Abstract
Purpose
Laryngopharyngeal reflux disease (LPRD) is mainly treated with proton pump inhibitors (PPI) such as esomeprazole, which have shortcomings like delayed absorption and increased osteoporosis. Fexuprazan is a novel potent potassium-competitive acid blocker that inhibits gastric acid secretion with rapid onset and long duration of action. To assess the efficacy and safety of fexuprazan compared to esomeprazole in patients with LPRD.
Methods
This prospective, randomized, double-blinded, multicenter, active-controlled trial was conducted in nine otolaryngologic clinics. Patients with reflux symptom index (RSI) ≥ 13 and reflux finding score (RFS) ≥ 7 were randomly assigned to the fexuprazan or esomeprazole groups, and received fexuprazan 40-mg or esomeprazole 40-mg once daily for 8 weeks. The outcomes were (1) mean change, change rate, and valid rate in RSI, RFS, and LPR-related questionnaires; and (2) adverse events.
Results
A total of 136 patients (fexuprazan n = 68, esomeprazole n = 68) were followed up for ≥ 1 month. Each parameter significantly improved after 4 and 8 weeks in each group, with no significant differences between the two groups. For those with severe symptoms (RSI ≥ 18), the fexuprazan group (n = 32) showed more improvement in the mean change and change rate in the RSI than esomeprazole group (n = 31) after 4 weeks (p = .036 and .045, respectively). This phenomenon was especially observed in hoarseness and troublesome cough.
Conclusion
Fexuprazan improved symptoms and signs without no serious adverse events in patients with LPRD. In patients with severe symptoms, fexuprazan resulted in a faster symptom improvement than PPI.
Trial registration
KCT0007251, https://cris.nih.go.kr/cris/search/detailSearch.do?seq=22100.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Laryngopharyngeal reflux disease (LPRD) and gastroesophageal reflux disease (GERD) are both caused by the reflux of gastric content, but two conditions have many differences in symptoms, manifestations, and treatment response [1, 2]. Patients with LPRD commonly complain of globus sensation, hoarseness, or throat clearing caused by daytime, upright, gaseous mixed reflux [2]. The diagnosis of LPRD is mainly based on symptoms and laryngoscopic findings followed by empirical PPI therapy [3]. The validated Reflux Symptom Index (RSI) questionnaire is commonly utilized to assess the severity of LPRD symptoms and to estimate treatment response [4]. The laryngoscopic findings-based Reflux Finding Score (RFS) tool has been used to assess clinical severity [5]. Patients with RSI and RFS ≥ 13 and 7 are considered to have LPRD [6].
PPIs are the most commonly prescribed class of medication for reflux diseases such as GERD, esophagitis, and LPRD [3, 7]. PPIs such as esomeprazole inhibit gastric acid secretion by binding covalently to the gastric acid pump H+/K+-ATPase [8]. While GERD is commonly pure gastric acid reflux, LPRD is a mixture of other refluxate such as bile acids and pepsin [9, 10]. Thus, a substantial proportion of patients with LPRD are more refractory to PPI than those with GERD, resulting in more aggressive and prolonged PPI therapy [11]. Long-term PPI therapy may increase osteoporosis, bone fracture, and community-acquired pneumonia [12]. Furthermore, because of diversity in PPI metabolism, PPI may not be effective in every patient [13].
Fexuprazan (Daewoong Pharmaceutical Co., Ltd., Seoul, South Korea), a novel potassium-competitive acid blocker (P-CAB) has been suggested as an alternative treatment for acid reflux diseases. P-CABs have an onset of action within 2 h, a prolonged half-life (about 9 h) and maximal effect from the first dose [14, 15]. In a phase I trial, fexuprazan showed rapid and sustained suppression of gastric acid secretion, resulting in intragastric pH > 4 for 24 h in healthy male subjects [13]. In a phase III trial, the fexuprazan group showed similar healing rate, symptom responses, and side effects at weeks 4 and 8 in GERD-related erosive esophagitis, compared with the esomeprazole group. Furthermore, chronic cough, an extraesophageal symptom, was significantly improved in the fexuprazan group compared to the esomeprazole group [16]. We hypothesized that fexuprazan would have a similar effect for erosive esophagitis in LPRD.
This prospective, randomized, double-blind, and actively controlled exploratory clinical trial was conducted to assess the efficacy and safety of fexuprazan compared to esomeprazole in patients with symptoms and signs of LPRD.
Subjects and methods
Trial design
This prospective, randomized, double-blinded, multicenter, active-controlled trial was conducted in nine otolaryngologic clinics in Korea between August 2022 and December 2023: Asan Medical Center, Kyung Hee University Medical Center, Kyung Hee University Hospital in Gangdong, Inha University Hospital, Seoul National University Bundang Hospital, Nowon Eulji University Hospital, Ajou University Hospital, Dong-A University Hospital, and Kosin University Gospel Hospital. This trial was registered with the Clinical Research Information Service of the Republic of Korea (ISRCTN, KCT0007251).
Table 1 shows the schedule of the different protocol phases according to the standard protocol items: recommendations for interventional trials (SPIRIT) guidance [17].
Participants presenting with LPRD symptoms at the otolaryngology clinic of each medical center were recruited competitively. Eligible participants were randomly allocated to either the experimental or the control group in a 1:1 ratio after screening based on the inclusion and exclusion criteria. Patients were assessed for up to 8 weeks after their first visit using the RSI, RFS, reflux symptom score-12 (RSS-12), and laryngopharyngeal reflux–health-related quality of Life (LPR-HRQoL) questionnaires.
Participants and eligibility
Inclusion and exclusion criteria
The inclusion criteria were: (1) age ≥ 19 years; (2) LPRD symptoms [18] including lump sensation in the throat, troublesome cough, frequent throat clearing, or hoarseness ≥ 1 month; (3) RSI ≥ 13; (4) RFS ≥ 7; and (5) provision of written informed consent.
The exclusion criteria were: (1) history of malignancy of the head and neck, esophagus, or stomach; (2) previous radiotherapy; (3) previous anti-reflux or gastroesophageal surgery; (4) gastrointestinal disorders such as erosive GERD, erosive esophagitis, Barrett’s esophagus, and Zollinger-Ellison syndrome; (6) administration of H2 blocker, PPI, P-CAB, antacid, or prokinetics within 2 weeks; (7) abnormal laboratory testing results at screening and follow up (alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase, gamma-glutamyl transferase, total bilirubin, creatinine, or blood urea nitrogen ≥ 2 times the upper limit of normal of the reference range for each participating hospital); (8) any other conditions or diseases considered inappropriate for this study by an investigator.
Ethics
The study was approved by the Institutional Review Board (IRB) of Kyung Hee University Hospital at Gangdong (approval no. KHNMC 2022-01-020), Seoul National University Bundang Hospital (B-2022-741-002), Nowon Eulji Medical Center (EMCS 2022-01-033), Ajou University Hospital (AJIRB-MED-CT2-21–681), Inha University Hospital (2022-01-005), Dong-A University Hospital (DAUHIRB-22-042), Kosin University Hospital (KUGH 2022-01-037), Kyung Hee University Medical Center (KHUH 2022-02-007), and Asan Medical Center (S2022-0025-0001).
All participants were provided with information regarding the study, and written informed consent was obtained from all eligible participants before enrolment.
Interventions
The experimental (fexuprazan) group received both 40 mg of fexuprazan and its matching placebo, whereas the control (esomeprazole) group received both 40 mg of esomeprazole and its matching placebo. The matching placebo in the fexuprazan group had the same size, color, and shape; thus, they were distinguishable from esomeprazole to ensure the double-blind nature of the study. The matched placebo in the esomeprazole group had the same properties as fexuprazan. All participants took two tablets before breakfast for 8 weeks.
Furthermore, patients received behavior modification education in dietary and lifestyle habits at each visit for 8 weeks. Education on dietary habits were: (1) avoid caffeine drinks, chocolate, sour fruit, and mint; (2) refrain from drinking alcohol; (3) refrain from eating spicy or greasy food; (4) refrain from overeating; (5) refrain from intense exercise within 2 h of eating; and (6) refrain from eating food within 3 h before bed. Education on lifestyle habits were: (1) quit smoking; (2) avoiding tight clothes and wearing comfortable clothes; (3) trying to maintain normal weight; (4) staying left when lying down; and (5) raising the bed at the head side if night reflux symptoms are severe.
Outcomes
Primary outcome
The primary outcome was the mean change in the RSI score at week 8.
Secondary outcomes
The secondary outcomes were:
-
(1)
Mean change in RSI scores at week 4.
-
(2)
Change rate and valid rate in RSI scores at weeks 4 and 8.
-
(3)
Mean change, change rate, and valid rate in RFS score at weeks 4 and 8.
-
(4)
Mean change and valid rate in RSS-12 total and quality of life (QoL) scores at weeks 4 and 8.
-
(5)
Mean change in LPR-HRQoL scores at weeks 4 and 8.
The RSS-12 questionnaire is a recently validated twelve-item self-administered questionnaire that assesses symptom severity in patients with LPRD [19]. The LPR-HRQoL questionnaire is a validated 43-item self-administered questionnaire that assesses HRQoL affected by LPRD based on the previous month [20].
Responders were defined as participants with a decrease of ≥ 50% in the total RSI or RFS scores [18]. The valid rate for the RSI or RFS scores was defined as the proportion of responders among patients with LPRD according to the RSI or RFS score [21]. Responders according to the RSS-12 total or QoL scores were defined as patients with a decrease of ≥ 20% in the RSS-12 total or QoL scores, and the valid rate for the RSS-12 total or QoL score was defined as the proportion of responders according to their respective scores [22].
Sample size
The effectiveness of fexuprazan was assumed to be similar to that of esomeprazole. Therefore, the sample size was calculated on the basis of the results of a previous study using omeprazole (40 mg), which has the same PPI formulation as esomeprazole [23]. Considering a two-sided 95% confidence interval of 1.220, 95% power, and 1:1 ratio of experimental and control groups, approximately 63 participants were required in each group. Assuming a 15% dropout rate, the required sample size was 150 participants (75 in each group).
Statistical methods
Patient groups for data analysis
All randomized sets (ARS) included randomly assigned participants. The full analysis set (FAS) consisted of participants who received at least one dose of the clinical trial drug and underwent an efficacy evaluation more than once during the treatment period. The per-protocol set (PPS) included participants who consumed > 70% of the prescribed doses of the clinical study drugs, completed follow-up visits, and underwent corresponding outcome measurements. The safety set (SS) included all participants who received the study drug at least once after randomization.
Statistical analysis
Data are presented as the mean ± standard deviation for continuous data or frequencies for categorical data. The demographic and baseline values of the experimental and control groups were compared and evaluated using analysis of variance (ANOVA) for continuous outcome measures and Pearson’s chi-square test or Fisher’s exact test for categorical outcome measures.
To analyze the primary and secondary outcomes, a paired t-test was performed to compare the changes or change rates in the treatment group. Differences in the changes or change rates of each score between the two groups were analyzed using an analysis of covariance (ANCOVA) model. In the ANCOVA model, each baseline score and treatment group were included as covariates and factors, respectively. Pearson’s chi-square test or Fisher’s exact test was used to compare the valid rates of each score.
For safety analysis, Pearson’s chi-square test or Fisher’s exact test was used to compare the differences in the incidence of adverse events (AEs) between the two groups. The R software package (http://www.r-project.org) was used for all statistical analyses. Statistical significance was set at p < 0.05.
Results
Patient allocation and baseline demographics
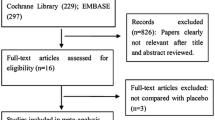
Of the 170 patients screened, 155 who met the inclusion criteria were included in the ARS and randomly allocated to the experimental (fexuprazan; n = 79) or control (esomeprazole; n = 76) groups. The FAS included 136 patients (n = 68 in each group), and the SS included 150 patients (n = 75 in each group). The detailed reasons for exclusion from each group are described in Fig. 1.
There were no significant differences in age, sex, smoking history, alcohol history, caffeine history, comorbidities, disease duration, baseline RSI, or RFS between the two groups (Table S1). The baseline RSI scores were 20 ± 6.354 and 19.842 ± 6.474 in fexuprazan and esomeprazole groups, respectively (p = 0.878).
Efficacy assessment in the FAS
In the FAS, the drug compliance rates throughout 8 weeks were 92.43 ± 20.775% and 93.98 ± 17.720% in the fexuprazan and esomeprazole groups, respectively. There were no significant differences in compliance rates between the two groups.
A summary of the efficacy in the FAS is presented in Table 2. In the FAS analysis, both groups showed a significant decrease in the mean change in the RSI and RFS scores at weeks 4 and 8 (Table 2 and Fig. 2). Furthermore, both groups showed significant improvement in the mean changes in the RSS-12 total, RSS-12 QoL, and LPR-HRQoL scores at weeks 4 and 8. However, there were no significant differences in the degree of mean change, change rate, or valid rate of each score at weeks 4 and 8 between the two groups.
Subgroup analysis
The distribution of the baseline RSI in the FAS ranged from 13 to 42, indicating that the severity of symptoms varied (Fig. 3). The median value of baseline RSI in FAS was 18, thus FAS was classified into severe (n = 63; RSI > 18) and mild (n = 73; RSI ≤ 18) groups.
The severe group in the FAS contained fexuprazan (n = 32) and esomeprazole (n = 31) groups, while mild group contained fexuprazan (n = 36) and esomeprazole (n = 37) groups.
Efficacy assessment in severe group
In the severe group, both groups showed a significant decrease in the degree of mean change and change rate in each score at weeks 4 and 8 (Table 3). There were no significant differences in the degree of mean change and change rate in the RSI score at week 8 between the two groups. However, at 4 weeks, the fexuprazan group showed more improvement than the esomeprazole group in the mean change and the change rate in the RSI score (p = 0.036 and 0.045; Fig. 4A). Furthermore, the fexuprazan group, compared to the esomeprazole group, showed significant improvement or an improvement tendency in the degree of the mean change, the change rate, and the valid rate in the RFS score not at week 4 but at week 8 (Fig. 4B).
Individual RSI items in the both groups were further analyzed (Table S2). After 4 weeks, both groups showed a significant decrease in the mean change in each RSI item except swallowing difficulty and breathing difficulty. Furthermore, the fexuprazan group, compared to the esomeprazole group, showed significant improvement or an improvement tendency in the mean change in hoarseness, swallowing difficulty, and troublesome cough at week 4 (p = 0.006, 0.050, and 0.033; Fig. 5).
There were no significant differences in the valid rate of the RSS-12 total score at weeks 4 and 8 (Figure S1A). However, the fexuprazan group, compared to the esomeprazole group, showed significant improvement in the valid rate of the RSS-12 QoL score at week 4 (p = 0.020; Figure S1B). There were no significant differences in the valid rate of the RSS-12 QoL score at week 8.
Efficacy assessment in mild group
Compared with baseline, each parameter was significantly improved at weeks 4 and 8 within each group, except for the mean change in the LPR-HRQoL score at week 4 (Table S3). There were no significant differences in the degree of improvement of each parameter between the two groups.
Adverse events
Adverse events were analyzed in the SS (n = 150). There were no significant differences in treatment-emergent adverse events (TEAEs) or adverse drug reactions (ADRs) between the groups (Table 4). TEAEs were identified in 16 patients in the fexuprazan group and 17 patients in the esomeprazole group. ADRs were identified in five patients in the fexuprazan group and in four patients in the esomeprazole group. No serious TEAEs or ADRs were identified in either patient group.
Discussion
PPIs are commonly used to treat acid-related reflux diseases, but ineffective for postprandial heartburn, extra-esophageal GERD symptoms, and may cause some complications with long-term therapy [24]. Thus, new gastric acid suppressant P-CABs such as fexuprazan are available as alternatives treatment for GERD [25]. LPRD, like GERD, is also caused by reflux of gastric contents and treated with PPI or P-CAB. However, LPRD requires at least 8 weeks of prolonged anti-reflux therapy to significantly improve major extraesophageal symptoms. Patients with LPRD can be initially treated with 40 mg esomeprazole once a day for 8 weeks [26]. In our trial, the efficacy and safety of fexuprazan 40 mg compared with esomeprazole 40 mg for 8 weeks, in patients with symptoms and signs of LPRD were examined. Both groups presented similar treatment responses, with different response patterns for some detailed features.
There are many differences in the action mechanisms between PPIs and P-CAB [27]. P-CAB can directly inhibit the proton pump without transformation to the active form and thus can be taken regardless of the meal. In addition, P-CAB can fully control the proton pump after the first dose, resulting in rapid action. Clinical trials on the effectiveness of P-CAB in GERD are being conducted; however, there is still a lack of information on the effectiveness of P-CAB in LPRD. In this study, fexuprazan resulted in faster symptom improvement than esomeprazole in patients with LPRD with severe symptoms, especially in terms of extra-esophageal symptoms. This may serve as the basis for the use of P-CAB in LPRD, which often requires a longer treatment than GERD.
In this study, the FAS was used for efficacy evaluation. The primary outcome was the mean change in the RSI score at week 8. Each group showed a significant decrease in the mean change in RSI score at week 8 and there were no significant differences between the two groups. The mean change in the RSI score at week 8 in the fexuprazan group was -9.029 ± 7.602. In previous studies of patients with LPRD, the mean change in the RSI score at week 8 was −7.1 and −5.18 for the PPIs rabeprozole [21] and omeprazole [23], respectively, similar to the present study, although with a slightly smaller change than with fexuprazan.
A previous double-blinded placebo-controlled study reported that patients with LPRD had a significant improvement in both RSI and RFS scores compared to placebo when treated with esomeprazole [28]. In our study, when LPRD was treated with esomeprazole or fexuprazan, both RSI and RFS scores at weeks 4 and 8 showed significant improvement. Both the fexuprazan and esomeprazole groups showed a valid rate of approximately 30% at week 4 and approximately 50% at week 8, without significant differences between the two groups. Both groups showed a greater response rate at week 8 than at week 4; Thus, long-term administration of PPI or P-CABs is required to treat LPRD.
There are several studies comparing the effects of PPI therapy and placebo in patients with suspected LPRD [29, 30]. Most studies did not show significant improvement in symptom relief or laryngeal findings compared to placebo when taking PPI in patients with suspected LPRD. This means that not only PPI therapy but also behavioral modification is very important in the treatment of LPRD. This is thought to be because PPI therapy suppresses reflux, but cannot eliminate the underlying etiology of LPRD [31]. Eating meals within two hours before bedtime, caffeine drinks, sour fruit, and spicy food is associated with prolonged acidity in the stomach, which could cause reflux. Additionally, increasing weight is significantly associated with increase in the risk for reflux diseases [32]. Thus, we tried to minimize the difference between behavior habits in the two groups by educating these habits and checking their performance at each patient’s visit in this study. Further studies comparing the fexuprazan group with the placebo group may helpful to systemize the effects of behavior modification in patients with LPRD.
The comparison between the fexuprazan and esomeprazole groups in the FAS does not reflect the therapeutic effects for patients with LPRD according to the degree of symptom severity. To compensate for this limitation, the FAS was classified into severe and mild symptom groups based on the median value of the baseline RSI. In the severe group, the fexuprazan group showed a greater improvement in the mean change in the RSI score (hoarseness, swallowing difficulty, and troublesome cough) at week 4 than the esomeprazole group. Similarly, the fexuprazan group showed greater improvement in the RSS-12 QoL score at week 4 than the esomeprazole group. This may relate to the rapid action and long-lasting effect of P-CAB compared with esomeprazole.
Our study had some limitations. First, to maintain the double-blind condition, all patients in both groups were administered the medication only before meals. PPIs can only be taken before meals, whereas P-CABs can be administered independently. Thus, the advantages of postprandial administration, such as convenience and patient compliance, were not considered. Second, 24-h MII-pH, which is considered the gold standard for the diagnosis of LPRD [33, 34], was not performed because of the need for common diagnostic tools in the study’s institutions. Further studies analyzing the therapeutic effect of fexuprazan after detailed classification of reflux types using 24-h MII-pH will be of value [35]. Finally, the total duration of this clinical trial was 8 weeks, which limits the analysis of long-term efficacy and side effects of fexuprazan. LPRD usually requires a longer and more aggressive treatment than GERD. Follow-up clinical trials should be conducted over a trial period of more than 3 months. Twice-daily dosing of PPIs presented better treatment results than once-daily dosing in some articles [36]. Although the therapeutic efficacy of P-CAB is sustained for 24 h, it is also necessary to evaluate the treatment response of twice-daily dosing of P-CAB in future studies.
To the best of our knowledge, this is the first multicenter prospective randomized controlled trial to compare the efficacy of P-CABs with PPIs for LPRD. The usefulness of fexuprazan in LPRD was confirmed, without significant side effects, and symptoms such as hoarseness, swallowing difficulty, and troublesome cough improved faster with fexuprazan than with esomeprazole in patients with more severe symptoms. Fexuprazan is a safe and effective alternative therapeutic option to PPIs in patients with LPRD.
Availability of data and materials
Data are available upon reasonable request to the corresponding author.
References
Mosli M, Alkhathlan B, Abumohssin A et al (2018) Prevalence and clinical predictors of LPR among patients diagnosed with GERD according to the reflux symptom index questionnaire. Saudi J Gastroenterol 24(4):236–241
Lechien JR, Akst LM, Hamdan AL et al (2019) Evaluation and management of laryngopharyngeal reflux disease: state of the art review. Otolaryngol Head Neck Surg 160(5):762–782
Gupta N, Green RW, Megwalu UC (2016) Evaluation of a laryngopharyngeal reflux management protocol. Am J Otolaryngol 37(3):245–250
Belafsky PC, Postma GN, Koufman JA (2002) Validity and reliability of the reflux symptom index (RSI). J Voice 16(2):274–277
Belafsky PC, Postma GN, Koufman JA (2011) The validity and reliability of the reflux finding score (RFS). Laryngoscope 111(8):1313–1317
Ford CN (2005) Evaluation and management of laryngopharyngeal reflux. JAMA 294(12):1534–1540
Altman KW, Stephens RM, Lyttle CS, Weiss KB (2005) Changing impact of gastroesophageal reflux in medical and otolaryngology practice. Laryngoscope 115(7):1145–1153
Sachs G, Shin JM, Howden CW (2006) Review article: the clinical pharmacology of proton pump inhibitors. Aliment Pharmacol Ther 23(s2):2–8
Bulmer DM, Ali MS, Brownlee IA, Dettmar PW, Pearson JP (2010) Laryngeal mucosa: its susceptibility to damage by acid and pepsin. Laryngoscope 120(4):777–782
Jung AR, Kwon OE, Park JM et al (2019) Association between pepsin in the saliva and the subjective symptoms in patients with laryngopharyngeal reflux. J Voice 33(2):150–154
Kim SI, Lechien JR, Ayad T et al (2020) Management of laryngopharyngeal reflux in Asia. Clin Exp Otorhinolaryngol 13(3):299–307
Lechien JR, Bock JM, Carroll TL, Akst LM (2020) Is empirical treatment a reasonable strategy for laryngopharyngeal reflux? A contemporary review. Clin Otolaryngol 45(4):450–458
Sunwoo J, Oh J, Moon S et al (2018) Safety, tolerability, pharmacodynamics and pharmacokinetics of DWP 14012, a novel potassium-competitive acid blocker, in healthy male subjects. Aliment Pharmacol Ther 48(2):206–218
Jenkins H, Sakurai Y, Nishimura A et al (2015) Randomised clinical trial: safety, tolerability, pharmacokinetics and pharmacodynamics of repeated doses of TAK-438 (vonoprazan), a novel potassium-competitive acid blocker, in healthy male subjects. Aliment Pharmacol Ther 41(7):636–648
Shibli F, Kitayama Y, Fass R (2020) Novel therapies for gastroesophageal reflux disease: beyond proton pump inhibitors. Curr Gastroenterol Rep 22:1–13
Lee KN, Lee OY, Chun HJ et al (2022) Randomized controlled trial to evaluate the efficacy and safety of fexuprazan compared with esomeprazole in erosive esophagitis. World J Gastroenterol 28(44):6294
Chan A-W, Tetzlaff JM, Altman DG et al (2013) SPIRIT 2013 statement: defining standard protocol items for clinical trials. Ann Intern Med 158(3):200–207
Kim SI, Jeong SJ, Kwon OE et al (2022) 24-hour multichannel intraluminal impedance—pH in proton pump inhibitor nonresponders vs responders in patients with laryngopharyngeal reflux. Otolaryngol Head Neck Surg 166(5):910–916
Lechien JR, Bobin F, Rodriguez A et al (2021) Development and validation of the short version of the reflux symptom score: reflux symptom score–12. Otolaryngol Head Neck Surg 164(1):166–174
Carrau RL, Khidr A, Gold KF et al (2005) Validation of a quality-of-life instrument for laryngopharyngeal reflux. Arch Otolaryngol Head Neck Surg 131(4):315–320
Lee YS, Choi S-H, Son YI, Park Y-H, Kim SY, Nam SY (2011) Prospective, observational study using rabeprazole in 455 patients with laryngopharyngeal reflux disease. Eur Arch Otorhinolaryngol 268:863–869
Lechien JR, Steffens Y, Calvo-Henriquez C, Mayo-Yanez M, Horoi M, Rodriguez A (2023) Impact of COVID-19 lockdown on stress and diet adherence in patients with laryngopharyngeal reflux. Maedica 18(2):190
Doshi K, Varghese A, Badyal DK (2015) Evaluation of omeprazole in the treatment of laryngopharyngeal reflux disease: a single center, prospective and randomized study. Int J Otorhinolaryngol Clin 7(2):45–50
Rettura F, Bronzini F, Campigotto M et al (2021) Refractory gastroesophageal reflux disease: a management update. Front Med 8:765061
Armstrong D (2023) Potassium-competitive acid blockers and gastroesophageal reflux disease. Gastroenterol Hepatol 19(5):295
DelGaudio JM, Waring JP (2003) Empiric esomeprazole in the treatment of laryngopharyngeal reflux. Laryngoscope 113(4):598–601
Leowattana W, Leowattana T (2022) Potassium-competitive acid blockers and gastroesophageal reflux disease. World J Gastroenterol 28(28):3608
Reichel O, Dressel H, Wiederänders K, Issing WJ (2008) Double-blind, placebo-controlled trial with esomeprazole for symptoms and signs associated with laryngopharyngeal reflux. Otolaryngol Head Neck Surg 139(3):414–420
Steward DL, Wilson KM, Kelly DH et al (2004) Proton pump inhibitor therapy for chronic laryngo-pharyngitis: a randomized placebo-control trial. Otolaryngol Head Neck Surg 131(4):342–350
Reimer C, Bytzer P (2008) Management of laryngopharyngeal reflux with proton pump inhibitors. Ther Clin Risk Manag 4(1):225–233
Yuksel ES, Vaezi MF (2013) Therapeutic strategies for laryngeal manifestations of gastroesophageal reflux disease. J Clin Gastroenterol 47(3):195–204
Dore MP, Maragkoudakis E, Fraley K et al (2008) Diet, lifestyle and gender in gastro-esophageal reflux disease. Dig Dis Sci 53:2027–2032
Kim SI, Jeong SJ, Kwon OE et al (2021) Pharyngeal reflux episodes in patients with suspected laryngopharyngeal reflux versus healthy subjects: a prospective cohort study. Eur Arch Otorhinolaryngol 278(9):3387–3392
Lechien JR, Vaezi MF, Chan WW et al (2024) The Dubai definition and diagnostic criteria of laryngopharyngeal reflux: The IFOS consensus. Laryngoscope 134(4):1614–1624
Lee JS, Jung AR, Park JM, Park MJ, Lee YC, Eun YG (2018) Comparison of characteristics according to reflux type in patients with laryngopharyngeal reflux. Clin Exp Otorhinolaryngol 11(2):141–145
Lechien JR, Mouawad F, Barillari MR et al (2019) Treatment of laryngopharyngeal reflux disease: a systematic review. World J Clin Cases 7(19):2995
Acknowledgements
Daewoong Pharmaceutical Co., Ltd. (Seoul, South Korea) supported all expenses of the clinical trial and provided the medication and matching placebo.
Funding
The authors have no financial relationship.
Author information
Authors and Affiliations
Contributions
S.I.K.: study design, data collection and analysis, writing, revising article, final approval of the version. Y.C.L.: data collection and analysis, final approval of the version. W.C.: data collection and analysis, final approval of the version. A.R.J.: data collection and analysis, final approval of the version. J.Y.J.: data collection and analysis, final approval of the version. J.-S.C.: data collection and analysis, final approval of the version. D.K.L.: data collection and analysis, final approval of the version. H.H.L.: data collection and analysis, final approval of the version. M.S.K.: data collection and analysis, final approval of the version. Y.S.L.: study design, data collection and analysis, revising article, final approval of the version, supervising this study. Y.-G.E.: study design, data collection and analysis, revising article, final approval of the version, supervising this study.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflicts interest.
Ethical approval
The study was approved by the Institutional Review Board (IRB) of Kyung Hee University Hospital at Gangdong (approval no. KHNMC 2022-01-020), Seoul National University Bundang Hospital (B-2022-741-002), Nowon Eulji Medical Center (EMCS 2022-01-033), Ajou University Hospital (AJIRB-MED-CT2-21-681), Inha University Hospital (2022-01-005), Dong-A University Hospital (DAUHIRB-22-042), Kosin University Hospital (KUGH 2022-01-037), Kyung Hee University Medical Center (KHUH 2022-02-007), and Asan Medical Center (S2022-0025-0001). All participants were provided with information regarding the study, and written informed consent was obtained from all eligible participants before enrolment.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kim, S.I., Lee, Y.C., Cha, W. et al. Efficacy and safety of fexuprazan in patients with symptoms and signs of laryngopharyngeal reflux disease: a randomized clinical trial. Eur Arch Otorhinolaryngol (2024). https://doi.org/10.1007/s00405-024-08877-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00405-024-08877-6