Abstract
Oxidative stress and immune dysregulation have been linked to schizophrenia and depression. However, it is unknown whether these factors are related to the pathophysiology or whether they are an epiphenomenon. Inconsistent oxidative stress-related findings in previous studies may have resulted from the use of different biomarkers which show disparate aspects of oxidative stress. Additionally, disease severity, medication, smoking, endocrine stress axis activation and obesity are potential confounders. In order to address some of these shortcomings, we have analyzed a broader set of oxidative stress biomarkers in our exploratory study, including urinary 8-iso-prostaglandin F2α (8-iso-PGF2α), 8-OH-2-deoyxguanosine (8-OH-2-dG), and blood levels of malondialdehyde (MDA), superoxide dismutase (SOD) and glutathione S-transferase (GST) in acutely ill drug-naïve first episode patients with schizophrenia (n = 22), major depression (n = 18), and controls (n = 43). Possible confounding factors were considered, and patients were followed-up after 6 weeks of treatment. No differences were observed regarding 8-OH-2-dG, MDA and GST. At baseline, 8-iso-PGF2α levels were higher in patients with schizophrenia (p = 0.004) and major depression (p = 0.037), with a trend toward higher SOD concentrations in schizophrenia (p = 0.053). After treatment, schizophrenia patients showed a further increase in 8-iso-PGF2α (p = 0.016). These results were not related to age, sex, disease severity, medication or adipose tissue mass. However, 8-iso-PGF2α was associated with smoking, endocrine stress axis activation, C-reactive protein levels and low plasma concentrations of brain-derived neurotrophic factor. This study suggests a role of lipid peroxidation particularly in drug-naïve acutely ill schizophrenia patients and highlights the importance of taking into account other confounding factors in biomarker studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The role of immunological factors in the pathophysiology of severe psychiatric diseases is still a matter of dispute. Increased blood levels of proinflammatory factors, such as C-reactive protein (CRP), have been observed in psychiatric disorders like schizophrenia and depression [17, 64]. Moreover, increased matrix metalloproteinase-9 (MMP-9) blood concentrations have been described [10, 53]. MMP-9 may provide a link between immune and neurotransmitter hypotheses of these major psychiatric disorders because of its involvement in the regulation of blood–brain-barrier integrity, synaptic plasticity and neuronal apoptosis [48, 63].
Scientific evidence for increased oxidative stress parameters would provide further support for the immune hypothesis of schizophrenia and depression due to the connection between inflammation and reactive oxygen species (ROS) production [49]. However, recent meta-analyses reported a considerable heterogeneity of results [8, 18, 38, 46]. The inconsistency of these findings may have been caused by technical difficulties to measure fleeting oxidative stress-related substances or by confounding factors such as long-term drug treatment, life style habits (e.g., smoking), endocrine stress axis activation, metabolic changes like obesity and severity of symptoms.
It is important to keep in mind that appropriate biomarkers should be employed to investigate specific aspects of oxidative stress [19, 30, 54]. Thus, 8-OH-2-deoyxguanosine (8-OH-2-dG) could be used as readout of oxidative damage to DNA, F2-isoprostanes (e.g., 8-iso-prostaglandin F2α/8-iso-PGF2α) could be used as a measure of arachidonic acid derivatization, and malondialdehyde (MDA) could be used as a biomarker of chemical modification of proteins subsequent to lipid oxidation [19, 30, 36, 54]. The activity of the antioxidant defense system can be estimated using enzymatic factors, such as superoxide dismutase (SOD), glutathione S-transferase (GST), glutathione peroxidase, glutathione reductase or non-enzymatic plasma antioxidants (albumin, bilirubin, ferritin, transferrin and uric acid) [30]. Therefore, broad analytical assessments such as determining the stable end-products of the generation of ROS and investigating different systems of oxidative damage and defense appears to be the best approach for oxidative stress determinations. For example, 8-iso-PGF2α, MDA and 8-OH-2-dG have been suggested as useful and stabile biomarkers in sleep apnea syndrome, a human disease model for studying the generation of ROS [30]. In addition, the impact of oxidative stress on the brain may be estimated by brain-derived neurotrophic factor (BDNF) or MMP-9 since these have been associated with synaptic plasticity [10, 15, 16]. Notably, these proteins have additional sources and functions. For example, approximately 75% of the circulating BDNF originates from the brain and the remainder arises from peripheral sources, including vascular endothelial cells, megakaryocytic cells and platelets [50]. Furthermore, MMP-9 blood levels have also been considered as a peripheral biomarker of inflammation and may be increased in cardiac, liver and pulmonary fibrosis [23, 29].
In our exploratory study, we have analyzed a broad set of several oxidative stress parameters in urine, plasma and serum from acutely ill drug-naïve first episode patients with schizophrenia or major depression. The biomarker set comprised stable analytes such as 8-iso-PGF2α, 8-OH-2-dG and MDA in addition to the antioxidant enzymes SOD and GST. Our main objective was to investigate whether or not these factors were present at different concentrations in acute first onset patients with schizophrenia or depression, compared to controls. In addition, the same parameters were also assessed after 6 weeks of follow-up to determine any effects of treatment. Finally, we tested the influence of several factors on oxidative stress-related parameters, such as age, sex, the severity of psychopathologic symptoms, medication, smoking, occasional use of cannabis, endocrine stress axis activation, adipose tissue mass and the levels of surrogate markers of inflammation and neuronal plasticity.
Materials and methods
Study design
The study was performed in accordance with German laws, the Declaration of Helsinki, and the guidelines of the local institutional review board. Written informed consent was obtained from all participants. Drug-naïve first episode patients were recruited at Magdeburg’s University hospital and service for psychiatry and psychotherapy and the psychiatric service of the city hospital Magdeburg by a trained study team responsible for standardized diagnostic assessments, psychometric ratings (psychologists: A. Dudeck and J. Schadow) and sample collection (study nurse: G. Meyer-Lotz). This team was supervised by J. Steiner and W. Jordan.
Parameters of oxidative stress were determined on hospital admission (time point T0) in acutely ill drug-naïve first episode patients with schizophrenia (n = 22), major depression (n = 18) and controls (n = 43) (see Table 1). Blood samples from acute disease phases were obtained within 24 h after admission in this naturalistic study. All acutely ill participants were treated as inpatients after admission and underwent therapeutic drug monitoring. Patients were followed-up after 6 weeks of inpatient treatment (time point T6). Patients with schizophrenia were started on the atypical antipsychotics olanzapine (n = 7), quetiapine (n = 7) or risperidone (n = 8). Depressed patients received mirtazapine (n = 10) or venlafaxine (n = 8). Only benzodiazepines were allowed as additional psychotropic medication (for ≤6 days). We had access to detailed clinical files, including the medical histories by proxy and referral letters from the general practitioners. Psychopathology was monitored using the Positive and Negative Syndrome Scale (PANSS) and the Hamilton Depression Scale (HAMD-21). Cumulative chlorpromazine and amitriptyline equivalent dosages were calculated for the treatment period of six weeks [3, 29, 51, 67]. A complete differential blood cell count, measures of CRP, creatinine, glucose, lipids, liver enzymes and a urine drug screen were done for all subjects. Exclusion criteria consisted of the presence of immune diseases, immunomodulating treatment, cancer, chronic terminal disease, cardiovascular disorders, diabetes or severe trauma. Controls were recruited from blood donors at the Institute of Transfusion Medicine at the University of Magdeburg and from students and staff members of the University of Magdeburg. We aimed to match these samples for age, sex, smoking and cannabis use. This was achieved except for the case of matching smoking habits. Controls were screened for personal or family history of neuropsychiatric disorders using the Mini-International Neuropsychiatric Interview [58].
Before definite inclusion of the blood samples into our scientific blood bank, as suggested by the diagnostic guidelines of the German Psychiatric Association [22], psychosis or depression resulting from other medical conditions and substance-induced psychosis were excluded by a thorough physical examination, routine blood analysis (including differential blood cell count, kidney function tests, and levels of C-reactive protein, glucose, lipids, liver enzymes and thyroid hormones), screening for illegal drugs and magnetic resonance imaging of the brain [2]. In addition, electroencephalography (EEG) was performed. The same tests were carried out in the control participants.
Morning urine was sampled, acidified between pH 2 and 4 and a 15 mL aliquot stored at −80 °C. Blood samples were taken from fasting subjects between 8:00 and 10:00 a.m. and collected into BD Vacutainer™ plasma and serum tubes (Becton–Dickinson, Heidelberg, Germany). Plasma tubes were centrifuged immediately at 1000 g for 10 min. The resulting supernatants were stored in Protein Low Binding microcentrifuge tubes (Eppendorf, Hamburg, Germany) at −80 °C. Serum tubes were left at room temperature for 2 h for blood coagulation and then processed as above for plasma.
Biochemical analyses
Commercially available assays were used for the measurement of most parameters according to the manufacturers’ instructions.
Urinary 8-iso-PGF2α was purified by immunoaffinity chromatography as described previously [62] and the concentration measured by gas chromatography–mass spectrometry (GC–MS). Briefly, urine samples were thawed and the labeled internal standard 2H4-8-iso-PGF2α added at a concentration of 1 ng/mL. Thereafter, the samples were run through immunoaffinity columns (Cayman Chemicals, Ann Arbor, Michigan, USA) and derivatized as described previously to obtain the pentafluorobenzyl ester and trimethylsilyl ether derivatives [56]. Detection of endogenous 8-iso-PGF2α targeted a mass-to-charge (m/z) ratio of 569.4 and the internal standard 2H4-8-iso-PGF2α was read at 573.4 m/z. The final results were expressed as pg of 8-iso-PGF2α per mg urinary creatinine. Quantitation of 8-OH-2-dG was done using an immunoassay kit from the Japan Institute for the Control of Aging (JaICA, Fukuroi, Shizuoka, Japan). The final results were calculated as ng of 8-OH-2-dG per mg creatinine. Urinary levels of metanephrine and normetanephrine were measured simultaneously by a commercially available high performance liquid chromatography (HPLC) method with electrochemical detection (Chromsystems Instruments & Chemicals GmbH, Munich, Germany). An internal standard (3-hydroxy-2-methylbutanoic acid) was used as a comparison to estimate both analyte concentrations. The limits of quantification for metanephrine and normetanephrine were 10 and 30 µg/L, respectively. The intra-assay coefficients of variation for metanephrine and normetanephrine were <2% at concentrations of 161 and 307 µg/L, respectively. The inter-assay coefficients of variation for metanephrine and normetanephrine were 2.8% at a concentration of 342 µg/L and 3.5% at a concentration of 269 µg/L. The ratio of metanephrines to creatinine for each urine sample was calculated to correct for internal dilution. Measurement of creatinine was carried out using standard methods on the Modular platform (Roche Diagnostics, Penzberg, Germany).
Total (free and bound) MDA was measured in plasma samples by derivatization with 2-4-dinitrophenylhydrazine as previously described [30, 47] followed by HPLC with UV detection. Plasma BDNF levels were determined using the Human BDNF DuoSet ELISA kit (R&D Systems, Wiesbaden, Germany). Samples were processed according to the kit instructions provided by the manufacturer.
Finally, the Human DiscoveryMAP™ multiplex immunoassay platform was used to measure the serum concentrations of SOD, GST, MMP-9 and cortisol as described previously [55]. High sensitivity CRP (hs-CRP) concentrations in serum were measured by a commercial enhanced latex technology immunoassay (Tina-quant® C-Reactive Protein Gen.3, Roche Diagnostics, Penzberg, Germany) on a fully automated MODULAR E170 system (Roche Diagnostics, Germany) as described by the manufacturer. The minimal detectable concentration of hs-CRP was 0.3 mg/L. All samples were assayed in duplicate.
Statistics
Statistical analysis was performed using the SPSS 15.0 package (Statistical Product and Service solutions, Chicago, USA). Fisher’s exact tests were used to calculate group differences regarding categorical variables such as sex, smoking and occasional cannabis use. Most of the data were not normally distributed, as indicated by Shapiro–Wilk tests. Thus, nonparametric Kruskal–Wallis H tests were applied to detect the overall effects of diagnostic group. In follow-up analyses, Bonferroni–Holm-corrected post hoc Mann–Whitney U tests were performed to identify which pair-wise diagnostic group differences contributed to the results detected as significant by the omnibus test. Paired Mann–Whitney U tests were performed to compare biomarker concentrations at baseline (T0) and after the 6-week treatment period (T6). Significant diagnosis- or time point-related differences in the oxidative stress measures were re-tested for the potential influence of confounding factors. For this, Mann–Whitney U tests were used to determine the effects of gender, smoking and cannabis use. In addition, Spearman rank correlation coefficients were calculated for the whole group of subjects and separately for each diagnostic group to assess the influence of age, waist circumference, body mass index (BMI) as well as the levels of metanephrine, normetanephrine, hsCRP, MMP-9, BDNF and cortisol. Similarly, Spearman rank correlation coefficients were calculated within both patient groups to determine the influence of cumulative drug dosage at T6 (chlorpromazine/amitriptyline units) and the severity of psychopathologic symptoms (PANSS, HAMD-21). All statistical tests were two-tailed, and p values of <0.05 were considered as significant, while p values of <0.10 were considered as a statistical trend.
There was no correction for multiple testing given that most of the assays were carried out in singlet (not multiplex) format. For this exploratory study with small group size, the results were presented without error probability correction. If a Bonferroni adjustment of the type I error probability had been applied, no significant differences would remain. However, if the error probability was adjusted the power of detecting existing mean differences would be too low.
Results
Demographic data
As summarized in Table 1, no significant differences were observed between the diagnostic groups regarding sex (p = 1.000) and the occasional use of cannabis (p = 0.212). However, the diagnostic groups differed regarding age (p = 0.014), particularly because of a younger age of drug-naïve patients with schizophrenia compared to those with major depression (p = 0.030). Differences regarding smoking habits (p = 0.005) were caused by a higher proportion of smokers in schizophrenia (~70% smokers) and major depression (~70% smokers) compared to controls (~35% smokers), although this was significant only in the case of the former (p = 0.024) with a trend seen for the latter (p = 0.055).
Oxidative stress
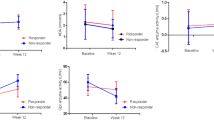
Details regarding oxidative stress-related parameters are displayed in Fig. 1 and Table 2. Urine 8-iso-PGF2α levels were significantly elevated in schizophrenia (p = 0.004) and major depression (p = 0.037) patients compared to controls at T0. An increase in 8-iso-PGF2α levels was observed in the schizophrenia cohort (p = 0.016) after 6 weeks of treatment (T6), while levels in the depressed patients were unchanged from baseline conditions. MDA plasma levels were not different across diagnostic groups at T0 (p = 0.416), but after 6 weeks of treatment the concentration of this analyte was decreased in schizophrenia patients in comparison with the baseline levels (p = 0.008). Serum SOD concentrations differed significantly between the diagnostic groups at T0 (p = 0.048) with higher levels in drug-naïve schizophrenia patients compared to controls, although this was a trend (p = 0.053). However, no significant differences in these parameters were observed after 6 weeks of treatment. Serum GST and urine 8-OH-2-dG showed no diagnosis- or time point-related changes. None of the other analytes showed significant differences at either timepoint.
Illustration of oxidative stress measures. a) urinary 8-iso-prostaglandin F2α (8-iso-PGF2α), b) urinary 8-OH-2-deoyxguanosine (8-OH-2-dG), c) plasma levels of malondialdehyde (MDA), d) serum concentrations of superoxide dismutase (SOD) and e) glutathione S-transferase (GST). Annotation: the box plots show the median, interquartile range, sample minimum and sample maximum, outliers are displayed as circles, * p < 0.05, ** p < 0.01
Potential confounding factors
At T0 and T6, oxidative stress measures were not affected by age, gender or occasional cannabis use in the whole group of subjects or for each diagnostic group separately. The urinary levels of 8-iso-PGF2α were increased significantly in the whole group of smokers compared to non-smokers at T0 (p = 0.001). However, a separate analysis of non-smokers confirmed the additional influence of diagnosis on this parameter (p = 0.040) as shown by higher 8-iso-PGF2α levels in non-smoking schizophrenia patients compared to non-smoking controls (p = 0.033). This was not found for the non-smoking patients with depression compared to non-smoking controls (p = 0.912). No other influences of smoking were observed on the remaining tested oxidative stress data.
To assess a potential influence of adipose tissue mass on oxidative stress measures, we checked for any correlation of the analytes measured with waist circumference (abdominal obesity) or BMI (overall fat mass). This showed that the levels of 8-iso-PGF2α, 8-OH-2-dG, MDA, SOD or GST were not correlated with either of these physical measurements despite the finding that waist circumference (p = 0.003) and BMI (p = 0.009) were significantly increased in schizophrenic patients between T0 and T6 (Table 1). A similar trend was found in the case of the major depression patients (waist circumference: p = 0.091, BMI: p = 0.094).
To address a potential role of biomarkers associated with inflammation, blood–brain-barrier integrity and synaptic plasticity in the different groups, we analyzed blood levels of hsCRP, MMP-9 and BDNF (Table 3). Serum hsCRP levels tended to be higher in depressed patients at baseline compared to controls (p = 0.062) and increased in both patient groups following treatment to reach significance only in schizophrenia patients (p = 0.012). Schizophrenia patients showed higher serum MMP-9 levels at T0 compared to controls (p = 0.020), and the levels of this analyte were significantly decreased after 6 weeks of treatment (p = 0.006), compared to the baseline. In contrast, baseline plasma BDNF levels were significantly lower in schizophrenia (p < 0.001) and tended to be lower in major depression (p = 0.087) patients compared to controls. Furthermore, the BDNF levels in both patient groups showed an increase from T0 to T6 but did not reach normal levels in either group. We found a positive correlation of serum hsCRP with urinary 8-iso-PGF2α (r = 0.280, p = 0.010) in the whole group of tested subjects at T0, but not at T6. Serum MMP-9 levels were correlated with those of SOD (T0 trend: r = 0.216, p = 0.052, T6: r = 0.353, p = 0.035), while plasma BDNF was inversely correlated with urinary 8-iso-PGF2α (T0: r = −0.259, p = 0.027, T6 trend: r = −0.333, p = 0.058) in the whole group of tested subjects. No significant associations were found in separate tests of the diagnostic groups.
Next, the potential influence of endocrine stress axis activation was assessed using urinary biomarkers of sympathetic nervous system activation. This showed significantly higher baseline levels of metanephrine (schizophrenia: p = 0.008, depression: p = 0.008) and normetanephrine (schizophrenia: p = 0.033, depression: p = 0.007) in both patient groups, and these did not change following 6 weeks of treatment, apart from a decrease in metanephrine levels in depressed patients (p = 0.015). In addition, the levels of urinary 8-iso-PGF2α were correlated with those of metanephrine (r = 0.404, p < 0.001) and normetanephrine (r = 0.401, p < 0.001) at T0 in the whole group of tested subjects, but not at T6. Similarly, 8-iso-PGF2α was correlated with metanephrine in depressed patients (r = 0.492, p = 0.038) and with normetanephrine in controls (r = 0.304, p = 0.048) at T0, but not at T6. Serum cortisol, which is another potential marker of stress, was not significantly different between the tested groups at baseline but decreased significantly in the schizophrenia group from T0 to T6 (p = 0.035) (Table 3). However, cortisol levels were not related to oxidative stress parameters at either T0 or T6.
Changes in the tested oxidative stress parameters and BDNF levels during the 6-week follow-up period were not correlated with cumulative drug dosage of antipsychotic (chlorpromazine units) or antidepressant drugs (amitriptyline units). Moreover, the severity of clinical symptoms or their change from T0 to T6 was not significantly associated with oxidative stress parameters in the respective patient groups, apart from an association of delta-PANSS-P-scores with delta GST (r = 0.588, p = 0.004).
Discussion
In the present study, we analyzed a panel of oxidative stress-related markers in acutely ill drug-naïve patients with schizophrenia and major depression. This included measurement of urinary biomarkers such as 8-iso-PGF2α and 8-OH-2-dG in addition to blood biomarkers like MDA, SOD and GST. Most importantly, we attempted to control for potential confounding factors on measurement of these analytes, including potential effects of age, gender, body mass index and treatment.
The finding that there was a trend toward higher SOD concentrations in patients with schizophrenia compared to controls suggested that oxidative stress was evident at this early stage in the disease, although no diagnosis-related differences were observed regarding the GST enzyme or in plasma MDA or urinary 8-OH-2-dG levels. Interestingly, the urinary marker 8-iso-PGF2α was significantly higher in both patient cohorts compared to controls and this was elevated further in schizophrenia patients after 6 weeks of treatment. Isoprostanes are formed during free radical-catalyzed oxidation of arachidonic acid (AA). The types of isoprostaglandins and their amount depend on oxygen tension and glutathione concentration [40]. In healthy humans, the major source of 8-iso-PGF2α is free radical-catalyzed lipid peroxidation. However, enzymatic over-expression due to inflammation and activation of cyclooxygenase 2 (Cox-2) can also contribute to 8-iso-PGF2α production under pathological conditions [65]. Recent investigations have suggested that F2-isoprostanes such as 8-iso-PGF2α are the most precise markers of oxidative stress in vivo [65], and several research groups have now associated changes in this analyte with schizophrenia and depression [8, 11, 37, 46]. A recent postmortem study has observed decreased levels of AA in the frontal cortex of schizophrenia patients [26]. In addition to the higher levels at baseline, we found that 8-iso-PGF2α was increased further after 6 weeks of treatment of schizophrenia patients, suggesting an accumulating damage of membrane lipids in psychosis. It is not likely that this was due a detrimental effect of treatment as we found no correlation between antipsychotic drug dosage and changes in 8-iso-PGF2α levels. As lipid peroxidation is ubiquitous, other conditions of oxidative stress including renal dysfunction have been ruled out in our study population. F2-isoprostane measurement by mass spectrometry is regarded as the best currently available surrogate marker of lipid peroxidation [25]. F2-isoprostanes can be measured in plasma, serum and urine. In contrast to urine, blood samples contain lipids and their handling requires a specific methodology, e.g., avoiding hemolysis, immediate analysis or immediate flash-freezing in liquid nitrogen [25]. The half-life of plasma 8-iso-PGF2α is very short, because it is rapidly metabolized and excreted into the urine [5]. Some authors have suggested that urinary F2-isoprostanes may partly be derived from local production in the kidney [68]. Moreover, an influence of renal blood flow cannot be excluded, especially since 8-iso-PGF2α is a potent vasoconstrictor in various vascular beds, including the kidney [4]. Nevertheless, urinary levels of 8-iso-PGF2α measured by GC–MS appear to be more reliable for the estimation of oxidative stress in clinical conditions than the measurement of other markers [19, 25]. It is of interest that recent clinical interventions attempting to mitigate the effects of lipid peroxidation have been tested in schizophrenia. These efforts include the administration of long-chain omega-3 fatty acids, eicosapentaenoic acid and N-acetyl cysteine (NAC) [1, 7, 21]. However, no placebo-controlled study to date has demonstrated that the resulting normalization of lipid peroxidation was associated with long-term symptom improvement [31].
In addition to oxidative stress effects, proinflammatory factors also appear to be associated with the pathogenesis of schizophrenia and major depression. The involvement of immunological mechanisms in the pathogenesis of these disorders is consistent with the observed beneficial effects of treatment with Cox-2 inhibitors, particularly when applied during early and acute disease stages [42]. Our finding of a positive correlation between the levels of urinary 8-iso-PGF2α with serum hsCRP further supports the hypothesis of a link between oxidative stress and a proinflammatory state in psychiatric patients.
We also investigated potential effects on neuronal growth factors in this study. Altered neuropsychological behavior in neurological diseases is believed to be associated with altered synaptic plasticity, and molecules such as BDNF are considered to be essential in mediating persistent changes in synaptic connectivity [14]. Thus, BDNF is considered to represent a potential blood biomarker for such neuronal changes in the brain. MMP-9 can convert proBDNF to mature BDNF, and it also cleaves and can thereby modulate the activity of other mediators of synaptic plasticity. We found lower BDNF levels at baseline in the first onset schizophrenia patients, consistent with the results of recent meta-analyses [16, 24], and these were significantly correlated with urinary 8-iso-PGF2α levels. These observations are in line with a recent study showing a negative correlation of BDNF levels with those of the oxidative stress marker SOD [70]. Although the findings were not significant, we also observed similar diagnosis-related changes of BDNF levels in the major depression patients. BDNF is largely stored in platelets [20] from where it is released into the bloodstream under activated conditions such as blood clot formation [52]. Thus, plasma may be a more reliable source of BDNF measures compared with serum as the former avoids the post-collection clot formation step. Although the brain may be the primary source of BDNF in blood plasma [33], the protein is also expressed in other cell types including myocytes, lymphocytes and endothelial cells, which are also likely to contribute to the circulating levels of this growth factor [6, 12, 32, 43, 66]. Although it cannot be proven whether plasma BDNF levels under pathological conditions result from reduced expression in the brain or periphery, it has been demonstrated that both expression and transport of BDNF are negatively regulated by oxidative stress [35, 41]. Furthermore, drug treatment leading to reduced lipid peroxidation under ischemic conditions was found to be associated with increased BDNF levels in the rat hippocampus [35] suggesting a link between lipid peroxidation and brain BDNF content. Especially, an interaction between serotonin signaling and BDNF has been emphasized [34]. Although there is growing evidence of a relationship between serotonin and BDNF [28], BDNF levels were not significantly influenced by medication in our study, possibly because of the limited specificity of the applied BDNF ELISA to distinguish between proBDNF and mature BDNF.
The significant increase of MMP-9 in schizophrenic patients with 8-iso-PGF2α and SOD at trend levels is consistent with previous studies and supports hypotheses which suggest a connection between a proinflammatory state, disturbances in blood–brain-barrier integrity and abnormal synaptic plasticity in schizophrenia patients [10, 69]. In this study, the elevated MMP-9 levels decreased to the range of healthy controls after 6 weeks of treatment, in line with earlier studies which found that some of these changes may occur only transiently during acute disease phases [61]. MMP-9 has been considered as a marker for pathological events in the brain [51]. Notably, increased blood levels of MMP-9 have been associated with an impairment of the blood–brain barrier [59]. Moreover, previous meta-analyses showed an increased production of pro-inflammatory cytokines in schizophrenia and major depression which is associated with an increased permeability of the blood–brain barrier [13, 39]. Thus, peripheral levels of MMP-9 and other parameters related to inflammation or oxidative stress may influence synaptic plasticity in the brain.
We also found that smoking and biomarkers of sympathetic nervous system activation were related to measures of 8-iso-PGF2α. This is consistent with the use of isoprostanes as biomarkers of oxidative stress in cigarette smokers [57]. However, even though both patient groups contained a higher percentage of smokers than the control group, we found a schizophrenia-related increase in 8-iso-PGF2α through a separate analysis of non-smokers. In contrast to findings by Black et al. [9], the increase of 8-iso-PGF2α in acutely ill patients with depression was not confirmed by a separate analysis of non-smoking patients with depression compared to non-smoking controls. Thus, one may speculate on a disease-inherent association of oxidative stress related to lipid peroxidation in schizophrenia, while smoking may be the driving factor for an increased production of 8-iso-PGF2α in acutely ill patients with depression. However, further studies using larger cohorts of smokers and non-smokers will be required to confirm this.
Although endocrine stress axis activation is a common feature of psychiatric disease, it is still not known whether or not this can induce oxidative stress. Catecholamines are most likely involved in the production of ROS and conditions causing increased catecholamine metabolism may therefore contribute to oxidative stress [60]. Accordingly, the levels of urinary 8-iso-PGF2α showed a significant correlation with those of metanephrine and normetanephrine, which are the degradation products of epinephrine and norepinephrine, respectively. Furthermore, 8-iso-PGF2α may have a modulatory effect on norepinephrine release from sympathetic nerves [44, 45].
Oxidative stress biomarkers did not correlate with improvements in clinical symptoms or cumulative drug dosage in our study and may thus be considered as trait markers. Alternatively, oxidative stress is an epiphenomenon which is not closely related to the pathogenesis of schizophrenia or major depression and is rather caused by an unhealthy lifestyle, including cigarette smoking, reduced physical activity and the preference of sweet and fatty food. Our study results may differ from previous ones since we have taken into account multiple potential confounding factors.
For this explorative study with small group size, the results were presented without error probability correction. Another potential limitation of the present study is that the oxidative stress measurements may be influenced by changes in energy metabolism or associated with weight gain in the patient cohorts. However, we attempted to account for such effects and did not observe an association of any of the analytes with these characteristics in drug-naïve patients with schizophrenia and depression. We did not control for sleep habits and/or the presence of obstructive sleep apnea in the study population which might possibly interfere with oxidative stress parameters. The tested oxidative stress parameters were not related to age, sex, disease severity or cumulative drug dosage over the treatment period. In addition, sympathetic nervous system activation and cigarette smoking could be identified as potential confounders. Finally, the potential influence of age, sex, cannabis use, adipose tissue mass, severity of symptoms and the use of modern antipsychotic and antidepressant drugs after 6 weeks of treatment on the oxidative stress measures cannot be ruled out because of the relatively low number of investigated cases in this study. This will require re-testing in a larger patient cohort. The lacking differentiation of the applied ELISA between proBDNF and mature BDNF measurements is another limitation of the present study [27].
Conclusion
Based on our study in drug-naïve patients with schizophrenia and major depression, we suggest a disease-inherent association of oxidative stress related to lipid peroxidation in schizophrenia, while smoking may be the driving factor for an increased production of 8-iso-PGF2α in acutely ill patients with depression. Nevertheless, it still remains unclear if oxidative stress is a causal factor or an epiphenomenon after the prodromal disease phase. For instance, endocrine stress axis activation (a common feature of psychiatric disease) may be related to oxidative stress. Taken together, the results of this study highlight the importance of accounting for potential confounding factors such as age, sex, smoking, stress levels and other physiometric characteristics in biomarker-related studies of psychiatric disorders.
References
Amminger GP, Schafer MR, Papageorgiou K, Klier CM, Cotton SM, Harrigan SM, Mackinnon A, McGorry PD, Berger GE (2010) Long-chain omega-3 fatty acids for indicated prevention of psychotic disorders: a randomized, placebo-controlled trial. Arch Gen Psychiatry 67:146–154
APA (2000) Diagnostic and statistical manual of mental disorders, 4th revised edition (dsm-iv-tr). American Psychiatric Press, Washington
Atkins M, Burgess A, Bottomley C, Riccio M (1997) Chlorpromazine equivalents: a consensus of opinion for both clinical and research applications. Psychiatr Bull 21:224–226
Badr KF, Abi-Antoun TE (2005) Isoprostanes and the kidney. Antioxid Redox Signal 7:236–243
Basu S (1998) Metabolism of 8-iso-prostaglandin f2alpha. FEBS Lett 428:32–36
Bayas A, Hummel V, Kallmann BA, Karch C, Toyka KV, Rieckmann P (2002) Human cerebral endothelial cells are a potential source for bioactive BDNF. Cytokine 19:55–58
Berk M, Copolov D, Dean O, Lu K, Jeavons S, Schapkaitz I, Anderson-Hunt M, Judd F, Katz F, Katz P, Ording-Jespersen S, Little J, Conus P, Cuenod M, Do KQ, Bush AI (2008) N-acetyl cysteine as a glutathione precursor for schizophrenia–a double-blind, randomized, placebo-controlled trial. Biol Psychiatry 64:361–368
Black CN, Bot M, Scheffer PG, Cuijpers P, Penninx BW (2015) Is depression associated with increased oxidative stress? A systematic review and meta-analysis. Psychoneuroendocrinology 51:164–175
Black CN, Penninx BW, Bot M, Odegaard AO, Gross MD, Matthews KA, Jacobs DR Jr (2016) Oxidative stress, anti-oxidants and the cross-sectional and longitudinal association with depressive symptoms: results from the cardia study. Transl Psychiatry 6:e743
Devanarayanan S, Nandeesha H, Kattimani S, Sarkar S (2016) Relationship between matrix metalloproteinase-9 and oxidative stress in drug-free male schizophrenia: a case control study. Clin Chem Lab Med 54(3):447–452
Dietrich-Muszalska A, Olas B (2009) Isoprostenes as indicators of oxidative stress in schizophrenia. World J Biol Psychiatry 10:27–33
Donovan MJ, Lin MI, Wiegn P, Ringstedt T, Kraemer R, Hahn R, Wang S, Ibanez CF, Rafii S, Hempstead BL (2000) Brain derived neurotrophic factor is an endothelial cell survival factor required for intramyocardial vessel stabilization. Development 127:4531–4540
Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, Lanctot KL (2010) A meta-analysis of cytokines in major depression. Biol Psychiatry 67:446–457
Edelmann E, Lessmann V, Brigadski T (2014) Pre- and postsynaptic twists in BDNF secretion and action in synaptic plasticity. Neuropharmacology 76 Pt C:610–627
Fernandes BS, Berk M, Turck CW, Steiner J, Goncalves CA (2014) Decreased peripheral brain-derived neurotrophic factor levels are a biomarker of disease activity in major psychiatric disorders: a comparative meta-analysis. Mol Psychiatry 19:750–751
Fernandes BS, Steiner J, Berk M, Molendijk ML, Gonzalez-Pinto A, Turck CW, Nardin P, Goncalves CA (2015) Peripheral brain-derived neurotrophic factor in schizophrenia and the role of antipsychotics: meta-analysis and implications. Mol Psychiatry 20:1108–1119
Fernandes BS, Steiner J, Bernstein HG, Dodd S, Pasco JA, Dean OM, Nardin P, Goncalves CA, Berk M (2016) C-reactive protein is increased in schizophrenia but is not altered by antipsychotics: meta-analysis and implications. Mol Psychiatry 21:554–564
Flatow J, Buckley P, Miller BJ (2013) Meta-analysis of oxidative stress in schizophrenia. Biol Psychiatry 74:400–409
Frijhoff J, Winyard PG, Zarkovic N, Davies SS, Stocker R, Cheng D, Knight AR, Taylor EL, Oettrich J, Ruskovska T, Gasparovic AC, Cuadrado A, Weber D, Poulsen HE, Grune T, Schmidt HH, Ghezzi P (2015) Clinical relevance of biomarkers of oxidative stress. Antioxid Redox Signal 23:1144–1170
Fujimura H, Altar CA, Chen R, Nakamura T, Nakahashi T, Kambayashi J, Sun B, Tandon NN (2002) Brain-derived neurotrophic factor is stored in human platelets and released by agonist stimulation. Thromb Haemost 87:728–734
Fusar-Poli P, Berger G (2012) Eicosapentaenoic acid interventions in schizophrenia: meta-analysis of randomized, placebo-controlled studies. J Clin Psychopharmacol 32:179–185
Gaebel W, Falkai P (2005) Interdisciplinary praxis guidelines: AWMF S3 guidelines of the german psychiatric association for schizophrenia. Steinkopff, Darmstadt
Giannandrea M, Parks WC (2014) Diverse functions of matrix metalloproteinases during fibrosis. Dis Model Mech 7:193–203
Green MJ, Matheson SL, Shepherd A, Weickert CS, Carr VJ (2011) Brain-derived neurotrophic factor levels in schizophrenia: a systematic review with meta-analysis. Mol Psychiatry 16:960–972
Halliwell B, Lee CY (2010) Using isoprostanes as biomarkers of oxidative stress: some rarely considered issues. Antioxid Redox Signal 13:145–156
Hamazaki K, Maekawa M, Toyota T, Iwayama Y, Dean B, Hamazaki T, Yoshikawa T (2016) Fatty acid composition and fatty acid binding protein expression in the postmortem frontal cortex of patients with schizophrenia: a case-control study. Schizophr Res 171:225–232
Hashimoto K (2015) Brain-derived neurotrophic factor (BDNF) and its precursor proBDNF as diagnostic biomarkers for major depressive disorder and bipolar disorder. Eur Arch Psychiatry Clin Neurosci 265:83–84
Hashimoto K (2016) Regulation of brain-derived neurotrophic factor (BDNF) and its precursor proBDNF in the brain by serotonin. Eur Arch Psychiatry Clin Neurosci 266:195–197
Hayasaka Y, Purgato M, Magni LR, Ogawa Y, Takeshima N, Cipriani A, Barbui C, Leucht S, Furukawa TA (2015) Dose equivalents of antidepressants: evidence-based recommendations from randomized controlled trials. J Affect Disord 180:179–184
Jordan W, Cohrs S, Degner D, Meier A, Rodenbeck A, Mayer G, Pilz J, Ruther E, Kornhuber J, Bleich S (2006) Evaluation of oxidative stress measurements in obstructive sleep apnea syndrome. J Neural Transm 113:239–254
Joshi YB, Pratico D (2014) Lipid peroxidation in psychiatric illness: overview of clinical evidence. Oxid Med Cell Longev 2014:828702
Kerschensteiner M, Gallmeier E, Behrens L, Leal VV, Misgeld T, Klinkert WE, Kolbeck R, Hoppe E, Oropeza-Wekerle RL, Bartke I, Stadelmann C, Lassmann H, Wekerle H, Hohlfeld R (1999) Activated human t cells, b cells, and monocytes produce brain-derived neurotrophic factor in vitro and in inflammatory brain lesions: a neuroprotective role of inflammation? J Exp Med 189:865–870
Klein AB, Williamson R, Santini MA, Clemmensen C, Ettrup A, Rios M, Knudsen GM, Aznar S (2011) Blood BDNF concentrations reflect brain-tissue BDNF levels across species. Int J Neuropsychopharmacol 14:347–353
Kronenberg G, Mosienko V, Gertz K, Alenina N, Hellweg R, Klempin F (2016) Increased brain-derived neurotrophic factor (BDNF) protein concentrations in mice lacking brain serotonin. Eur Arch Psychiatry Clin Neurosci 266:281–284
Lee CH, Park JH, Yoo KY, Choi JH, Hwang IK, Ryu PD, Kim DH, Kwon YG, Kim YM, Won MH (2011) Pre- and post-treatments with escitalopram protect against experimental ischemic neuronal damage via regulation of BDNF expression and oxidative stress. Exp Neurol 229:450–459
Lopresti AL, Maker GL, Hood SD, Drummond PD (2014) A review of peripheral biomarkers in major depression: the potential of inflammatory and oxidative stress biomarkers. Prog Neuropsychopharmacol Biol Psychiatry 48:102–111
Mahadik SP, Mukherjee S, Scheffer R, Correnti EE, Mahadik JS (1998) Elevated plasma lipid peroxides at the onset of nonaffective psychosis. Biol Psychiatry 43:674–679
Michel TM, Pulschen D, Thome J (2012) The role of oxidative stress in depressive disorders. Curr Pharm Des 18:5890–5899
Miller BJ, Buckley P, Seabolt W, Mellor A, Kirkpatrick B (2011) Meta-analysis of cytokine alterations in schizophrenia: clinical status and antipsychotic effects. Biol Psychiatry 70:663–671
Morrow JD, Scruggs J, Chen Y, Zackert WE, Roberts LJ 2nd (1998) Evidence that the e2-isoprostane, 15-e2t-isoprostane (8-iso-prostaglandin e2) is formed in vivo. J Lipid Res 39:1589–1593
Mukherjee A, Jenkins B, Fang C, Radke RJ, Banker G, Roysam B (2011) Automated kymograph analysis for profiling axonal transport of secretory granules. Med Image Anal 15:354–367
Müller N (2010) Cox-2 inhibitors as antidepressants and antipsychotics: clinical evidence. Curr Opin Investig Drugs 11:31–42
Nakahashi T, Fujimura H, Altar CA, Li J, Kambayashi J, Tandon NN, Sun B (2000) Vascular endothelial cells synthesize and secrete brain-derived neurotrophic factor. FEBS Lett 470:113–117
Nakamura K, Okada S, Ono K, Yokotani K (2003) Effects of 8-iso-prostaglandin e2 and 8-iso-prostaglandin f2 alpha on the release of noradrenaline from the isolated rat stomach. Eur J Pharmacol 470:73–78
Opere CA, Ford K, Zhao M, Ohia SE (2008) Regulation of neurotransmitter release from ocular tissues by isoprostanes. Methods Find Exp Clin Pharmacol 30:697–701
Palta P, Samuel LJ, Miller ER 3rd, Szanton SL (2014) Depression and oxidative stress: results from a meta-analysis of observational studies. Psychosom Med 76:12–19
Pilz J, Meineke I, Gleiter CH (2000) Measurement of free and bound malondialdehyde in plasma by high-performance liquid chromatography as the 2,4-dinitrophenylhydrazine derivative. J Chromatogr B Biomed Sci Appl 742:315–325
Pirici D, Pirici I, Mogoanta L, Margaritescu O, Tudorica V, Margaritescu C, Ion DA, Simionescu C, Coconu M (2012) Matrix metalloproteinase-9 expression in the nuclear compartment of neurons and glial cells in aging and stroke. Neuropathol Off J Jpn Soc Neuropathol 32:492–504
Popa-Wagner A, Mitran S, Sivanesan S, Chang E, Buga AM (2013) Ros and brain diseases: the good, the bad, and the ugly. Oxidative Med Cell Longev 2013:963520
Rasmussen P, Brassard P, Adser H, Pedersen MV, Leick L, Hart E, Secher NH, Pedersen BK, Pilegaard H (2009) Evidence for a release of brain-derived neurotrophic factor from the brain during exercise. Exp Physiol 94:1062–1069
Rey MJ, Schulz P, Costa C, Dick P, Tissot R (1989) Guidelines for the dosage of neuroleptics. I: chlorpromazine equivalents of orally administered neuroleptics. Int Clin Psychopharmacol 4:95–104
Rosenfeld RD, Zeni L, Haniu M, Talvenheimo J, Radka SF, Bennett L, Miller JA, Welcher AA (1995) Purification and identification of brain-derived neurotrophic factor from human serum. Protein Expr Purif 6:465–471
Rybakowski JK, Remlinger-Molenda A, Czech-Kucharska A, Wojcicka M, Michalak M, Losy J (2013) Increased serum matrix metalloproteinase-9 (mmp-9) levels in young patients during bipolar depression. J Affect Disord 146:286–289
Sawa A, Sedlak TW (2016) Oxidative stress and inflammation in schizophrenia. Schizophr Res 176:1–2
Schwarz E, Guest PC, Steiner J, Bogerts B, Bahn S (2012) Identification of blood-based molecular signatures for prediction of response and relapse in schizophrenia patients. Transl Psychiatry 2:e82
Schwedhelm E, Tsikas D, Durand T, Gutzki FM, Guy A, Rossi JC, Frolich JC (2000) Tandem mass spectrometric quantification of 8-iso-prostaglandin f2alpha and its metabolite 2,3-dinor-5,6-dihydro-8-iso-prostaglandin f2alpha in human urine. J Chromatogr B Biomed Sci Appl 744:99–112
Seet RC, Lee CY, Loke WM, Huang SH, Huang H, Looi WF, Chew ES, Quek AM, Lim EC, Halliwell B (2011) Biomarkers of oxidative damage in cigarette smokers: which biomarkers might reflect acute versus chronic oxidative stress? Free Radic Biol Med 50:1787–1793
Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC (1998) The mini-international neuropsychiatric interview (m.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 59(Suppl 20):22–33
Shigemori Y, Katayama Y, Mori T, Maeda T, Kawamata T (2006) Matrix metalloproteinase-9 is associated with blood-brain barrier opening and brain edema formation after cortical contusion in rats. Acta Neurochir Suppl 96:130–133
Smythies J (2000) Redox aspects of signaling by catecholamines and their metabolites. Antioxid Redox Signal 2:575–583
Steiner J, Bogerts B, Sarnyai Z, Walter M, Gos T, Bernstein HG, Myint AM (2012) Bridging the gap between the immune and glutamate hypotheses of schizophrenia and major depression: potential role of glial nmda receptor modulators and impaired blood-brain barrier integrity. World J Biol Psychiatry 13:482–492
Tsikas D, Schwedhelm E, Suchy MT, Niemann J, Gutzki FM, Erpenbeck VJ, Hohlfeld JM, Surdacki A, Frolich JC (2003) Divergence in urinary 8-iso-pgf(2alpha) (ipf(2alpha)-iii, 15-f(2t)-IsoP) levels from gas chromatography-tandem mass spectrometry quantification after thin-layer chromatography and immunoaffinity column chromatography reveals heterogeneity of 8-iso-PGF(2alpha). Possible methodological, mechanistic and clinical implications. J Chromatogr B Analyt Technol Biomed Life Sci 794:237–255
Vafadari B, Salamian A, Kaczmarek L (2016) Mmp-9 in translation: From molecule to brain physiology, pathology and therapy. J Neurochem 139(Suppl 2):91–114
Valkanova V, Ebmeier KP, Allan CL (2013) Crp, il-6 and depression: a systematic review and meta-analysis of longitudinal studies. J Affect Disord 150:736–744
van ‘t Erve TJ, Lih FB, Kadiiska MB, Deterding LJ, Eling TE, Mason RP (2015) Reinterpreting the best biomarker of oxidative stress: the 8-iso-pgf(2alpha)/pgf(2alpha) ratio distinguishes chemical from enzymatic lipid peroxidation. Free Radic Biol Med 83:245–251
Wang H, Ward N, Boswell M, Katz DM (2006) Secretion of brain-derived neurotrophic factor from brain microvascular endothelial cells. Eur J Neurosci 23:1665–1670
Woods SW (2003) Chlorpromazine equivalent doses for the newer atypical antipsychotics. J Clin Psychiatry 64:663–667
Wu JH, Ward NC, Indrawan AP, Almeida CA, Hodgson JM, Proudfoot JM, Puddey IB, Croft KD (2007) Effects of alpha-tocopherol and mixed tocopherol supplementation on markers of oxidative stress and inflammation in type 2 diabetes. Clin Chem 53:511–519
Yamamori H, Hashimoto R, Ishima T, Kishi F, Yasuda Y, Ohi K, Fujimoto M, Umeda-Yano S, Ito A, Hashimoto K, Takeda M (2013) Plasma levels of mature brain-derived neurotrophic factor (BDNF) and matrix metalloproteinase-9 (mmp-9) in treatment-resistant schizophrenia treated with clozapine. Neurosci Lett 556:37–41
Zhang XY, Chen DC, Tan YL, Tan SP, Wang ZR, Yang FD, Okusaga OO, Zunta-Soares GB, Soares JC (2015) The interplay between BDNF and oxidative stress in chronic schizophrenia. Psychoneuroendocrinology 51:201–208
Acknowledgements
Anke Dudeck, Gabriela Meyer-Lotz and Jeanette Schadow participated in the sample characterization and collection. Katrin Borucki provided helpful advice during manuscript preparation. This study has been performed without extra funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
SB is a director of Psynova Neurotech Ltd and PsyOmics Ltd. All other authors declare no conflicts of interest.
Rights and permissions
About this article
Cite this article
Jordan, W., Dobrowolny, H., Bahn, S. et al. Oxidative stress in drug-naïve first episode patients with schizophrenia and major depression: effects of disease acuity and potential confounders. Eur Arch Psychiatry Clin Neurosci 268, 129–143 (2018). https://doi.org/10.1007/s00406-016-0749-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00406-016-0749-7