cessation for smokers without an intention to quit: an updated systematic review and meta-analysis of randomized controlled. Int J Environ Res Public Health 12:10235–10253. doi:10.3390/ijerph120910235
Thorndike FP, Friedman-Wheeler DG, Haaga DAF (2006) Effect of cognitive behavior therapy on smokers’ compensatory coping skills. Addict Behav 31:1705–1710. doi:10.1016/j.addbeh.2005.12.005
Glasgow RE, Gaglio B, Estabrooks PA, Marcus AC, Ritzwoller DP, Smith TL, Levinson AH, Sukhanova A, O’Donnell C, Ferro EF, France EK (2009) Long-term results of a smoking reduction program. Med Care 47:115–120. doi:10.1097/MLR.0b013e31817e18d1
Estabrooks PA, Gaglio B, Morse EF, Smith T, Edwards A, Glasgow RE (2010) Defining and understanding success at smoking reduction: a mixed-methods study. Addict Behav 35:1113–1119. doi:10.1016/j.addbeh.2010.08.006
Gelkopf M, Noam S, Rudinski D, Lerner A, Behrbalk P, Bleich A, Melamed Y (2012) Nonmedication smoking reduction program for inpatients with chronic schizophrenia: a randomized control design study. J Nerv Ment Dis 200:142–146. doi:10.1097/NMD.0b013e3182438e92
Bernard PP, Esseul EC, Raymond L, Dandonneau L, Xambo JJ, Carayol MS, Ninot GJ (2013) Counseling and exercise intervention for smoking reduction in patients with schizophrenia: a feasibility study. Arch Psychiatr Nurs 27:23–31. doi:10.1016/j.apnu.2012.07.001
Tang YY, Tang R, Posner MI (2013) Brief meditation training induces smoking reduction. Proc Natl Acad Sci USA 110:13971–13975. doi:10.1073/pnas.1311887110
West R, McNeill A, Raw M (2000) Smoking cessation guidelines for health professionals: an update. Thorax 55:987–999
Fiore M, Jaen CR, Baker T, Bailey W, Benowitz N, Curry Se, emsp14, al, Dorfman S, Froelicher E, Goldstein M, Healton C (2008) Treating tobacco use and dependence: 2008 update. US Department of Health and Human Services, Rockville, MD
Fiore MC, Bailey WC, Cohen SJ, Dorfman SF, Goldstein MG, Gritz ER, Heyman RB, Jaén CR, Kottke TE, Lando HA, Mecklenburg RE, Mullen PD, Nett LM, Robinson L, Stitzer ML, Tommasello AC, Villejo L, Wewers ME (2008) Treating tobacco use and dependence. A clinical practice guideline. U.S. Department of Health and Human Services, Rockville, MD
Dt. Ges. f. Suchtforschung und Suchttherapie DGfPPuN (ed) (2004) Tabakbedingte Störungen: „Leitlinie Tabakentwöhnung“, Leitlinien der Dt. Ges. f. Suchtforschung und Suchttherapie (DG-Sucht) und der Dt. Ges. f. Psychiatrie, Psychotherapie und Nervenheilkunde (DGPPN)
Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO (1991) The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict 86:1119–1127
Ackenheil M, Stotz-Ingenlath G, Dietz-Bauer R (1999) M.I.N.I. Mini International Neuropsychiatric Interview. German Version 5.0.0. DSM-IV. In, München
Koegelenberg CFN, Noor F, Bateman ED, Zyl-Smit RNV, Bruning A, O’Brien JA, Smith C, Abdool-Gaffar MS, Emanuel S, Esterhuizen TM, Irusen EM (2014) Efficacy of varenicline combined with nicotine replacement therapy vs varenicline alone for smoking cessation: a randomized clinical trial. JAMA 312:155–161. doi:10.1001/jama.2014.7195
Faul F, Erdfelder E, Lang AG, Buchner A (2007) G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 39:175–191
IBM (2015) IBM SPSS Statistics. Version 23, Armonk, NY
Moher D, Hopewell S, Schulz KF, Montori V, Gøtzsche PC, Devereaux PJ, Elbourne D, Egger M, Altman DG (2010) CONSORT 2010 Explanation and Elaboration: updated guidelines for reporting parallel group randomised trials. BMJ. doi:10.1136/bmj.c869
Cohen A (1988) Statistical power analysis for the behavioral sciences. Erlbaum, Hillsdale
Batra A, Mühlig S, Kröger C, Ratje U, Andreas S, Rüther T, Schweizer C, Thürauf N, Ulbricht S, Bartsch G, Lindinger P, Jähne A, Gohlke H, Effertz T, Pötschke-Langer M, Petersen KU, Neumann T (2016) S3-Leitlinie Screening, Diagnostik und Behandlung des schädlichen und abhängigen Tabakkonsums. http://www.dg-sucht.de/fileadmin/user_upload/pdf/leitlilnien/AWMF_76-006_S3-Leitlinie_Tabak.pdf. Accessed 21 Nov 2016
Stead LF, Bergson G, Lancaster T (2008) Physician advice for smoking cessation. Cochrane Database Syst Rev. doi:10.1002/14651858.CD000165.pub3
Roethlisberger FJ, Dickson WJ (2003) Management and the worker. Psychology Press, New York
Grawe K (1995) Changes in psychotherapy. Discussion comments on the contribution by Ulrike Hoffmann-Richter. Psychiatr Prax 22:37
Falba T, Jofre-Bonet M, Busch S, Duchovny N, Sindelar J (2004) Reduction of quantity smoked predicts future cessation among older smokers. Addiction 99:93–102. doi:10.1111/j.1360-0443.2004.00574.x
Broms U, Korhonen T, Kaprio J (2008) Smoking reduction predicts cessation: longitudinal evidence from the Finnish adult twin cohort. Nicotine Tob Res 10:423–427. doi:10.1080/14622200801888988
Klemperer EM, Hughes JR, Solomon LJ, Callas PW, Fingar JR (2016) Motivational, reduction, and usual care interventions for smokers who are not ready to quit: a randomized controlled trial. Addiction. doi:10.1111/add.13594
Acknowledgements
The authors thank Jacquie Klesing, Board-certified Editor in the Life Sciences (ELS), for editing assistance with the manuscript and Melanie Düerkop, and Marija Miros, (Tobacco Dependence Outpatient Clinic of the Medical Center of the University of Munich) for their help during the recruitment phase of the study.
Funding
The authors have no support or funding to report.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
T Rüther has been a consultant for, received grant/research support and honoraria from and been a speaker for or on the advisory board of Johnson & Johnson, and Pfizer. O. Pogarell has been on the advisory board of Lundbeck and received speaker’s honoraria from Lundbeck, Desitin, and Otsuka. A. Kiss, K. Eberhardt, A. Linhardt and C. Kröger declare no conflicts of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Rüther, T., Kiss, A., Eberhardt, K. et al. Evaluation of the cognitive behavioral smoking reduction program “Smoke_less”: a randomized controlled trial. Eur Arch Psychiatry Clin Neurosci 268, 269–277 (2018). https://doi.org/10.1007/s00406-017-0818-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00406-017-0818-6