Abstract
Purpose
It is commonly recommended that tooth extraction should be performed prior to radiotherapy (RT) in patients with head neck cancer to prevent osteoradionecrosis (ORN). However, doctors still occasionally encounter patients who require tooth extraction during RT. This study aimed to determine the risk of ORN in patients who undergo tooth extraction during RT.
Methods
Data were collected from Taiwan's National Health Insurance Research Database. We retrospectively enrolled 24,412 patients with head and neck cancer treated with radiotherapy between 2011 and 2017. The associations between ORN and demographic characteristics, timing of tooth extraction, and treatments were examined using univariate and multivariable Cox proportional hazards regression models.
Results
A total of 24,412 head and neck cancer patients were enrolled; 133 patients underwent tooth extraction during RT and 24,279 patients did not undergo tooth extraction during RT. Tooth extraction during RT was not associated with a significantly higher risk of ORN (hazard ratio [HR] = 1.303, P = 0.4862). Tumor site, RT dose ≥ 60 Gy, age < 55 y/o, mandibulectomy, chronic periodontitis, and chemotherapy were significantly associated with a higher risk of ORN.
Conclusion
The risk of ORN in head and neck cancer is not significantly different between patients who undergo tooth extraction during RT and patients who do not undergo tooth extraction during RT.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Head and neck cancer (HNC) has been one of the most common cancers in Taiwan for several decades and represents a serious national socioeconomic burden [1]. Radiotherapy (RT) is a current treatment option for HNC. However, common clinical complications after RT not only include fibrosis of the surrounding tissue, but also an increased risk of trismus, hearing loss, xerostomia, dysphagia, oral mucositis, and even osteoradionecrosis (ORN) [2, 3]. Previous systematic reviews indicate the total incidence of ORN after tooth extraction in patients who subsequently received RT is nearly 7% [4]. ORN is a serious RT complication that reduces the quality of life (QoL) of patients [5]. Therefore, there is a crucial need to determine the best form of management for preventing and dealing with ORN.
Tooth extraction after RT is an independent risk factor for ORN [6, 7]. Therefore, in order to prevent ORN, it is commonly recommended that tooth extraction should be performed prior to RT for cancer patients with poor oral hygiene or uncontrollable dental issues. However, pre-RT extractions have been shown to have a profound negative impact, leaving many patients devastated [8]. Even though most patients with dental issues have affected teeth extracted prior to RT, doctors still occasionally encounter patients who require tooth extraction during RT [8]. These cases may be associated with a patient’s unwillingness to have a tooth extracted prior to RT due to their awareness that the extraction may decrease their QoL or the inconvenience of undergoing pre-RT tooth extraction. A recent study by Saito et al. revealed that the risk of ORN was significantly higher in patients who experienced a longer duration of time between RT and tooth extraction [9]. Thus, it was concluded that tooth extraction should not be delayed, and should even be performed when patients are receiving RT. However, this assumption may have a major implication on clinical practice, as there are currently no data on the effect of tooth extraction during RT on the risk of developing ORN in patients with HNC. A better understanding of the impact of tooth extraction in HNC would help to establish more effective approaches and potentially improve patient outcomes. Thus, the purpose of this national-based cohort study was to investigate the risk factors associated with ORN in patients with HNC undergoing RT; in particular, to explore whether tooth extraction during RT increases the risk of ORN in patients with HNC.
Materials and methods
Ethical consideration
This nationwide, population-based, retrospective study examined data from the Taiwan Cancer Registry Database (TCRD) and Cause of Death Statistics, which are subsets of the National Health Insurance Research Database (NHIRD). The NHIRD comprises de-identified data and this study was performed retrospectively, so informed consent did not need to be requested from the patients. The ethics committee of Chung Shan Medical University Hospital provided ethical approval to carry out the study (CS1-20171); the hospital also provided financial support (Grant No.CSH-2021-C-002).
Data source and population
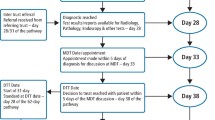
This study examined data collected between 2011 and 2017 using the NHIRD. The prevalence of national health insurance coverage in Taiwan is high (more than 99%)[10]. The inclusion and exclusion criteria for this study were similar to the criteria used in our previous studies [6, 7] (Fig. 1). The definition of ORN was exposed irradiated bone that had failed to heal over a period of 3 months in the absence of a local tumor [7]. All patients diagnosed with HNC between 2011 and 2017 were identified; the first day of RT was defined as the index day. The exclusion criteria were the same as our previous study [11], as follows: patients with incomplete or missing data, aged less than 20-years-old, no RT, duration from diagnosis to index day > 180 days, delayed surgery and/or incomplete RT, a history of other cancers, early death, which was defined as < 90 days between the index day and date of death, or ORN occurring < 3 months after the index day. The outcome of interest in this study was the occurrence of ORN associated with tooth extraction during RT. Thus, we divided the enrolled patients into two groups based on the timing of tooth extraction (if any): the exposure group (tooth extraction during RT) versus the control group (no tooth extraction during RT).
Statistical analysis
All enrolled patients were followed-up from the index date until occurrence of the study outcome (i.e., ORN), death, or the end of the study (December 31, 2019). Univariate and multivariable Cox proportional hazards regression were used to estimate the hazard ratio (HR) for the effect of tooth extraction during RT on the risk of ORN. All statistical analyses were conducted using SAS 9.4 (SAS Inc., Cary, NC, USA); two-sided P < 0.05 were considered statistically significant.
Results
Table 1 shows the demographic and clinical data for the study population. In total, 133 patients with HNC underwent tooth extraction during RT (exposure group) and 24,279 patients with HNC did not undergo tooth extraction during RT (control group). The mean observation time was 42.46 months in the exposure group and 42.70 months in the control group. The maximal observation time was 103 months in both groups. The rates of smoking, betel nut chewing, and post-RT tooth extraction were higher in the exposure group than the control group. Overall, 8.3% and 4.7% of patients in the exposure group and control group, respectively, underwent tooth extraction between 12 and 24 months after RT (P = 0.0481). There were no significant differences between the two groups in terms of gender, age, clinical T or N stage, dose of RT, BMI, level of hospital, alcohol drinking, or any of the comorbidities listed below.
Multivariate analysis showed that tumor located in oral cavity or oropharynx (P < 0.0001), age < 55 years old (P = 0.0108), a RT dose ≥ 60 Gy (P < 0.0001), chronic periodontitis (P = 0.0166), mandibulectomy (P < 0.0001), and chemotherapy (P = 0.0017) were all significantly associated with a higher risk of ORN (Table 2). However, the risk of ORN was not significantly different between patients who underwent tooth extraction during RT and the control group (adjusted odds ratio = 1.303, P = 0.4862). Therefore, tooth extraction during RT was not identified as a risk factor for ORN.
Discussion
This nationwide population-based cohort study identified tumor site, age < 55 years old, RT dose ≥ 60 Gy, periodontitis, mandible surgery, and chemotherapy as independent risk factors for ORN in patients with HNC treated with RT. Tooth extraction during RT was not associated with an increased risk of ORN in patients with HNC. Jansma et al. concluded that some protocols can prevent a variety of oral sequelae following RT in HNC, including the extraction of all teeth with a questionable dental prognosis before the start of RT [12]. Although teeth with a poor dental prognosis are commonly extracted prior to RT in order to restore healing capacity, controversy still remains regarding the proper timing of tooth extraction relative to RT [13]. Tooth extraction during RT is usually considered to be contraindicated during the clinical treatment of patients with HNC. However, as treating physicians, we still encounter patients who require tooth extraction during RT in daily practice. Some patients hesitate to undergo tooth extraction prior to RT as they believe the extraction may negatively affect their QoL, such as being unable to chew properly. Additionally, as the pathological course of HNC progresses, any teeth that were considered stable prior to RT may subsequently become unstable during RT. These reasons may explain why some patients still require tooth extraction during RT. Moreover, long-term poor oral hygiene, which can result in a carious stump, was also identified as another major risk factor for ORN [14]. In a recent study, Saito et al. found that tooth extraction at a longer period of time after RT was associated with a higher risk of ORN, and suggested it is not necessary to postpone tooth extraction—even among patients undergoing RT [9]. This observation is consistent with our study and suggests that extraction of teeth during RT may be a better option than choosing to wait until later.
Periodontitis is a well-known risk factor of ORN [5, 15,16,17,18]. In our study, we also obtained a similar result that periodontitis was an independent risk factor of ORN. Periodontitis is common in adults, and radiotherapy could exacerbate it because of hyposalivation and the loss of the protective effects of saliva [5]. Pre-RT oral health evaluation is crucial to detect pre-existing dental disease and to minimize the risk of ORN for head and neck cancer patients by early management of the dental problems.
Although RT is one of the most important forms of treatment for patients with HNC, it can lead to adverse effects including mucositis, salivary gland dysplasia, trismus, dysphagia, and even ORN. Currently, the ideal dose of RT remains controversial. Higher doses of RT are thought to increase the risk of ORN [14, 19]. Tsai et al. reported that a RT dose higher than 50–60 Gy to the mandible significantly increased the risk of ORN of the mandible [20]. This finding is consistent with our data, which showed that a RT dose higher than 60 Gy to target tissue is associated with a significantly higher risk of ORN compared to a dose less than 60 Gy. However, the actual dose of RT should be accurately calculated because any reduction in the RT dose may reduce the effectiveness of irradiation.
In our study, tumor sites of the enrolled patients were divided into oral cavity, oropharynx, hypopharynx, larynx, and others. Tumor located in oral cavity or oropharynx had significant higher risk of ORN. Besides, for patients with oral cavity caner, tongue cancers had a lower risk of ORN compared with other types of oral cavity cancers. This result was compatible with previous published studies [6, 21, 22]. These finding might be explained by the inclusion of the mandible in the high-dose therapeutic area [6, 20, 23, 24]. Kubota et al. revealed that the volume of the irradiated jaw was larger in the oral cavity or oropharynx than in the other tumor sites [24]. Many studied showed that higher dose RT to the mandible can lead to ORN [19,20,21], and one of the cause might be that radiation could result in severe acute mucositis, which can exacerbate bony exposure and lead to bone damage [25]. Another interesting finding of this study is that tumor site categorized as “others” also had higher risk of ORN. For those patients with tumor sites categorized as “others”, most of them were patients with cancer of major salivary gland (ICD-10-CM codes: C07 or C08). According to the anatomical localization, this result might be related to the involvement of mandible in radiation field.
This study also found that performing mandibulectomy was an independent risk factor associated with the development of ORN. Many other studies have obtained similar results [26, 27]. Large segmental resection of the mandible may impair blood supply to the mandible, and surgery-induced damage to the periosteum may possibly lead to a further decrease in blood supply [26].
There have been conflicting opinions as to the impact of chemotherapy on the risk of ORN in patients receiving radiotherapy. Shim et al. reported that chemotherapy was associated with increased prevalence of osteonecrosis, but Nabil et al. suggested that chemotherapy show no increase in osteoradionecrosis risk [28, 29]. In our study, chemotherapy was an independent risk factor for ORN. More attention should be paid to those patients who received chemotherapy, and more study might be needed to clarify this relationship between chemotherapy and development of ORN.
We also found that age < 55 years old was an independent risk factor for development of ORN. This might be explained by the fact that younger patients may have longer life expectancy for development of ORN.
There are some limitations to this study. First, the sample size in the exposure group was extremely small compared to the size of the control group, thus statistical bias may be inevitable. Therefore, additional studies with a larger sample size is necessary in the future. Second, data on the patients’ dental condition, irradiated volume of the mandible, oral hygiene status, and type of RT protocol are not recorded in the NHIRD. Third, in this study, all the enrolled patients with ORN had grades 2–4 of Tsai’s classification (grade 2, minor debridement received; grade 3, hyperbaric oxygen needed; grade 4, major surgery required) [20]. Because those patients with grade 1 ORN (grade 1, with conservative management only) did not receive any procedures, we could not identify these patients from NHIRD. Lastly, more effort must be devoted to gain a better understanding of the factors associated with the relative risk of ORN in patients undergoing tooth extraction before, during, and after RT. Despite, the present study is the first investigation in Taiwan to compare the risk of ORN among patients with HNC who underwent or did not undergo tooth extraction during RT. These findings may contribute to the development of treatment guidelines for patients with HNC in the near future. However, large-scale prospective studies are needed to confirm the results of this retrospective study.
Conclusion
The risk of ORN was not significantly different between patients who had a tooth extraction procedure during RT (the exposure group) and patients who did not undergo tooth extraction during RT (the control group). Although the sample size of the exposure group was small, this retrospective study suggests that tooth extraction during RT is not absolutely contraindicated in patients with HNC.
Availability of data and materials
Data are obstained from the National Health Insurance Research Database (NHIRD).
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A et al (2021) Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 71:209–249
Strojan P, Hutcheson KA, Eisbruch A, Beitler JJ, Langendijk JA, Lee AWM et al (2017) Treatment of late sequelae after radiotherapy for head and neck cancer. Cancer Treat Rev 59:79–92
Lalla RV, Brennan MT, Gordon SM, Sonis ST, Rosenthal DI, Keefe DM (2019) Oral mucositis due to high-dose chemotherapy and/or head and neck radiation therapy. J Natl Cancer Inst Monogr 2019(53):lgz011
Nabil S, Samman N (2012) Risk factors for osteoradionecrosis after head and neck radiation: a systematic review. Oral Surg Oral Med Oral Pathol Oral Radiol 113:54–69
Sroussi HY, Epstein JB, Bensadoun RJ, Saunders DP, Lalla RV, Migliorati CA et al (2017) Common oral complications of head and neck cancer radiation therapy: mucositis, infections, saliva change, fibrosis, sensory dysfunctions, dental caries, periodontal disease, and osteoradionecrosis. Cancer Med 6:2918–2931
Liao PH, Chu CH, Hung YM, Tang PL, Kuo TJ (2021) Tumor subsites and risk of osteoradionecrosis of the jaw in patients with oral cavity cancer: a national-based cohort study. Eur Arch Otorhinolaryngol 278:3425–3433
Kuo TJ, Leung CM, Chang HS, Wu CN, Chen WL, Chen GJ et al (2016) Jaw osteoradionecrosis and dental extraction after head and neck radiotherapy: a nationwide population-based retrospective study in Taiwan. Oral Oncol 56:71–77
Clough S, Burke M, Daly B, Scambler S (2018) The impact of pre-radiotherapy dental extractions on head and neck cancer patients: a qualitative study. Br Dent J 225:28–32
Saito I, Hasegawa T, Kawashita Y, Kato S, Yamada SI, Kojima Y et al (2021) Association between dental extraction after radiotherapy and osteoradionecrosis: a multi-centre retrospective study. Oral Dis 28(4):1181–1187
Lin LY, Warren-Gash C, Smeeth L, Chen PC (2018) Data resource profile: the National Health Insurance Research Database (NHIRD). Epidemiol Health 40:e2018062
Lin C, Shih YJ, Kuo TJ, Liao PH (2022) Letter to the editor - Reply to the “Association between dental extraction after radiotherapy and osteoradionecrosis.” Oral Dis 28:1741–1742
Jansma J, Vissink A, Spijkervet FK, Roodenburg JL, Panders AK, Vermey A et al (1992) Protocol for the prevention and treatment of oral sequelae resulting from head and neck radiation therapy. Cancer 70:2171–2180
Eliyas S, Al-Khayatt A, Porter RW, Briggs P (2013) Dental extractions prior to radiotherapy to the jaws for reducing post-radiotherapy dental complications. Cochrane Database Syst Rev 2013(2):CD008857
Kojima Y, Yanamoto S, Umeda M, Kawashita Y, Saito I, Hasegawa T et al (2017) Relationship between dental status and development of osteoradionecrosis of the jaw: a multicenter retrospective study. Oral Surg Oral Med Oral Pathol Oral Radiol 124:139–145
Schuurhuis JM, Stokman MA, Witjes MJH, Reintsema H, Langendijk JA, Vissink A et al (2018) Patients with advanced periodontal disease before intensity-modulated radiation therapy are prone to develop bone healing problems: a 2-year prospective follow-up study. Support Care Cancer 26:1133–1142
Irie MS, Mendes EM, Borges JS, Osuna LG, Rabelo GD, Soares PB (2018) Periodontal therapy for patients before and after radiotherapy: A review of the literature and topics of interest for clinicians. Medicina oral, patologia oral y cirugia bucal 23:e524–e530
Katsura K, Sasai K, Sato K, Saito M, Hoshina H, Hayashi T (2008) Relationship between oral health status and development of osteoradionecrosis of the mandible: a retrospective longitudinal study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 105:731–738
Schuurhuis JM, Stokman MA, Roodenburg JL, Reintsema H, Langendijk JA, Vissink A et al (2011) Efficacy of routine pre-radiation dental screening and dental follow-up in head and neck oncology patients on intermediate and late radiation effects. A retrospective evaluation. Radiother Oncol 101:403–409
Chang DT, Sandow PR, Morris CG, Hollander R, Scarborough L, Amdur RJ et al (2007) Do pre-irradiation dental extractions reduce the risk of osteoradionecrosis of the mandible? Head Neck 29:528–536
Tsai CJ, Hofstede TM, Sturgis EM, Garden AS, Lindberg ME, Wei Q et al (2013) Osteoradionecrosis and radiation dose to the mandible in patients with oropharyngeal cancer. Int J Radiat Oncol Biol Phys 85:415–420
Chen JA, Wang CC, Wong YK, Wang CP, Jiang RS, Lin JC et al (2016) Osteoradionecrosis of mandible bone in patients with oral cancer–associated factors and treatment outcomes. Head Neck 38:762–768
Wang TH, Liu CJ, Chao TF, Chen TJ, Hu YW (2017) Risk factors for and the role of dental extractions in osteoradionecrosis of the jaws: a national-based cohort study. Head Neck 39:1313–1321
Jiang Y-m, Zhu X-d, Qu S (2014) Incidence of osteoradionecrosis in patients who have undergone dental extraction prior to radiotherapy: a systematic review and meta-analysis. J Oral Maxillofac Surg Med Pathol 26:269–275
Kubota H, Miyawaki D, Mukumoto N, Ishihara T, Matsumura M, Hasegawa T et al (2021) Risk factors for osteoradionecrosis of the jaw in patients with head and neck squamous cell carcinoma. Radiat Oncol 16:1
Studer G, Studer SP, Zwahlen RA, Huguenin P, Grätz KW, Lütolf UM et al (2006) Osteoradionecrosis of the mandible: minimized risk profile following intensity-modulated radiation therapy (IMRT). Strahlentherapie und Onkologie Organ der Deutschen Rontgengesellschaft [et al] 182:283–288
Renda L, Tsai TY, Huang JJ, Ito R, Hsieh WC, Kao HK et al (2020) A nomogram to predict osteoradionecrosis in oral cancer after marginal mandibulectomy and radiotherapy. Laryngoscope 130:101–107
Iqbal Z, Kyzas P (2020) Analysis of the critical dose of radiation therapy in the incidence of Osteoradionecrosis in head and neck cancer patients: a case series. BDJ Open 6:18
Shim K, MacKenzie MJ, Winquist E (2008) Chemotherapy-associated osteonecrosis in cancer patients with solid tumours: a systematic review. Drug Saf 31:359–371
Nabil S, Samman N (2011) Incidence and prevention of osteoradionecrosis after dental extraction in irradiated patients: a systematic review. Int J Oral Maxillofac Surg 40:229–243
Funding
The authors report no commercial, proprietary or financial interest in the products or companies mentioned in this article.
Author information
Authors and Affiliations
Contributions
P-HL: conceptualization, investigation, methodology, project administration, validation, and writing—original draft and editing. CL: conceptualization, data curation, formal analysis, investigation, resources, writing—original draft, and editing. J-YH: data curation, formal analysis, software. H-ML: conceptualization, data curation, resources. T-JK: conceptualization, investigation, methodology, project administration, supervision, validation, visualization, and writing—review and editing.
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liao, PH., Lin, C., Huang, JY. et al. Association between tooth extraction during radiotherapy and the risk of osteoradionecrosis in patients with head and neck cancers. Eur Arch Otorhinolaryngol 280, 2945–2952 (2023). https://doi.org/10.1007/s00405-023-07885-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00405-023-07885-2