Abstract
Introduction
The ATA guidelines for differentiated thyroid cancer (DTC) are one of the most widely referred to. Their 2015 edition proposed a new risk stratification system and modified the indications for radioactive iodine (RAI) ablation especially for the low risk category. We attempted to analyze whether the new guidelines altered referral practices for RAI ablation at our institute.
Methodology
Patients who underwent total or completion thyroidectomy for DTC during 2016–2017 were included. Relevant demographical and pathological data was tabulated. Patients were classified as per the new stratification system and referral practice for RAI ablation documented.
Results
238 patients were included. Of these 20.6% were low risk, 44.1% were intermediate and 35.3% were high risk as per modified guidelines. All patients within the intermediate and high-risk group and 77.8% of the low risk group were referred for RAI ablation. Analysis of risk factors revealed that within the low risk group there were three patients with < 5 metastatic nodes, all within 3 cm in size—a category that the ATA failed to stratify appropriately. Among those labeled as Intermediate risk due to microscopic extra thyroidal extension (ETE), 85% had no other risk factors and were upstaged solely due to microscopic ETE, which is interestingly no longer included in the TNM staging.
Conclusion
Majority of low risk patients continue to receive RAI ablation due to persistent belief emanating from literature that remnant ablation improves outcomes and aids in follow up. The issue of RAI ablation for low risk group and prognostic implications of microscopic ETE and limited nodal disease need to be revisited.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Differentiated thyroid cancers (DTC) constitute the most common variety of thyroid cancers. Surgery in the form of hemithyroidectomy alone or total thyroidectomy followed by radioactive-iodine (RAI) ablation, whenever indicated, forms the mainstay of treatment for these cancers [1,2,3,4]. The decision to remove the entire gland, as well as to administer RAI post-operatively is dependent on a multitude of prognostic factors. The relative importance of these prognosticators has evolved over the decades, thereby influencing the referral practice for RAI ablation. The recent increase in the incidence of thyroid cancer has seen a parallel increase in the literature pertaining to the same. This coupled with a paradigm shift towards lesser treatment as advocated by the 2015 American Thyroid Association (ATA) guidelines [4], have resulted in global variations in practice. The 2009 ATA guidelines advocated that for DTC, any nodule greater than 1 cm in size warranted a Total Thyroidectomy [3]. Remnant ablation was advocated post total thyroidectomy to improve the sensitivity of serum thyroglobulin and neck sonography during subsequent follow up [5]. The 2015 ATA guidelines saw a significant change in recommendations, especially for the above two treatment aspects (extent of surgery and indications for RAI administration), with an overall shift towards lesser treatment. To guide the decision for post-operative adjuvant RAI ablation, a modified risk stratification system was proposed. Those tumors that fell under the category of low risk, need not be referred for RAI/Remnant ablation, while patients under the moderate or high-risk category warrant adjuvant RAI therapy. Since these guidelines are widely referred to worldwide, the extent to which they would bring about a change in practice, especially with respect to referral patterns for RAI ablation, formed an interesting research question. With this pertinent question in mind, we reviewed our institutional practice subsequent to the implementation of the 2015 ATA guideline to assess whether the change in guidelines had significantly modified the referral practice for RAI ablation being followed at our institute.
Methodology
We performed a retrospective review of the referral practices for RAI ablation for patients with DTC who underwent treatment from January 2016 until December 2017 in the Department of Head and Neck Surgical Oncology at our Institute.
The inclusion criteria for patients selected were as follows
-
1.
Patients without any prior treatment who underwent total thyroidectomy at our institute
-
2.
Patients who underwent hemithyroidectomy as the index procedure at our institute followed by a completion thyroidectomy (as warranted by the histopathology from the index surgery)
-
3.
Patients who underwent hemithyroidectomy as the index procedure outside and were referred to our center for a completion thyroidectomy and had complete records of pre-operative investigations and histopathology details from the earlier procedure.
Patients were excluded from the review for any of the following features
-
1.
Histopathology apart from DTC: medullary thyroid cancer (MTC), poorly differentiated thyroid cancer (PDTC) and anaplastic thyroid cancer.
-
2.
Only hemithyroidectomy performed for DTC
-
3.
Patients operated for hemithyroidectomy at an outside center and referred with incomplete prior clinical and pathological details
-
4.
Revision surgeries apart from completion thyroidectomy (e.g., thyroid bed exploration, lateral neck exploration)
All relevant demographic and clinicopathological details were obtained from the hospital’s electronic medical record system.
Results
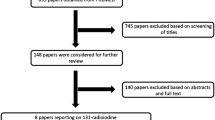
From January 2016 until December 2017, 572 patients underwent thyroid surgery at our institute. Of these, 238 patients were suitable for analysis based on the eligibility criteria mentioned earlier. Majority of the patients were females (63%) and the mean age of the cohort was 39.4 years (range 18–77 years). Total thyroidectomy was performed in 78 % of patients, while the rest underwent completion thyroidectomy. The most common histology was classical variant of papillary thyroid carcinoma (PTC) (66 %) followed by follicular variant of papillary thyroid carcinoma (22.3 %) and follicular carcinoma (6%). Aggressive variants of PTC formed the remainder of patients. Demographic details of the cohort are mentioned in Table 1.
Distribution of risk stratification (mentioned in Table 2)
Based on the ATA 2015 risk stratification system, all the patients were stratified accordingly. Of the entire 238 patients, 49 (20.6%) were categorized as low risk, 104 (44.1%) as intermediate risk and 85 (35.3%) were categorized as high risk. Majority of our patients fell within the intermediate or high-risk category. This was an expected finding given that majority of patients who present to a tertiary oncology center like ours are either loco regionally advanced or are referred from elsewhere due to their aggressive nature.
Referral practices in each risk category
Within the low risk category, 38 out of 49 (77.6%) patients were referred for remnant ablation, while the rest 11 (22.4%) were observed. Of 104 Intermediate category patients, 1 was lost to follow up, while all the rest 103 patients were referred for RAI ablation. All 85 patients within the high-risk category were referred for RAI ablation.
Tumor characteristics guiding risk stratification
Low risk category
Of the 38 patients referred for RAI ablation, majority was p T1–T2 N0 (n = 26/38, 68.4%) followed by p T3N0 (n = 8, 16.3%). Of the 49 patients, 4 were pN1a among which one patient had micro-metastasis to a single node, (defined as metastatic focus within the node < 0.2 cm) and the remaining 3 patients had less than 5 metastatic nodes, all being less than 3 cm in size.
Intermediate risk category
The criteria for being classified as intermediate risk category were—microscopic extra thyroidal extension (54.8%), greater than five metastatic nodes with largest diameter < 3 cm (43.26%) and aggressive variants of PTC (7.7%). Of the 48 patients with microscopic ETE, 41(85%) had nodule size less than 4 cm with no other high-risk features and the remaining 7 (15%) had nodule size ≥ 4 cm.
High risk category
Criteria for being classified as high risk were gross ETE (66%), metastatic nodes > 3 cm (36.5%), vascular emboli (> 4) in follicular cancers (2.4%) and incomplete (R+) resection (7.2%). (The above characteristics are summarized in Table 3).
Discussion
Surgery forms the mainstay of treatment for differentiated thyroid cancers, with RAI ablation being used as an adjunct to improve outcomes. The indications for total thyroidectomy as well as that for RAI ablation have evolved over the last decade with a focus on lesser treatment given the excellent overall prognosis of these tumors.
There are three broad goals of post-operative RAI Therapy (ablation) as described by Tuttle et al. [5]. The first being ablation of remnant/residual thyroid tissue which improves the diagnostic accuracy of follow up with serum thyroglobulin (Sr.Tg) and whole body RAI scans in the future. The second goal is for adjuvant therapy in cases, where there has been complete surgical ablation of disease, yet the patient is at high risk for harboring residual microscopic disease with a greater propensity for loco regional or distant recurrence. Thirdly, it may be used as ablative treatment for known RAI avid metastatic disease.
There are multiple patient and tumor factors in addition to the standard TNM criterion that are of prognostic importance in DTC and have been used to risk stratify patients appropriately. While the AJCC TNM staging system is based on predicting overall survival, risk stratification systems have the added benefit of predicting the risk of persistent/recurrent disease. Various such prognostication models have been used in the past (AGES, MACIS, GAMES, etc.), most of which were difficult to apply and use in clinical practice. The simplified low, intermediate and high-risk categories adopted by the ATA incudes response to post-surgical RAI ablation in addition to tumor characteristics and extent of surgical ablation [3, 4].
The 2015 ATA guidelines for DTC show a marked departure from previous editions with respect to criteria for risk stratification as well as the need for RAI ablation in low risk patients [6].
The modified risk stratification system does not consider size of nodule (s), though nodule size is still an important determinant of T stage. Moreover, nodal disease has been distributed over all the 3 categories based on the number and size of nodes as well as size of metastatic foci within smaller nodes. The low risk group has been expanded to include, intrathyroidal encapsulated follicular variant of papillary thyroid cancer, intrathyroidal well differentiated follicular cancer with capsular or minor vascular invasion (< 4 vessels involved), and intrathyroidal papillary micro carcinomas that are either BRAF wild type or BRAF mutant (a simplified version of the risk stratification system is depicted in Table 4). This implies that a significant subset of patients would now be placed at a lesser risk than before with implications on the need for RAI ablation, as well as intensity of follow up and TSH suppression. Recommendation 51 of the ATA 2015 indicates that remnant ablation is now not routinely recommended for low risk patients and that the decision should be tailor made for the individual patient [4, 6].
This study was specifically conducted to analyse whether these recommendations modified the referral practice for RAI ablation post total thyroidectomy/completion thyroidectomy at our institution. While primarily being focussed at the course of treatment for low risk patients, our findings did encourage us to review the literature on other issues that the ATA 2015 failed to provide such as convincing answers pertaining to the current prognostic role of microscopic ETE as well as limited nodal disease. Each of these issues have been discussed below in the light of our findings.
The need for RAI ablation in low risk group
Contrary to expectation, a significant majority of patients within the low risk category were referred for remnant ablation (77.6%). While 163 /238 (69.5%) of patients within the entire cohort harboured nodal metastasis, only four of these were placed in the low risk category based on the modified risk criteria. One of these four patients had a microscopic focus of 0.2 mm in a single node. Three of the remaining had less than five nodes with metastatic foci being less than 3 cm in largest diameter. Interestingly, the ATA 2015 fails to place these patients (with less than five nodes and having a metastatic focus of less than 3 cm) into any particular category. All these four patients still underwent RAI ablation with adjuvant intent.
While the ATA 2015 recommends that RAI ablation may be withheld in the low risk group of patients, it is noteworthy that the strength of this recommendation is weak due to “low quality evidence”. In a meta-analysis of 18 studies, Sawka et al. analyzed the benefit of remnant ablation in DTC and concluded that the benefit was doubtful in the low risk category [7]. Moreover, the study demonstrated that benefit of RAI ablation was seen only in series, where the median follow up was more than 10 years thus implying that drawing inferences from studies with shorter follow up duration may not be scientifically correct. The ATA 2015 referred to three retrospective studies which attempted to validate the 2009 risk stratification system and showed no detriment in disease outcomes when remnant ablation was omitted [8,9,10]. Interestingly we note that only 2 [8, 9] of these dealt with low risk patients alone, while the third included patients with intermediate and high risk as well. Literature is indeed divided on this contentious issue, with studies based on the SEER database showing definite benefit of remnant ablation in low risk categories as well [11]. There are three ongoing randomized trials on the need for RIA in low risk category [IoN [12], Estambl 2 [13] and CLERAD PROBE [14] trials]. Although their primary endpoints have been planned at 5 year follow-up, the indolent nature and long survival of patients with low risk disease necessitates at least a 10 year follow-up for meaningful results as demonstrated by Sawka [7]. Until the debate is answered, the general consensus at our institute remains to offer remnant ablation for most patients so as to improve the sensitivity of serum thyroglobulin and RAI scan during follows up. Post-operative serum thyroglobulin and low dose whole body scans may be used to identify select patients who may be observed, especially those with papillary thyroid microcarcinoma (PTMC).
Prognostic role of microscopic ETE
Among the intermediate risk patients in our cohort, it was seen that 54.8% had microscopic ETE of whom 85% of them had nodule size less than 2 cm. The remaining 15% had nodule size greater than 4 cm making them p T3, which serves as an indication for RIA at our institute. Recent literature has questioned the importance of microscopic ETE [15] and the current AJCC 8th TNM staging has been modified from the previous edition to exclude microscopic ETE from the T stage for thyroid cancers [16]. It is to be noted that the present AJCC was published subsequent to the ATA 2015. If microscopic ETE is excluded from future risk stratification systems, this would imply that those T1–T2 tumors who were being upstaged solely based on microscopic ETE need not be subjected to adjuvant RAI ablation. However, recent studies based on large datasets from both SEER and NCDB database continue to maintain that microscopic ETE does have an impact on prognosis for differentiated thyroid cancers [17, 18]. There remains a lack of consensus regarding the prognostic role of microscopic ETE and future risk stratification systems need to address this contentious issue to adequately guide the need for adjuvant treatment in this group of patients.
Role of RAI ablation in presence of limited nodal metastasis
Presence of nodal metastasis has remained an important indication for adjuvant RAI ablation. The 2015 ATA guidelines have distributed nodal metastasis across all the 3 risk stratifications. In an exhaustive review of literature, Randolph et al. have provided a predictive model for recurrence based on the size and number of nodes as well as presence of extra nodal extension (ENE). The current ATA recommendations seem to be largely inspired by this model with the exception that ENE has not been included into the stratification system. While increasing nodal burden is a known to confer a higher risk of recurrence, the exact nodal cut off varies in literature. The ATA 2015, inspired by the review by Randolph and colleague’s places this cut off at five nodes, whereas Adams and Sosa in their retrospective study on 47,902 patients ≤ 45 years of age conclude that nodal burden beyond six nodes ceases to have a corresponding detrimental effect on survival. In our study, three patients were identified as having less than five metastatic nodes with size ≤ 3 cm and referred for RAI ablation based on our institutional practice of offering RAI ablation for all p N + patients. The ATA fails to place this group of patients in any of the three categories and requires clarification in future guidelines.
While the ATA guidelines for differentiated thyroid cancer remain one of the most well researched and widely followed recommendations, publication of the 2015 edition was not universally endorsed by other parallel societies. Verburg et al., cited that limiting the role of RAI ablation in low risk category patients was one of the two most important reasons for the European Association of Nuclear Medicine (EANM) not endorsing these guidelines [19]. Moreover, recognizing that there was a need for greater dialogue between concerned societies that formulate treatment policies for DTC, a joint meeting of the ATA, the European Thyroid Association (ETA), Society of Nuclear Medicine and Molecular Imaging (SNMMI) and the EANM was held to discuss contentious issues with respect to the role of RIA.Results of this discussion have been published as the “Martinique principles” [20]. Until greater clarity is achieved, clinicians need to interpret the literature in the correct light and decision for RAI ablation post total thyroidectomy needs to be tailor made for the individual patient taking into account multiple risk factors and patient preferences as well.
Conclusion
Our study indicated that recommendations of the 2015 ATA guidelines failed to significantly change practice in patients with DTC categorized as low risk at our institute. Indications for RAI ablation in this group of patients have been stretched with the belief stemming from existing literature that offering RIA in these patients does improve the accuracy of post-operative serum thyroglobulin and diagnostic whole-body scans. Until randomized evidence on this issue is available, treatment should be individualized and select patients may be observed based on post ablation thyroglobulin levels. The role of microscopic ETE as a prognosticator remains unclear and there is a need for further clarity in future guidelines. Limited nodal disease has been classified as low risk. Policy makers need to account for other evidence that indicates nodal metastasis does have a detrimental effect on survival in younger patients. While recommendations from well-formed guidelines form an important basis for guiding decisions, clinicians need to interpret literature with care and incorporate all the available evidence when dealing with contentious issues.
References
Haddad RI, Nasr C, Bischott L, Busaidy NL, Byrd D, Callevder G et al (2018) NCCN guidelines insight: thyroid carcinoma, version 2.2018. J Natl Compr Canc Netw 16(12):1429–1440
Perros P, Boelaert K, Colley S, Evans C, Evans RM, Ba GG et al (2014) Guidelines for the management of thyroid cancer. Clin Endocrinol (Oxf) 81(s1):1–122
Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, Mandel SJ et al (2009) Revised American thyroid association management guidelines for patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association (ATA) guidelines taskforce on thyroid nodules and differentiated thyroid cancer. Thyroid 19(11):1167–1214
Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE et al (2016) 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid 26(1):1–133
Tuttle RM, Lopez N, Leboeuf R, Minkowitz SM, Grewal R, Brokhin M et al (2010) Radioactive iodine administered for thyroid remnant ablation following recombinant human thyroid stimulating hormone preparation also has an important adjuvant therapy function. Thyroid 20(3):257–263
Kim BW, Yousman W, Wong WX, Cheng C, McAninch EA (2016) Less is more: comparing the 2015 and 2009 American Thyroid Association guidelines for thyroid nodules and cancer. Thyroid 26(6):759–764
Sawka AM, Thephamongkhol K, Brouwers M, Thabane L, Browman G, Gerstein HC (2004) A systematic review and metaanalysis of the effectiveness of radioactive iodine remnant ablation for well-differentiated thyroid cancer. J Clin Endocrinol Metab 89(8):3668–3676
Schvartz C, Bonnetain F, Dabakuyo S, Gauthier M, Cueff A, Fieffé S et al (2012) Impact on overall survival of radioactive iodine in low-risk differentiated thyroid cancer patients. J Clin Endocrinol Metab 97(5):1526–1535
Durante C, Montesano T, Attard M, Torlontano M, Monzani F, Costante G et al (2012) Long-term surveillance of papillary thyroid cancer patients who do not undergo postoperative radioiodine remnant ablation: is there a role for serum thyroglobulin measurement? J Clin Endocrinol Metab 97(8):2748–2753
Vaisman F, Shaha A, Fish S, Michael TR (2011) Initial therapy with either thyroid lobectomy or total thyroidectomy without radioactive iodine remnant ablation is associated with very low rates of structural disease recurrence in properly selected patients with differentiated thyroid cancer. Clin Endocrinol (Oxf) 75(1):112–119
Orosco RK, Hussain T, Brumund KT, Oh DK, Chang DC, Bouvet M (2015) Analysis of age and disease status as predictors of thyroid cancer-specific mortality using the surveillance, epidemiology, and end results database. Thyroid 25(1):125–132
IoN-Is ablative radio-iodine necessary for low risk differentiated thyroid cancer patients—full text view—ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT01398085. Accessed 13 Nov 2019
Differentiated thyroid cancer: is there a need for radioiodine ablation in low risk patients?—full text view—ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT01837745. Accessed 13 Nov 2019
I-124 PET/CT Based remnant radioiodine ablation decision concept in differentiated thyroid cancer—full text view—ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT01704586. Accessed 13 Nov 2019
Nixon IJ, Ganly I, Patel S, Palmer FL, Whitcher MM, Tuttle RM et al (2011) The impact of microscopic extrathyroid extension on outcome in patients with clinical T1 and T2 well-differentiated thyroid cancer. Surgery 150(6):1242–1249
Amin MB, American Joint Committee on Cancer, American Cancer Society, editors. AJCC cancer staging manual. Eight edition/editor-in-chief, Mahul B. Amin, MD, FCAP; editors, Stephen B. Edge, MD, FACS [and 16 others]; Donna M. Gress, RHIT, CTR-Technical editor; Laura R. Meyer, CAPM-Managing editor (2017). American Joint Committee on Cancer, Springer, Chicago
Youngwirth LM, Adam MA, Scheri RP, Roman SA, Sosa JA (2017) Extrathyroidal extension is associated with compromised survival in patients with thyroid cancer. Thyroid 27(5):626–631
Liu Z, Huang Y, Chen S, Hu D, Wang M, Zhou L et al (2019) Minimal extrathyroidal extension affects the prognosis of differentiated thyroid cancer: is there a need for change in the AJCC classification system? PLoS ONE 14(6):e0218171
on behalf of the EANM, and the EANM Thyroid Committee, Verburg FA, Aktolun C, Chiti A, Frangos S, Giovanella L et al (2016) Why the European Association of Nuclear Medicine has declined to endorse the 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer. Eur J Nucl Med Mol Imaging 43(6):1001–1005
Tuttle RM, Ahuja S, Avram AM, Bernet VJ, Bourguet P, Daniels GH et al (2019) Controversies, consensus, and collaboration in the use of 131 I therapy in differentiated thyroid cancer: a joint statement from the American Thyroid Association, the European Association of Nuclear Medicine, the Society of Nuclear Medicine and Molecular Imaging, and the European Thyroid Association. Thyroid 29(4):461–470
Funding
None.
Author information
Authors and Affiliations
Contributions
Study concepts: ST, DC, HD. Study design: ST, HD. Data acquisition: AY, HD, SSN. Quality control of data and algorithms: ST, DC. Statistical analysis: ST, HD. Manuscript preparation: all authors. Manuscript editing: ST, HD, DC. Manuscript reviewing: all authors.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interest.
Ethical approval
Exempted, as it was a clinical audit of an existing database. No patient contact was made. All received the standard of care for their condition and was as per the ethical standards.
Informed consent
No identifying information about participants is available in the article. However, all patients have given consent for the treatment they have received.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dhar, H., Thiagarajan, S., Yousuf, A. et al. Referral Practice for Radioactive Iodine Ablation (RAI) after ATA guidelines 2015: results from a Tertiary Cancer Care Centre. Eur Arch Otorhinolaryngol 277, 2521–2526 (2020). https://doi.org/10.1007/s00405-020-05946-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00405-020-05946-4