Abstract
Purpose
First-generation bone bridges (BBs) have demonstrated favorable safety and audiological benefits in patients with conductive hearing loss. However, studies on the effects of second-generation BBs are limited, especially among children. In this study, we aimed to explore the surgical and audiological effects of second-generation BBs in patients with bilateral congenital microtia.
Methods
This single-center prospective study included nine Mandarin-speaking patients with bilateral microtia. All the patients underwent BCI Generation 602 (BCI602; MED-EL, Innsbruck, Austria) implant surgery between September 2021 and June 2023. Audiological and sound localization tests were performed under unaided and BB-aided conditions.
Results
The transmastoid and retrosigmoid sinus approaches were implemented in three and six patients, respectively. No patient underwent preoperative planning, lifts were unnecessary, and no sigmoid sinus or dural compression occurred. The mean function gain at 0.5–4.0 kHz was 28.06 ± 4.55-dB HL. The word recognition scores improved significantly in quiet under the BB aided condition. Signal-to-noise ratio reduction by 10.56 ± 2.30 dB improved the speech reception threshold in noise. Patients fitted with a unilateral BB demonstrated inferior sound source localization after the initial activation.
Conclusions
Second-generation BBs are safe and effective for patients with bilateral congenital microtia and may be suitable for children with mastoid hypoplasia without preoperative three-dimensional reconstruction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Microtia is a congenital anomaly of the ear that ranges in severity from mild structural abnormalities to complete absence of the ear. More than 90% of individuals with microtia experience conductive hearing loss (CHL) on the affected side, with an air-bone-conduction gap (ABG) of up to approximately 60 dB HL [1, 2]. In patients with bilateral microtia, early hearing intervention, such as bone-conduction hearing aids, is important for providing adequate stimulation for the development of the central auditory system. Bone conduction implants are considered one of the best options for improving hearing when a patient grows older [3]. The first-generation bone bridge (BB), MED-EL (Austria), was introduced in 2012. It is a semi-implantable hearing system comprising two major parts. The implantable part contains a bone-conduction floating mass transducer (BC-FMT) that applies vibrations directly to the bone, and the external part is an audio processor that digitally processes sound and sends information through the coil to the internal part. The first-generation BB, BCI601, has demonstrated favorable safety and significantly improved hearing thresholds and speech perception in children with CHL [4, 5].
Congenital microtia is often accompanied by anatomic malformations [6], such as mastoid hypoplasia, sigmoid sinus antidisplacement, temporomandibular joint retroposition, and a low middle fossa tegmen plate, which pose a challenge to the transducer’s implantation space. The penetration depth due to the 8.7-mm FMT thickness of BCI601 makes preoperative three-dimensional (3D) reconstruction of the temporal bones and virtual 3D BC-FMT models necessary, especially in children with microtia.
In patients with malformations, poorly pneumatized mastoids, and after canal wall-down surgery, BCI601 is associated with a considerable risk of depressing the sinus and/or dura [7]. Furthermore, a lift is required to reduce the drilling depth in patients with microtia [8]. The latest generation, BCI602, addressed these disadvantages as it is almost half as thick compared with the previous generation and allows implantation with a 4.5-mm drilling depth; thus, rendering presurgical planning redundant and allowing for more individual positioning options. Current research on BCI602 is mostly focused on adult patients with single-sided-deaf (SSD) and mixed/conductive hearing loss (M/CHL). There is a lack of research reports on the surgical effect and sound source localization ability in children with bilateral CHL [9, 10].
In this study, we aimed to share our surgical experience associated with the novel BCI602 in patients with bilateral congenital microtia. Moreover, using audiological evaluation, we aimed to explore whether a decrease in FMT thickness could maintain sufficient auditory gain in patients with congenital microtia.
Materials and methods
Nine patients, including seven males and two females with bilateral microtia and atresia, with a mean age of 9.78 (range 6–16) years, were included in this single-center prospective study. All patients were Mandarin-speaking and underwent BCI Generation 602 (BCI602; MED-EL, Innsbruck, Austria) implant surgery at [BLINDED FOR REVIEW] between September 2021 and June 2023. This study was conducted in accordance with the ethical standards of the 1964 Declaration of Helsinki and its later amendments. Ethical approval for the present study was obtained from the Institutional Ethics Committee of our hospital (no. Z171100001017079).
The patient characteristics are presented in Table 1. The inclusion criteria were as follows: (1) age ≥ 5 years and bilateral congenital malformations of the middle ear with or without atresia; (2) air bone gap (ABG) > 60 dB HL, bone-conduction threshold (BCT) ≤ 20 dB HL between 0.25 and 4 kHz, and a BCT difference between both ears of < 15 dB HL; (4) patients with a Jahrsdoerfer score of ≤ 7; (5) no inner-ear malformation or central auditory processing disorders; and (6) a sound understanding of Mandarin and the ability to repeat words. Patients who did not meet these criteria were excluded from the analysis.
Surgical intervention and test conditions
All the participants signed an informed consent form before surgery. Preoperative computed tomography (CT) images were obtained to evaluate the development of the patient’s temporal bone and the Jahrsdoerfer score. The optimal surgical approach was selected based on preoperative CT findings and the original plastic incision. The implantation side of the BB depended on the hearing threshold and the implant space, which is determined by the skull development and degree of mastoid pneumatization. The patients included in this study had bilateral symmetrical CHL, and the choice of implant side was mainly based on the development of the bilateral temporal bone and patient's preference. For patients with differences in bilateral temporal bone development, the relatively well-developed side was chosen through the evaluation of preoperative temporal bone CT, which can acquire a larger surgical implant space. In patients with no significant differences in binaural hearing and temporal bone development, the implant side of the BB was freely chosen by the patient and their family following preoperative soft band trying. Some patients and their families chose the right side for the convenience of wearing as they were right-handed. The initial activation and fitting of the audio processor were performed approximately 2 weeks after surgery, following the complete disappearance of any swelling over the receiver coil and magnet. Audiological and sound source localization tests were performed under unaided conditions before surgery and BB-aided conditions 1 month after surgery.
Hearing threshold and the speech perception test
Pure-tone audiometry (PTA), sound-field hearing thresholds (SFTs), speech reception thresholds in noise (SRTs), and word recognition scores (WRSs) under quiet conditions were collected and compared. PTA was measured using a US GSI-61 audiometer to determine the air threshold and BCT at 0.25, 0.5, 1, 2, and 4 kHz. SFT was evaluated using a trill presented from the front at 0.25, 0.5, 1, 2, and 4 kHz. The average SFTs at 0.5, 1, 2, and 4 kHz were calculated and compared. The SRT for noise presented at 65 dB was determined using an adaptive test with speech and noise proceeding from the front and was expressed using the signal noise ratio (SNR, dB), which is defined as the difference between speech presentation and the noise level when the patient achieves 50% speech recognition. The WRS under quiet conditions was measured using Mandarin speech test materials. Fifty monosyllabic words, 50 disyllabic words, and 10 short sentences were dictated at a 65-dB sound pressure level (SPL). The percentages of correctly identified words and sentences were calculated.
Sound localization test
The sound localization test methods were consistent with those in a previous study [11]. Briefly, seven loudspeakers located at the front at ± 90°, with an average interval of 30° for all loudspeakers, were positioned in a horizontal plane in a sound-proof room. Patients wearing eye masks were seated at 0° azimuth toward the middle of the loudspeaker. The stimulus level was presented at 65-dB SPL with a level roving of ± 5 dB. Each stimulus level was presented twice in random order. Overall, 42 presentations were made for each test condition. The patients were asked to indicate the number of loudspeakers that presented the stimuli. Sound localization was tested after BB activation in three patients. In our study, the sound source localization test was based on the premise that the sound could be received by the patients (stimulus level ≥ 65 dB SPL). Although patients aided by a unilateral BB have lateralized sound source localization, they achieved substantial hearing threshold benefits.
Statistical analysis
Data analysis was performed using SPSS® statistical software version 22.0. Quantitative indicators were described as mean ± standard deviation (SD) or median (range). Normally distributed data were analyzed using a paired-sample t test and a repeated measures analysis of variance (ANOVA), and non-normally distributed data were analyzed using a Wilcoxon signed rank test. A P value less than 0.05 was considered statistically significant. The localization accuracy was determined by calculating the mean absolute error (MAE; see Eq. 1) between the response angle indicated by the patient and the actual stimulus angle presented by the loudspeaker. The best linear fit of the stimulus–response relationship for azimuth was determined using Eq. (2), where αRESP and αSTIM are the response and stimulus azimuths, respectively, expressed in degrees; b is the response bias (offset in degrees); and g is the response gain (slope, dimensionless). If all data points in the stimulus–response plot formed a diagonal, resulting in an MAE < 10°, a gain close to 1, and a bias close to 0, the patient was considered a good performer.
Results
Surgical approaches and complications
A footprint sizer was used to mark the position of the implant on intact skin. A skin incision was made 0.5–1.0 cm posterior to the latter helix or at the residual incision of the aesthetic auricular reconstruction. A V-shaped incision was made in the myofascial region of the temporalis. A subperiosteal pocket was created directly on the skull for the coil and the size was verified using a footprint sizer. The thickness of the skin flap above the coil was limited to 7 mm. A transducer sizer was used to outline the position and measure the depth of the implant bed for BC-FMT. A 4.5-mm deep recess for the BC-FMT was drilled, and the bottom and sides of the implant bed were examined to determine whether exposure of the dura or sigmoid sinus was present. Thereafter, the implant was bent according to the implant position and skull curvature and placed in the implant bed. To ensure that the fixation wings with anchor holes were laid flat on the bone without any gaps or soft tissue, two self-drilling screws were used to fix the implant through the anchor holes. The planar position of the BC-FMT was verified by palpation, followed by wound closure. No lifts were necessary among the nine patients, and no complications occurred.
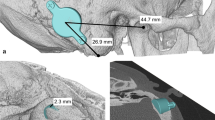
The mean overall surgery time was 34 (range 30–40) min. The transmastoid approach was used in patients 1 (P1), 3 (P3), and 4 (P4), and the retrosigmoid (RS) approach was used in patients 2 (P2), 5 (P5), 6 (P6), 7 (P7), 8 (P8), and 9 (P9). None of the patients underwent preoperative 3D planning for BCI602 or used a lift, and no sigmoid sinus or dural compression occurred. P2, P5, P6, P7, P8, and P9 had already undergone aesthetic auricular reconstruction using the skin-expanding method and had a residual incision (Fig. 1). To avoid creating a new postauricular incision, the FMT was implanted using the RS sinus approach through a residual plastic incision. P3 exhibited bilateral congenital aural atresia accompanied by mastoid hypoplasia. We simulated the BCI601 implant using 3D reconstruction (see Figure, Supplemental Digital Content 1). The BCI601 implant space in the mastoid area was limited by the BCI601 obviously protruding from the bone surface; thus, suggesting the necessity of a lift for this patient, with a considerable possibility of compression of the regional dura or sigmoid sinus. The patient was successfully implanted with the BCI602 via the transmastoid approach without lifting, and no compression occurred. Due to mastoid hypoplasia, the sigmoid sinus was partially exposed but not compressed, and there was no exposure of the dura mater. There was no exposure of any sigmoid sinus or dura mater in the other patients, except in P3.
The preoperative, perioperative, and postoperative images of patient 2. a Pre-operation image of patient 2 6 months after total auricular reconstruction using the total expanded flap technique. The surgical incision can be observed posterior to the hairline. b Post-operation image of Patient 2 fitted with a new generation bone bridge processor (Samba 2). c To avoid creating another post-ear incision, the FMT was implanted using the retrosigmoid approach through the original plastic incision. The implant bed was drilled without the exposure of the dura or sigmoid sinus. d The FMT was implanted without the use of lift. FMT floating mass transducer
Free SFT
The average preoperative pure tone audiometry threshold of the patients was 63.67 ± 5.92 dB on the left side and 62.39 ± 4.95 dB on the right side, with no difference in hearing threshold between bilateral ears (paired samples t test, P < 0.01). The difference between the pre-and post-operative BCTs of the patients was within 5-dB of HL (Table 1), suggesting that BCI602 implantation was a safe surgical approach. The mean SFTs of the patients were 60.56 ± 3.96-dB HL and 32.50 ± 4.28-dB HL under unaided and BB-aided conditions, respectively. The mean function gain at 0.5–4.0 kHz was 28.06 ± 4.55-dB HL (Table 1). The frequency-specified sound field thresholds for different listening conditions are shown in Fig. 2. The average functional gain at 1.0 and 4.0 kHz was higher than that at other frequencies, with mean values of 32.78 ± 7.95-dB HL and 32.22 ± 6.18-dB HL, respectively (paired samples t test, P < 0.05). The mean FG was lowest at 0.25 kHz, with a mean value of 16.11 ± 9.61-dB HL (paired samples t test, P < 0.01).
Speech reception scores
The WRSs under quiet conditions are shown in Fig. 3. The mean monosyllabic, disyllabic, and sentence WRSs were 1.78 ± 3.67%, 4.44 ± 9.32%, and 11.11 ± 24.64%, respectively, under unaided conditions. These values significantly improved to 78.55 ± 11.13%, 90.67 ± 8.89%, and 99.11 ± 2.67%, respectively, under BB-aided conditions. The mean gains for monosyllabic words, disyllabic words, and sentences were 77%, 86%, and 88%, respectively. The mean SRT in noise of the patients was 9.78 ± 1.92-dB SNR under “unaided” conditions and − 0.8 ± 0.97-dB SNR under BB-aided conditions. The SRT in noise was improved by lowering the SNR by a mean value of 10.56 ± 2.30-dB SNR with BB upon activation (Wilcoxon signed rank test, P < 0.05).
Sound localization
The mean unaided MAE of the children with CHL was 36.83 ± 16.74°. Under “aided” conditions, the mean MAE was 70.32 ± 10.67°. Patients in the unaided condition exhibited superior directional hearing and a lower MAE than those with unilateral fitting (paired samples t test, P < 0.05). The sound source localization under the BB aided condition at three sound intensities (65, 70, and 75 dB) was 70.00 ± 16.18, 69.29 ± 15.11, and 71.67 ± 9.65 dB, respectively. In the unaided condition, the MAE values at 65, 70, and 75 were 49.52 ± 15.58, 35.48 ± 19.23, and 25.48 ± 20.00 dB, respectively. The patients showed the best localization performance when a suprathreshold sound intensity of 75 dB was applied (repeated measures ANOVA, P < 0.05). However, there was no significant difference in sound source localization ability under the BB-aided condition among the different sound intensities (repeated measures ANOVA, P > 0.05) (Fig. 4).
The localization results under the unaided and aided conditions are shown by the stimulus–response plots displayed in Fig. 5. There were individual differences in sound localization among patients. Patients P5 and P9 exhibited poor sound localization under the unaided condition. The hearing threshold of the two patients before surgery was relatively poor; the preoperative hearing thresholds were 63.75 and 65 dB, respectively. Except for P3, P5, and P9, the gains of the patients in the unaided condition were better (close to 1) than those in the BB-aided condition. Sound localization was biased toward the implantation side in all patients (a negative bias value represents left lateralization; a positive bias value represents right lateralization).
Discussion
The dimensions of the BC-FMT are based on the limitations of the implant space and the requirement for adequate vibration. The optimized geometry of the transducer has two main advantages. First, the decreased implant depth expands the anatomical indications and makes it more suitable for patients with congenital microtia, who often have limited implant space due to mastoid hypoplasia. Based on 3D reconstructions, the BCI-601 can be adequately fitted in < 50% of children < 8 years of age, while the BCI 602 can be virtually implanted in 100% of patients aged ≥ 12 years and in 75% of children aged 3–5 years [12]. Considering congenital microtia is always accompanied by a limited implant space resulting from temporal bone dysplasia, BCI601 implantation in these patients often requires detailed preoperative CT-based surgical planning. Additionally, alternative surgical approaches, such as the middle fossa or RS approaches, were selected, which may occasionally be necessary to compress the dura and distance the microphone from the pinna [13, 14].
Second, the decreased thickness reduced the usage of the lift and exposure of the sinus and dura. The use of lifts and sigmoid sinuses or dura compression is necessary to accommodate the BCI601 in most cases [15]. With the BCI601, it is almost always necessary to expose and even compress the regional dura or sigmoid sinus, especially in younger children and difficult cases [12, 16]. In a previous study of 110 patients with bilateral congenital microtia, a lift was used in 38% of patients during BCI601 implantation, and 39% had dural and/or sigmoid sinus compression [8]. In this study, no lift was used, and no compression of the dura or sigmoid sinus occurred in either the transmastoid or RS approach. Only patient P3, who had bilateral mastoid hypoplasia with limited implant space in the mastoid area for BCI601, was successfully implanted with the BCI602 via the transmastoid approach with dural exposure without compression.
The resonance frequency is indirectly and exponentially related to the mass of the BC-FMT [7]. The volume of the BC-FMT is crucial for achieving adequate skull acceleration; therefore, a lower penetration depth of the BC-FMT into the temporal bone requires a larger BC-FMT diameter to ensure adequate acceleration. The novel BCI addresses this problem by increasing the diameter to 2.4 mm and partially translocating the BC-FMT above the skull surface.
Through an audiological evaluation, we verified whether sufficient functional gain was achieved. Previous studies on individuals with CHL, mixed hearing loss, and single-sided deafness demonstrated that the mean FG with BCI602 at activation was 25- to 28.0-dB HL [9, 17]. In our study on bilateral microtia, this value was 28.06 ± 4.55-dB HL. Previous studies have shown that the BCI601 has greater hearing gain at high frequencies [18]. In this test, the mean FG was lowest at 0.25 kHz, with mean value of 16.11 ± 9.61-dB HL, and was better at 1.0 kHz and 4.0 kHz, with mean values of 32.78 ± 7.95-dB HL and 32.22 ± 6.18-dB HL, respectively; however, a longer follow-up and larger sample are required.
Speech intelligibility significantly improved in all patients after BCI602 implantation. The mean gains for monosyllabic words, disyllabic words, and sentences were 77%, 86%, and 88%, respectively. The SRT in noise was improved by lowering the SNR by a mean value of 10.56 ± 2.30-dB SNR with BB upon activation (P < 0.05). Research on BCI602 implantation in 13 adults and 10 children with either CHL or MHL showed a 7.02 dB improvement in the mean SRT in noise and 68.0% in the WRS in quiet conditions [19]. Sprinzl et al. [9] reported significantly increased FG and speech intelligibility in patients with M/CHL after 3 months, suggesting that further improvement may occur over time.
In our study, the sound source localization ability during activation was worse than that under unaided conditions. With unilateral fitting, patients with bilateral CHL demonstrated difficulties discriminating horizontal sound positions and tended to be biased toward the implantation side when identifying the sound source. The reasons for this are as follows. First, when only one BB was applied, the stimulated vibrations were transmitted to the bilateral cochleae with only minimal differences in the level and time delay. Cross-stimulation interferes with the interaural time or intensity differences, thereby reducing the individuals’ ability to extract binaural cues [20]. Second, the phenomenon of sound source localization bias may be worse during activation and can be improved after long-term adaptation and training.
In summary, the geometric changes and use of the device’s self-drilling screws improved surgical handling and reduced sigmoid sinus or dura compression and lift use. Patients with congenital microtia benefited significantly from the BCI602 treatment in terms of safety and audiological performance.
Methodological considerations and limitations
The implantation of this new generation was first reported globally in 2019 and introduced in China in 2021; therefore, this study is a preliminary report on the application of this novel implant. However, this study only included nine patients; hence, further large-sample studies are warranted. Although the audiological results demonstrated significant hearing benefits, long-term results, especially of speech intelligibility in noise and sound source localization, require further evaluation over time.
Conclusion
Second-generation BBs are safe and effective solutions for hearing reconstruction in patients with bilateral congenital microtia. With optimization of the FMT, it can be safely applied to children with mastoid hypoplasia, without preoperative 3D reconstruction of the temporal bones, and potentially yields satisfactory audiological gain.
Data, materials and/or code availability
The data are available from the corresponding author on reasonable request.
References
Luquetti DV, Heike CL, Hing AV et al (2012) Microtia: epidemiology and genetics. Am J Med Genet 158A:124–139. https://doi.org/10.1002/ajmg.a.34352
Shonka DC, Livingston WJ, Kesser BW (2008) The Jahrsdoerfer grading scale in surgery to repair congenital aural atresia. Arch Otolaryngol Head Neck Surg 134:873. https://doi.org/10.1001/archotol.134.8.873
Lo JFW, Tsang WSS, Yu JYK et al (2014) Contemporary hearing rehabilitation options in patients with aural atresia. Biomed Res Int 2014:1–8. https://doi.org/10.1155/2014/761579
Yang J, Chen P, Zhao C et al (2020) Audiological and subjective outcomes of 100 implanted transcutaneous bone conduction devices and preoperative bone conduction hearing aids in patients with bilateral microtia-atresia. Acta Otolaryngol 140:667–673. https://doi.org/10.1080/00016489.2020.1762929
Irmer C, Volkenstein S, Dazert S, Neumann A (2022) The bone conduction implant BONEBRIDGE increases quality of life and social life satisfaction. Eur Arch Otorhinolaryngol 279:5555–5563. https://doi.org/10.1007/s00405-022-07384-w
Takano K, Takahashi N, Ogasawara N, Himi T (2016) The association of external and middle ear anomaly and mandibular morphology in congenital microtia. Otol Neurotol 37:889–894. https://doi.org/10.1097/MAO.0000000000001048
Plontke SK, Götze G, Wenzel C et al (2020) Implantation of a new active bone conduction hearing device with optimized geometry. HNO 68:106–115. https://doi.org/10.1007/s00106-020-00877-2
Yang J, Zhao C, Liu Y et al (2020) The effect of anatomical variables and use of the Lifts system on hearing outcomes after implantation of an active transcutaneous bone conduction device in bilateral congenital conductive hearing loss. J Otolaryngol Head Neck Surg 49:57. https://doi.org/10.1186/s40463-020-00452-3
Sprinzl GM, Schoerg P, Ploder M et al (2021) Surgical experience and early audiological outcomes with new active transcutaneous bone conduction implant. Otol Neurotol 42:1208–1215. https://doi.org/10.1097/MAO.0000000000003230
Kim H, Park MK, Park SN et al (2023) Efficacy of the Bonebridge BCI602 for adult patients with single-sided deafness: a prospective multicenter study. Otolaryngol Head Neck Surg. https://doi.org/10.1002/ohn.520
Zhao C, Yang J, Liu Y et al (2020) Horizontal sound localisation and speech perception in Bonebridge-implanted single-sided deafness patients. J Laryngol Otol 134:814–821. https://doi.org/10.1017/S0022215120001899
Rahne T, Schilde S, Seiwerth I et al (2016) Mastoid dimensions in children and young adults: consequences for the geometry of transcutaneous bone-conduction implants. Otol Neurotol 37:57–61. https://doi.org/10.1097/MAO.0000000000000881
Zernotti ME, Di Gregorio MF, Zernotti M (2021) Alternative inverted middle fossa approach in Bonebridge surgery. Technique results and complications. Int Arch Otorhinolaryngol 25:e374–e378. https://doi.org/10.1055/s-0040-1715152
Kong TH, Park YA, Seo YJ (2017) Image-guided implantation of the Bonebridge™ with a surgical navigation: a feasibility study. Int J Surg Case Rep 30:112–117. https://doi.org/10.1016/j.ijscr.2016.11.057
Law EKC, Bhatia KSS, Tsang WSS et al (2016) CT pre-operative planning of a new semi-implantable bone conduction hearing device. Eur Radiol 26:1686–1695. https://doi.org/10.1007/s00330-015-3983-x
Lassaletta L, Sanchez-Cuadrado I, Muñoz E, Gavilan J (2014) Retrosigmoid implantation of an active bone conduction stimulator in a patient with chronic otitis media. Auris Nasus Larynx 41:84–87. https://doi.org/10.1016/j.anl.2013.04.004
Šikolová S, Urík M, Hošnová D et al (2022) Two Bonebridge bone conduction hearing implant generations: audiological benefit and quality of hearing in children. Eur Arch Otorhinolaryngol 279:3387–3398. https://doi.org/10.1007/s00405-021-07068-x
Huber AM, Sim JH, Xie YZ et al (2013) The Bonebridge: preclinical evaluation of a new transcutaneously-activated bone anchored hearing device. Hear Res 301:93–99. https://doi.org/10.1016/j.heares.2013.02.003
Sprinzl G, Toner J, Koitschev A et al (2023) Multicentric study on surgical information and early safety and performance results with the Bonebridge BCI 602: an active transcutaneous bone conduction hearing implant. Eur Arch Otorhinolaryngol 280:1565–1579. https://doi.org/10.1007/s00405-022-07792-y
Yost WA (2016) Sound source localization identification accuracy: level and duration dependencies. J Acoust Soc Am 140:EL4–EL9. https://doi.org/10.1121/1.4954870
Acknowledgements
This work was supported by a Grant from the National Natural Science Foundation of China (no. 81770989) and the Capital Health Research and Development of Special (no. 2020-2-2057).
Author information
Authors and Affiliations
Contributions
Conceptualization: PC, SZ, DW, XF, RD; methodology: PC, LY, YL, JY; formal analysis and investigation: DW, RR, YL; writing—original draft preparation: PC; writing—review and editing: SZ; funding acquisition: SZ; resources: SZ, DW, XF, RD; supervision: DW.
Corresponding author
Ethics declarations
Conflict of interest
We have no financial disclosures that may pose or create a conflict of interest regarding the information presented in this article.
Ethical approval
Ethical approval for the execution of the present study was obtained from the Institutional Ethics Committee of Beijing Tongren Hospital, Capital Medical University (no. Z171100001017079).
Informed consent
All the participants signed an informed consent form before surgery.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, P., Liu, Y., Yang, J. et al. A new active bone-conduction implant: surgical experiences and audiological outcomes in patients with bilateral congenital microtia. Eur Arch Otorhinolaryngol 281, 4039–4047 (2024). https://doi.org/10.1007/s00405-024-08523-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00405-024-08523-1