Abstract
Purpose
Local allergic rhinitis (LAR) is characterized by a localized nasal allergic response without evidence of systemic atopy. LAR is an underdiagnosed entity and is a diagnostic and therapeutic challenge for clinicians. This study aimed to investigate the prevalence and clinical characteristics of patients with LAR to house dust mites (LAR-HDM) in Korea.
Methods
We performed a retrospective chart review of 336 adult patients with rhinitis symptoms who visited the Rhinologic Clinic at Korea University Guro Hospital from October 2019 to April 2021. Using results of the skin prick test, serologic test, and nasal provocation test, patients were classified as allergic rhinitis (AR) to HDM (AR-HDM), AR to other allergens, non-allergic rhinitis (NAR), or LAR-HDM. We excluded patients with AR to other allergens and compared the clinical characteristics of the remaining three groups. Patient demographic data were reviewed, and patients’ nasal symptoms, olfactory function, serum total IgE, and severity of accompanying rhinosinusitis were evaluated.
Results
In total, 336 patients were examined. AR-HDM was diagnosed in 138 (41.1%) patients, AR to other allergens in 36 (10.7%) patients, NAR in 21 (42.0%) patients, and LAR-HDM in 21 (6.3%) patients. The mean age of patients with LAR-HDM was significantly higher than that of patients with AR-HDM. There were no significant differences in sex, smoking history, asthma, and family history of allergic diseases between the groups. Compared to NAR patients, there were significantly more patients with LAR-HDM who had persistent nasal symptoms. The frequency of nasal itching and sneezing was significantly higher in the LAR-HDM group than in the NAR group. The olfactory function score in the LAR-HDM group was significantly worse than that in the AR-HDM group, and the Lund-Mackay score was significantly higher in the LAR-HDM group than in the other groups.
Conclusion
Clinical history and nasal symptoms are very similar in LAR-HDM and AR-HDM. Clinicians should take more care to differentiate them. LAR-HDM should also be considered in patients with persistent and severe nasal symptoms without systemic atopy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic rhinosinusitis is a common chronic inflammatory disease in Rhinologic clinics and affects approximately 30% of the total population. It is characterized by nasal symptoms such as nasal obstruction, rhinorrhea, sneezing, and itching [1]. Chronic rhinitis can be classified into the following four types according to etiology: allergic rhinitis (AR), non-allergic rhinitis (NAR), infectious rhinitis, and mixed rhinitis. In other words, non-infectious chronic rhinitis is generally divided into AR and NAR [2]. AR is an immunoglobulin E (IgE)-mediated chronic inflammatory disease and can be diagnosed based on the results of the skin prick test and serum allergen-specific IgE (sIgE) test [1]. However, even when the results of these two tests are negative, some patients show nasal symptoms suggestive of AR. This condition suggests a localized allergic reaction of the nose in the absence of systemic atopy, and termed as local allergic rhinitis (LAR) [3]. Although the pathophysiology of LAR is not fully understood, it has been reported that exposure to aeroallergens causes localized type 2 inflammation in the nasal epithelium and results in the production of nasal sIgE with levels not detected in peripheral mast cells of the blood [4].
The proportion of LAR among rhinitis patients has been reported to be relatively higher in Western countries than in East Asia. LAR accounts for 3.5–8.2% of all rhinitis patients in Asia, whereas it accounts for 17.6–25.7% in Western countries [5,6,7,8,9]. In addition, studies on LAR have mostly been conducted in Western countries over the past 10 years, and reports on the prevalence and clinical features of LAR seem to be lacking in Asia. Meanwhile, LAR, a distinct type of rhinosinusitis, has a natural course of worsening rhinitis severity and increasing the frequency of comorbidities such as asthma and conjunctivitis [10]. Hence, timely management of LAR is very important through a precise diagnosis, and the nasal provocation test (NPT) is known to be a safe and the most important test for diagnosis of LAR [11,12,13]. The position paper of the European Academy of Allergy and Clinical Immunology (EAACI) provided a standardized guideline for NPT in 2018, aiming to reduce the previous differences in NPT results [14].
Therefore, the purpose of this study was to investigate the prevalence of LAR using a standardized NPT and evaluate the clinical features of LAR compared to other rhinitis subtypes in Korea.
Materials and methods
Study design and subjects
A retrospective chart review was conducted for all adult patients with rhinosinusitis symptoms who visited the Rhinologic Clinic of Korea University Guro Hospital between October 2019 and April 2021. All patients aged 19 years or older who met the criteria for chronic rhinitis from the EAACI were included in this study: at least two nasal symptoms (nasal obstruction, rhinorrhea, sneezing, or itching) should be present for at least 1 h daily for a minimum of 12 weeks per year [2]. The following cases were excluded: previous sinonasal surgery, immunologic diseases, sinonasal malignant diseases, or upper airway infections in the previous 4 weeks, previous treatment at another clinic within 4 weeks (including oral medications or nasal sprays), or patients with incomplete data.
Measures
Demographic characteristics were reviewed, including age, sex, smoking history, asthma, and family history of allergic diseases. Nasal symptoms were evaluated according to the classification presented by ARIA (Allergic Rhinitis and its Impact on Asthma): intermittent or persistent, and mild or moderate to severe. We also reviewed which symptoms each patient presented with among the four rhinitis symptoms (nasal obstruction, rhinorrhea, sneezing, and itching), and the severity of complaints of nasal symptoms was evaluated using the visual analog scale (VAS) with a total score of 10. Olfactory function was assessed using the YSK olfactory function test (YOF test; Kimex Co, Suwon, Korea), which included thresholds, discrimination, and identification (TDI) tests. The total TDI score was 36 points, and the cut-off scores for anosmia and hyposmia were set to 14.5 and 21.0 points respectively, as suggested by the criteria of the previous study [15]. The serum level of total IgE serum level was measured using UniCAP system (Pharmacia, Uppsala, Sweden).
Skin prick test and serum allergen-specific IgE
The results of the skin prick test (SPT) and serum allergen-specific IgE test were reviewed for all enrolled patients, and patients with at least one positive result were defined as having systemic atopy. SPT was performed using multiple allergen panels, including house dust mites (HDM), tree pollen, grass pollen, weed pollen, mold, dog, cat, and cockroach (Allergopharma, Reinbek, Germany). The test was interpreted as a positive result if the wheal was ≥ 3 mm or ≥ 25% of the positive control. Serum allergen-specific IgE levels were measured using a fluoro-enzyme immunoassay method (UniCAP; Pharmacia, Uppsala, Sweden) to aeroallergens, including HDM, common ragweed, cat, dog, and cockroach. The cut-off value of the positive result was set to 0.35 U/mL.
Nasal provocation test
NPT was performed according to the standardization presented by the EACCI position paper in 2018. Baseline measurements were evaluated using subjective and objective assessments after 15 min to acclimate to room conditions. First, we performed a control challenge to exclude patients with nasal hyperreactivity, in which saline was sprayed into both nostrils with two puffs and reevaluated the subjective and objective assessments after 10 min. Next, an allergen challenge was performed on patients with negative control challenge results. The allergen solution was sprayed with two puffs into both nostrils and the subjective and objective assessments were reevaluated after 10 min. If a positive result was obtained, the test was stopped. But if a negative result was obtained, an allergen test was performed again after 10 min. If it was negative until the second allergen test, it was evaluated as a negative result.
We used the standardized allergen extract from Hollister Stier (Spokane, WA, USA), a mixture of D. pteronyssinus (DP), and D. farina (DF; 5,000 AU/ml each species). This allergen solution was diluted 1:10 with diluent (sterile albumin saline with phenol) and sprayed into the nostrils with a pump-aerosol spray offering (0.05 ml at each puff).
Acoustic rhinometry (Eccovision, Hood Laboratories, Pembroke, MA, USA) was used as an objective assessment to evaluate nasal patency, and the total nasal symptom score was used as a subjective assessment of clinical symptoms. The result of the test (control or allergen challenge) was evaluated as a positive result when either one of the objective and subjective assessments was clearly positive, or when both were moderately positive. The definition of clearly positive is decrease of cross section area − 2 ≥ 40% in acoustic rhinometry (objective assessment) and increase of ≥ 5 points in total nasal symptom score (subjective assessment). The moderately positive is decrease in the sum of nasal volume2–6 (cm3) ≥ 27% bilaterally in acoustic rhinometry and increase of ≥ 3 points in Total nasal symptom score.
Group classification
All enrolled patients were classified into four subgroups based on the results of the SPT, serum-specific IgE test, and NPT (Fig. 1). The AR-HDM group included patients with systemic atopy for DP or DF, regardless of the results for other allergens. The AR-others group included patients with systemic atopy to other allergens with negative results for DP and DF. Among patients without systemic atopy for all allergens, patients with positive NPT were classified into the LAR-HDM group, and patients with negative NPT were classified into the NAR group. Since we performed NPT using only HDM, the demographic and clinical characteristics were compared among the remaining three groups (AR-HDM, NAR, and LAR-HDM) except for the AR-others group.
Statistical analyses
Statistical analyses were performed using IBM SPSS (version 22.0, IBM Corp., Armonk, NY, USA). Numerical data are expressed as the mean ± standard deviation. One-way analyses of variance were used, followed by a post-hoc test (Tukey) for comparison of continuous variables with normality among groups. The Kruskal–Wallis test and Mann–Whitney U test were performed to compare continuous variables without normality among the groups. For categorical data, the chi-square test and Fisher's exact test were used to compare groups. Statistical significance was set at p < 0.05 (Fig. 2).
Results
Prevalence of LAR
A total of 336 patients were enrolled in the study. According to the diagnostic algorithm presented above, 138 (41.1%) patients were diagnosed with AR-HDM, 36 (10.7%) patients with AR-other, 141 (42.0%) patients with NAR, and 21 (6.3%) patients with LAR-HDM. Therefore, LAR-HDM accounts for 6.3% of all patients with chronic rhinitis symptoms.
Demographic characteristics
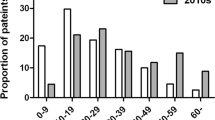
Table 1 shows the demographic characteristics of the three groups (AR-HDM, NAR, and LAR-HDM), including age, sex, smoking history, asthma, and family history of allergic diseases. The mean age of LAR-HDM (54.8 years) patients was significantly higher than that of AR-HDM patients (39.5 years; p = 0.001) and was not significantly different from that of NAR patients (54.1 years; p = 0.742). In particular, more than half of the patients (52.4%) in the LAR-HDM group were in their 60 s or above. Although not significantly, LAR-HDM showed a slight female predominance, unlike other patient groups. There were no significant differences in smoking history, asthma, or family history of allergic diseases.
Nasal symptoms, serum total IgE, and olfactory function test
Table 2 shows nasal symptoms in the three groups. With regard to ARIA classification, the duration and severity of LAR-HDM patients exhibited similar patterns to those of AR-HDM patients. Compared to NAR patients, there were significantly more patients with LAR-HDM who had persistent (p = 0.018), and although not significantly, more LAR-HDM patients had moderate to severe symptoms than NAR patients (p = 0.090). Serum total IgE level was significantly lower in LAR-HDM than in AR-HDM patients (p = 0.001) and was not significantly different between LAR-HDM and NAR patients (p = 0.120).
YOF test demonstrated the presence of olfactory dysfunction (both hyposmia and anosmia) in 13 patients (61.9%) with LAR-HDM; four of them showed anosmia and nine had hyposmia. In AR-HDM and NAR, 54 patients (39.1%) and 69 patients (48.9%) presented with olfactory dysfunction. There was a significant difference in the comparison between the LAR-HDM and AR-HDM groups (p = 0.010). The mean TDI (sum of threshold, odor discrimination, and odor identification) score was 22.5 in the AR-HDM (SD = 5.73; Hi = 33.0, Low = 9.0), 21.3 in the NAR (SD = 5.67; Hi = 33.0, Low = 7.5), and 19.2 in the LAR-HDM (SD = 5.13; Hi = 27.0, Low = 8.0) groups. A significant difference was noted between the AR-HDM and LAR-HDM groups (p = 0.01).
PNS CT imaging findings
In the analysis using CT scan of paranasal sinuses, paranasal sinusitis was confirmed in 28.3% of AR-HDM patients (n = 39), 37.6% of NAR patients (n = 53), and 42.9% of LAR-HDM patients (n = 9), with no significant difference among the three groups (Fig. 3A). For patients with paranasal sinusitis confirmed by CT, the severity of rhinosinusitis was evaluated using the Lund-Mackay scoring system. The mean score of LAR-HDM patients (13.67 ± 6.87) was significantly higher than that of AR-HDM patients (6.87 ± 5.06) and NAR patients (7.11 ± 5.48; p = 0.011 and p = 0.007, respectively).
Accompanying sinusitis was evaluated by CT scan of the paranasal sinuses, and patients with sinusitis were assessed for severity of sinusitis using Lund-Mackay scoring system. Percentage of patients with sinusitis in the three groups (A). Lund-Mackay score of patients with sinusitis (B). AR allergic rhinitis, NAR non-allergic rhinitis, LAR local allergic rhinitis, HDM house dust mite, ns not significant
Discussion
This study was designed to demonstrate the prevalence of LAR among patients with chronic rhinitis and to analyze the clinical characteristics of LAR patients compared with other rhinitis patients. We found that LAR accounted for about 6.3% of all chronic rhinitis in Korean adults when NPT was performed according to the procedure presented by EAACI Position Paper in 2018. LAR group had a higher mean age than the AR group, and the nasal symptoms of the two groups showed similar pattern. Meanwhile, compared with NAR, nasal itching and sneezing are characteristic nasal symptoms of allergic reactions and were more frequent in LAR. Serum sIgE in LAR, which is associated with systemic allergic status, was lower than that in the AR group and similar to that in the NAR group. Interestingly, olfactory dysfunction and accompanying paranasal sinusitis were the most severe conditions in LAR among the three groups.
As various studies have been conducted on the prevalence of LAR over the past decade, the results vary from 5 to 50% worldwide. According to reports from Spain and Poland, LAR accounted for approximately 25.7% and 17.6% of all patients with rhinitis, respectively [5, 6]. In addition, 3,400 and 648 patients were analyzed in a systematic literature review by Hamizan et al., and the prevalence of LAR was 24.7% and 10.2%, respectively [16, 17]. Its prevalence has been reported to be relatively low in Asian countries compared with Western countries, with 4.0% in Korea and 7.7% in China [8, 18]. Ethnic differences account for a large proportion of the differences between these results, but the inconsistency of the diagnostic method is also supposed to have contributed in part [14]. The present study is meaningful in that it was conducted according to the NPT procedure suggested by EAACI Position Paper in 2018, and the result was similar to that of a previous study in Korea at 6.3%.
In contrast, the LAR was significantly older than the AR. There was no significant difference between the LAR and NAR. This was consistent with previous studies conducted in Eastern countries such as Korea and China [8, 18]. However, in the study reported by Rondone et al., patients with LAR had a lower mean age than patients with NAR and no significant difference with patients with AR in Spain [6]. Bozek et al. also demonstrated that the LAR patients in Poland were younger than other rhinitis patients [5]. It is interesting to note that the age distributions of rhinitis subtypes in the East and West are different from each other. Ethnic and genetic factors are also thought to play a major role in this difference, as previously described. But further consideration is needed. Regarding gender, smoking, asthma, and family history of allergic diseases, there were no significant differences between groups, as described by other reports [5, 6, 8, 18].
In this study, LAR showed clinical nasal symptoms similar to those of AR, including duration, severity, and all four representative nasal symptoms. This similarity is consistent with other studies that previously analyzed the nasal symptoms of LAR [6, 8]. Although only HDM was included in the allergen, it can be considered that there is no significant difference in the localized nasal pathophysiology of LAR and AR. Meanwhile, compared to NAR, LAR involved more persistent symptoms and more frequent nasal itching and sneezing symptoms, as characteristic symptoms caused by an allergic reaction to HDM. Serum total IgE has been known to be one of the indicators to evaluate the status of systemic atopy, and, as expected, did not increase in LAR patients in our study [19, 20].
In this study, we analyzed the olfactory function and severity of accompanying rhinosinusitis depending on subtypes of chronic rhinitis patients, which has not been described in previous studies. The results of YOF olfactory test showed that LAR-HDM patients were more likely to have olfactory dysfunction compared to AR-HDM patients. Olfactory dysfunction can be caused by various factors and is mainly considered to be conductive or sensorineural [21]. In particular, rhinosinusitis or nasal polyps are strongly associated with olfactory dysfunction and are a representative cause of conductive or mixed olfactory dysfunction [21]. AR can also be an aggravating factor of an olfactory dysfunction, since it has been known that 20–40% of AR patients complain of olfactory impairments [22]. Examples of sensorineural loss have been reported to be due to age-related deterioration of olfaction, as well as conditions such as genetic mutation, head trauma, or viral upper respiratory tract infections [21, 23]. In our study, LAR patients being older and having severe accompanying rhinosinusitis might be the possible explanation for worse olfactory function. However, more research is needed to understand why rhinosinusitis is more severe in patients with LAR than in other rhinitis patients. Since various factors such as the presence of AR, patients’ age, duration of illness, and the type of inflammation can affect the severity and clinical features of paranasal sinusitis, there is a limitation in elucidating the direct relationship between LAR and CRS. Nevertheless, we believe that this study can serve as a basis for future research on the relationship between rhinitis, olfactory function, and paranasal sinusitis [21, 24].
Our study has some limitations. First, the prevalence of LAR may have been underestimated because we performed NPT using only HDM among various aeroallergens. A study to investigate the prevalence and clinical features of NPT containing more antigens such as pollen will be needed in the future. Second, pediatric patients were excluded from this study due to technical and clinical environmental limitations. The clinical features of pediatric LAR are not described in this study, and this may have led to a bias in the prevalence of LAR. Finally, the LAR patients were few, which limited our ability to demonstrate a more comprehensive clinical feature.
In conclusion, clinical history and nasal symptoms of AR and LAR patients are very much alike. LAR should be differentiated by performing NPT in patients who present with AR symptoms without systemic atopy. Further studies investigating the effect of LAR on olfactory dysfunction and rhinosinusitis should be performed in the future.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author onreasonable request.
References
Bousquet J, Khaltaev N, Cruz AA, Denburg J, Fokkens WJ, Togias A et al (2008) Allergic rhinitis and its impact on asthma (ARIA) 2008. Allergy 63(Supplement 86):8–160
Hellings PW, Klimek L, Cingi C, Agache I, Akdis C, Bachert C et al (2017) Non-allergic rhinitis: position paper of the European Academy of Allergy and Clinical Immunology. Allergy 72(11):1657–1665
Rondón C, Canto G, Blanca M (2010) Local allergic rhinitis: a new entity, characterization and further studies. Curr Opin Allergy Clin Immunol 10(1):1–7
Rondón C, Campo P, Togias A, Fokkens WJ, Durham SR, Powe DG et al (2012) Local allergic rhinitis: concept, pathophysiology, and management. J Allergy Clin Immunol 129(6):1460–1467
Kim YH, Jang TY (2010) Clinical characteristics and therapeutic outcomes of patients with localized mucosal allergy. Am J Rhinol Allergy 24(4):e89–e92
Tao XY, Ng CL, Chen D, Lin ZB, Wu SL, Liang MJ et al (2018) Clinical characteristics and allergen sensitization patterns of patients with local allergic rhinitis in southern China. Int Arch Allergy Immunol 175(1–2):107–113
Cheng KJ, Xu YY, Liu HY, Wang SQ (2013) Serum eosinophil cationic protein level in Chinese subjects with nonallergic and local allergic rhinitis and its relation to the severity of disease. Am J Rhinol Allergy 27(1):8–12
Bozek A, Scierski W, Ignasiak B, Jarzab J, Misiolek M (2019) The prevalence and characteristics of local allergic rhinitis in Poland. Rhinology 57(3):213–218
Rondón C, Campo P, Galindo L, Blanca-López N, Cassinello MS, Rodriguez-Bada JL et al (2012) Prevalence and clinical relevance of local allergic rhinitis. Allergy 67(10):1282–1288
Rondon C, Campo P, Eguiluz-Gracia I, Plaza C, Bogas G, Galindo P et al (2018) Local allergic rhinitis is an independent rhinitis phenotype: the results of a 10-year follow-up study. Allergy 73(2):470–478
Rondón C, Eguiluz-Gracia I, Campo P (2018) Is the evidence of local allergic rhinitis growing? Curr Opin Allergy Clin Immunol 18(4):342–349
Jang TY, Kim YH (2015) Nasal provocation test is useful for discriminating allergic, nonallergic, and local allergic rhinitis. Am J Rhinol Allergy 29(4):e100–e104
Campo P, Eguiluz-Gracia I, Bogas G, Salas M, Plaza Serón C, Pérez N et al (2019) Local allergic rhinitis: implications for management. Clin Exp Allergy 49(1):6–16
Augé J, Vent J, Agache I, Airaksinen L, Campo Mozo P, Chaker A et al (2018) EAACI Position paper on the standardization of nasal allergen challenges. Allergy 73(8):1597–1608
Ha JG, Kim J, Nam JS, Park JJ, Cho HJ, Yoon JH et al (2020) Development of a Korean culture-friendly olfactory function test and optimization of a diagnostic cutoff value. Clin Exp Otorhinolaryngol 13(3):274–284
Hamizan AW, Rimmer J, Alvarado R, Sewell WA, Kalish L, Sacks R et al (2017) Positive allergen reaction in allergic and nonallergic rhinitis: a systematic review. Int Forum Allergy Rhinol Wiley Online Lib 7(9):868–877
Hamizan AW, Rimmer J, Husain S, Alvarado R, Tatersall J, Sewell W et al (2019) Local specific immunoglobulin E among patients with nonallergic rhinitis: a systematic review. Rhinology 57(1):10–20
Jung CG, Lee JH, Ban GY, Park HS, Shin YS (2017) Prevalence and clinical characteristics of local allergic rhinitis to house dust mites. Yonsei Med J 58(5):1047–1050
Jung YG, Kim KH, Kim HY, Dhong HJ, Chung SK (2011) Predictive capabilities of serum eosinophil cationic protein, percentage of eosinophils and total immunoglobulin E in allergic rhinitis without bronchial asthma. J Int Med Res 39(6):2209–2216
Li Y, Wu R, Tian Y, Bao T, Tian Z (2016) The correlation of serum eosinophil cationic protein level with eosinophil count, and total IgE level in Korean adult allergic rhinitis patients. Asian Pac J Allergy Immunol 34(1):33–37
Goncalves S, Goldstein BJ (2016) Pathophysiology of olfactory disorders and potential treatment strategies. Curr Otorhinolaryngol Rep 4(2):115–121
Stuck BA, Hummel T (2015) Olfaction in allergic rhinitis: a systematic review. J Allergy Clin Immunol 136(6):1460–1470
Pinto JM, Wroblewski KE, Kern DW, Schumm LP, McClintock MK (2015) The rate of age-related olfactory decline among the general population of older US adults. GERONA 70(11):1435–1441
Rosati MG, Peters AT (2016) Relationships among allergic rhinitis, asthma, and chronic rhinosinusitis. Am J Rhinol Allergy 30(1):44–47
Author information
Authors and Affiliations
Contributions
Conceptualization: HML. Data curation: SJK, JWM, YMJ. Formal analysis HML. Writing original draft: SJK. Writing—review and editing: all authors.
Corresponding author
Ethics declarations
Conflict of interest
None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kim, SJ., Moon, J.W., Cho, Y. et al. Clinical characteristics of local allergic rhinitis sensitized to house dust mites in Asia. Eur Arch Otorhinolaryngol 281, 2413–2420 (2024). https://doi.org/10.1007/s00405-023-08394-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00405-023-08394-y