Abstract
Purpose
To investigate the feasibility of lymph drainage mapping (LDM) using SPECT/CT to help select head and neck cancer (HNSCC) patients for unilateral elective neck irradiation (ENI). Patients with lateralized HNSCC treated with radiotherapy routinely undergo bilateral ENI, despite the incidence of contralateral regional failure being relatively low even after unilateral ENI. We hypothesized that patients with a lateralized tumor without visible lymph drainage to the contralateral neck have an extremely low risk of contralateral involved nodes. Excluding the contralateral neck from elective irradiation will reduce radiation-induced toxicity and improve quality-of-life.
Methods
Fifty-five patients with lateralized cT1-3N0-2bM0 HNSCC not crossing the midline underwent LDM. Radiolabeled 99mTc-nanocolloid was injected in 4–5 depots around and in the primary tumor. Lymph drainage patterns were visualized using planar scintigraphy and SPECT/CT after 4 h. We report on the incidence of contralateral drainage, the location of draining areas, and the size of underlying nodes.
Results
Lymphatic drainage was successfully visualized in 54 patients (98%). In 11 patients (20%) with visible contralateral drainage, 14 draining areas (16 nodes; median volume 0.50 cc, diameter 8.0 mm) were identified. Neck levels with contralateral drainage were level II (88%), III (25%), and IV (13%). Contralateral drainage was significantly higher in T3 compared to T1–2 tumors (45 and 14%, respectively, P = 0.035).
Conclusion
SPECT/CT-guided LDM is feasible and can be used to guide unilateral ENI in HNSCC patients in prospective studies. In addition, the anatomical confidence in visualization of contralateral drainage indicates a potential for ENI limited to draining levels alone.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The prevalence of regional lymph node metastases in head and neck squamous cell carcinoma (HNSCC) is high, also to the contralateral side of the neck, and is an important prognostic factor for survival [1, 2]. Out of concern for contralateral regional failure (cRF), all patients with HNSCC who are treated primarily with radiotherapy are irradiated electively on both sides of the neck, with the exception of T1 laryngeal and very lateralized tonsillar fossa cancer. This treatment strategy stems from the era when nodal staging was solely based on clinical examination. The currently used advanced diagnostic imaging techniques have significantly improved the accuracy of nodal staging. The number of patients with missed small nodal deposits is nowadays rapidly declining [3,4,5]. Nevertheless, the treatment paradigm of bilateral elective nodal irradiation (ENI) remains basically unchanged.
Bilateral irradiation and the use of large radiation treatment volumes are, together with concurrent chemotherapy, the most important predictors of increased toxicity and deterioration of quality-of-life [6,7,8]. Reversely, exclusion of the contralateral neck from the irradiation fields significantly reduces radiation-related toxicity [9, 10]. A recently published review of 11 studies on oropharyngeal cancer (OPC) (1116 patients in aggregate) shows that for tumors without midline involvement, the average incidence of cRF was 2.4% [11]. Surgical series with cN0 HNSCC have reported incidences of cRF < 15% [12,13,14,15]. Hypopharyngeal carcinoma (HPC) and laryngeal carcinoma (LC) might have higher incidence of contralateral metastases than OPC [16]. However, a recent report suggests that in case of lateralized LC, the incidence of cRF is < 10% [17]. These excellent oncologic outcomes led us to hypothesize that a less conservative approach with regard to the selection of patients for unilateral ENI might be well justified in patients with lateralized HNSCC [18].
One potential way to select candidates for unilateral ENI is to evaluate lymph drainage patterns. Preoperative lymphoscintigraphy for lymph drainage mapping (LDM) in the context of the sentinel node (SN) procedure has been shown to reliably identify draining nodes of HNSCC. In the literature, the false-negative rate of SNB in HNSCC is < 10% [13, 19,20,21,22]. Single-photon-emission computed tomography coupled with a computed tomography scan (SPECT/CT) has the potential to detect more SNs than planar lymphoscintigraphy alone [23], thereby theoretically increasing the diagnostic accuracy. An even higher rate of SN identification was found in a multicenter study using 99mTc-tilmanocept for sentinel node biopsy (SNB) procedure in patients with intraoral and cutaneous HNSCC. The reported negative predictive value, false negative, and overall accuracy rates were 97.8, 2.56, and 98.8%, respectively [24]. Importantly, most false negatives in SNB are caused by lymph nodes in close proximity to the primary tumor (e.g., in floor-of-mouth tumors). The lymph drainage pattern seen on SPECT/CT is thus very accurate, especially in the contralateral neck, which is positioned away from the primary tumor. We hypothesize that in the absence of visible lymph drainage to the contralateral neck on SPECT/CT, the incidence of contralateral involved lymph nodes will be very low, and well below the incidence in patients that do have visible lymph drainage. Therefore, LDM with SPECT/CT might guide safe exclusion of the contralateral neck from ENI.
At our institution, we initiated a proof-of-concept study, the SUSPECT study (mapping of sentinel lymph node drainage using SPECT to tailor ENI in node-negative neck of patients with head and neck cancer) (ClinicalTrials.gov Identifier NCT02572661). The aim of this study was to investigate the role of SPECT/CT for the LDM in patients with lateralized HNSCC; to identify the levels at risk to harbor occult metastases; and to exclude the contralateral neck from ENI when there was no draining SN on that side. The accrual started in July 2015 and closed in October 2017. While awaiting maturation of the outcome and toxicity data, we present the findings of LDM using SPECT/CT.
Materials and methods
Ethical consideration
This study was conducted in accordance with the Declaration of Helsinki and approved by the local research ethics committee (Medical Research Ethics Committee of the Netherlands Cancer Institute/Antoni van Leeuwenhoek, protocol ID: NL15706.031.14). Informed consent was obtained from all individual participants included in the study.
Patient inclusion
In the SUSPECT study, LDM was performed using SPECT/CT scans prior to radiotherapy of lateralized HNSCC. Eligible for inclusion was newly diagnosed patients with primary HNSCC (T1-3N0-2bM0, American Joint Committee on cancer 7th edition [25]) located in the oral cavity, oropharynx, larynx (except T1 glottic), and hypopharynx, not crossing the midline and planned for treatment with (chemo)radiotherapy in curative setting. Patients with extra-capsular extension, patients with N2b disease with more than three involved lymph nodes, and patients with clinically positive contralateral lymph nodes were excluded. All patients received the standard work-up for diagnosis and staging of HNSCC, consisting of ultrasound-guided fine-needle aspiration cytology, contrast-enhanced CT scan and/or MRI, FDG-PET/CT, and endoscopy under general anesthesia.
Lymph drainage mapping
The radioactive tracer was administered during endoscopy under general anesthesia (or in case of an accessible tumor of the oral cavity and oropharynx under local anesthesia) by a head and neck surgeon with expertise in the SN technique. Prior to injection, it was clinically confirmed that the primary tumor was not crossing the midline. A syringe with 180 MBq (4.86 mCi) 99mTechnetium-labelled nanocolloid (Nanocoll, Nycomed Amersham, Sorin, Italy), in a volume of 2 cc with 0.05 mg nanocolloid, was on-site connected by the nuclear medicine physician to a long biopsy needle (18G × 25 cm, Bard Peripheral Vascular, Tempe, USA) using a flexible 15 cm luer-lock connectable line (Lectro-cath, Vygon, Ecouen, France). The activity was pushed towards the tip of the needle. The tip of the needle was positioned at four locations in the mucosa around the primary tumor at 3 mm from macroscopic tumor edges, and preferably at a fifth location deep in the center of the tumor in case of larger tumors. The needle placement at each location was well-verified to avoid tracer spillage. Then, a volume of 0.2 cc was injected in each location, to create 4–5 depots of approximately 18 Mbq (0.49 mCi) each. Remaining activity in the syringe and needle was measured after the injection procedure to calculate the net administered dose, and was then discarded according to radiation hygiene regulations.
Planar scintigraphic imaging and SPECT/CT of the neck were acquired using a dual-head SPECT/CT gamma camera (Symbia T, Siemens, Erlangen, Germany). Imaging was performed at 4 ± 1 h after administration, to allow for adequate tracer distribution with maximum sensitivity for contralateral drainage. Planar images were acquired from anterior, left anterior oblique with the head turned to the right, and right anterior oblique with the head turned to the left. SPECT acquisition parameters were 256 × 256 matrix, zoom of 1.0, 2 heads, 180° rotation with 20 views per head (30 s per view). Low-dose CT parameters included 40 mAs, 130 kV, B30s kernel, axial reconstruction with 2 mm slice thickness and interval. The low-dose CT images were applied for anatomical correlation with SPECT, and for attenuation correction and scatter correction of SPECT images. For image reading SPECT, CT and fused SPECT/CT were displayed using orthogonal multiplanar reconstruction, maximum intensity projection, and volume rendering. The planar and SPECT images were evaluated visually for lymphatic drainage to the contralateral neck side, noting all positive nodal levels on both sides of the neck. The hotspot containing the tracer accumulation at SPECT/CT will further be denoted as ‘draining area’.
The SPECT/CT images were compared with the radiotherapy planning CT scan to localize anatomical substrates that correlated with tracer accumulation, and visible nodes were delineated. The volume and greatest diameter in the axial plane were then calculated for each substrate. Clinically positive lymph nodes, based on prior imaging, were excluded from the volumetric analysis of the anatomical substrates seen on the corresponding slices of the CT scan.
Results
Sixty-one patients were included in the SUSPECT study. Six patients were excluded during further clinical work-up for having a T4 tumor (n = 3), N2c neck (n = 2), or histology other than squamous cell carcinoma (n = 1), leaving 55 patients who are the subject of the current analysis (Fig. 1). Figure 2 shows the location and extension of the tumor of the first patient included in the study.
Table 1 shows the characteristics of the included patients. All 55 patients underwent the procedure of LDM, and finished the whole procedure of tracer injection and SPECT/CT without additional pain, discomfort, complications, or hospital admission. In nine patients with accessible tumors, the tracer was injected under local anesthesia. In all other patients, the injection was done during planned endoscopy under general anesthesia.
In one patient, no drainage was seen on either side of the neck, indicating a failed procedure (non-visualization), resulting in a visualization rate by SPECT/CT of 98%. In the other 54 patients, at least one draining area was identified (median 2, range 1–9) (Table 2).
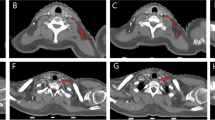
A total of 137 draining areas were visualized on the SPECT scan. Of these, 123 were ipsilateral and 14 contralateral. When correlating these draining areas with the corresponding CT slices, no measurable anatomical substrates were identified in 17 areas (12%), while in the other 120 areas, 133 measurable substrates were identified that were considered the corresponding lymph nodes (mean of 2.44 nodes per patient). Median volume and median largest diameter of these draining lymph nodes were 0.39 cc (range 0.01–2.64 cc) and 8.0 mm (range 3–20 mm), respectively. The SPECT/CT scan of the first patient included in the study is shown in Fig. 3, demonstrating the tracer depot at the site of the primary tumor and the three draining areas with gradually decreasing intensity of the tracer accumulation by increasing distance from the primary tumor. Figure 4 demonstrates the correlation of draining areas with the presence or the absence of underlying anatomical substrates in two different patients.
SPECT/CT images of the first patient. Planar lymphoscintigraphy (a) and SPECT/CT images (b–d) of the first patient included in the study. The tracer depot is visible (green arrow), as are draining areas in level 2 (b, adjacent to the injection site), level 3 (c) and level 4 (d), indicated by red arrows
Presence and absence of anatomical substrate. Example of a patient, where a draining area in level 2 on the right (a) corresponds to a lymph node that is clearly visible on low-dose CT (b, white arrow) and another patient with a draining area low in level 4 (c), without a visible substrate on low-dose CT (d, white arrow)
Of the 54 evaluable patients, 43 (80%) had only ipsilateral drainage. In 11 patients (20%), bilateral drainage was seen on SPECT/CT; 9 of them had only 1 draining area, and the other 2 patients had more than one draining area contralaterally. In these 11 patients, we visualized 14 draining areas on the contralateral side, with 16 underlying lymph nodes (median volume 0.50 cc, median diameter 8.0 mm). Table 3 provides information about the number of draining areas per patient, the size, and volume of the nodal substrate if visualized and shows the distribution of the visualized draining areas among the neck levels of the whole group and by tumor sites. Contralateral drainage was predominantly seen in level 2 (64%) and in levels 3 and 4 (both 27%). In patients with OPC and oral cavity cancer (OCC), the contralateral drainage was mainly seen in level 2 (88%) and level 3 (25%) and in patients with LC and HPC in level 4 (67%) and level 3 (33%).
Table 4 illustrates the incidence of contralateral drainage by different tumor characteristics. A significantly higher incidence of contralateral drainage was seen in T3 tumors, compared to T1 and T2 (45 vs. 14%, respectively, P = 0.035). Logistic regression showed no significant effect of tumor volume, tumor thickness or distance to the midline on the likelihood of contralateral drainage.
Discussion
To the best of our knowledge, this is the largest series to date reporting on the findings of SPECT/CT-based LDM in patients with lateralized HNSCC treated primarily with radiotherapy. In this proof-of-concept study, we investigated the role of SPECT/CT-guided LDM in identifying the contralateral drainage from lateralized HNSCC to more accurately select patients for unilateral ENI.
The visualization rate of draining areas was 98%. The pattern of ipsilateral lymph drainage reported in our study (OCC and OPC draining into levels 2 and 3 and LC and HPC draining into levels 3, 2, and 4) is fairly comparable with that reported in studies, where the identified SN was removed for pathological examination [21, 26, 27].
Contralateral drainage was seen in 20% of our patients, predominantly in level 2. In patients with OPC and OCC, the contralateral drainage was mainly seen in levels 2 and 3 and in patients with LC and HPC in levels 4 and 3. In the literature, the incidence of contralateral lymphatic drainage was found to be 38–40% in patients with LC, HPC, and OPC near the midline [21, 26], and ranging from 8 to 38% in patients with lateralized OCC and OPC [13, 14, 19, 20, 27,28,29]. When correlating the incidence of contralateral drainage with T-classification, N-classification, tumor site, tumor thickness, the distance of the primary tumor from the midline, and HPV status, only T-classification showed significant correlation (Table 4). These figures should be interpreted with caution because of the small number of patients with contralateral drainage and the absence of the pathological confirmation of negative or positive status of draining nodes on the SPECT/CT scan. Still, according to the SUSPECT study protocol, all neck levels harboring draining nodes would receive elective irradiation.
Over the last few decades, oncologic outcomes of patients with HNSCC have improved as a result of the use of chemoradiation and cetuximab and altered fractionation schemes of radiotherapy [30]. However, this was achieved at the cost of important toxic effects. As a consequence of improved survival and the increasing incidence of HPV-related OPC in young patients, reduction of treatment-related toxicity and the impact of disease and treatment on patient’s quality-of-life have become important secondary considerations. One way to significantly reduce the frequency, severity, and duration of radiation-related toxicity is reducing the radiation fields by the use of unilateral ENI. The main concern when excluding the contralateral N0 neck from the ENI in HNSCC is the potential increased risk of cRF. However, the incidence of contralateral metastases in HNSCC not crossing the midline is < 10%, both in studies, where unilateral ENI was applied [18], and in those where the neck dissection was guided by SNB [13, 14, 26, 31], and around 20% when the resection of the primary tumor was combined with neck dissection without SNB [32, 33]. Since these figures are still below the generally accepted level to justify ENI in HNSCC (15–20%) and below the level, where observation was advocated instead of elective neck dissection in the surgical series (20% by Weiss et al. [34] and 44% by Okura et al. [35]), we hypothesize that the paradigm of unilateral ENI might be well justified in lateralized HNSCC not extending beyond the midline, since midline involvement is the most important prognosticator for cRF [9, 10, 36].
To justify research with this approach, we need to select patients with a very low likelihood of contralateral involved nodes. The SNB approach is widely investigated in HNSCC [13, 26, 27, 37], although it is generally applied to detect nodal involvement of the first echelon nodes by pathological confirmation, rather than to exclude it by imaging alone. Furthermore, SNB is an invasive procedure, and in patients treated with radiotherapy alone, a non-invasive approach needs to be developed to safely guide the exclusion of the contralateral neck from the irradiation fields.
Since several studies have shown that the sensitivity of SPECT/CT to detect an SN which might harbor occult disease would be around 90% [20, 29, 38], we hypothesize that patients with no detectable lymph drainage to the contralateral side will have a very low likelihood of involved nodes. Therefore, we adopted this minimally invasive technique to guide the unilateral ENI in lateralized HNSCC in the current study.
In conclusion, SPECT/CT-guided LDM in patients with HNSCC treated primarily with radiotherapy seems to be feasible, the procedure was well tolerated and the incidence and the pattern of lymphatic drainage to both sides of the neck was comparable with literature, where pathological confirmation of the SN was performed. This justifies further research to guide exclusion of the contralateral neck from ENI in patients with lateralized HNSCC. The oncologic and toxicity outcomes of patients treated by this approach within the framework of the SUSPECT study will be published as soon as the data is mature.
References
Roberts TJ, Colevas AD, Hara W et al (2016) Number of positive nodes is superior to the lymph node ratio and American Joint Committee on Cancer N staging for the prognosis of surgically treated head and neck squamous cell carcinomas. Cancer 122:1388–1397. https://doi.org/10.1002/cncr.29932
Snow GB, van den Brekel MW, Leemans CR, Patel P (1994) Surgical management of cervical lymph nodes in patients with oral and oropharyngeal cancer. Recent Results Cancer Res 134:43–55
Yamazaki Y, Saitoh M, Notani K et al (2008) Assessment of cervical lymph node metastases using FDG-PET in patients with head and neck cancer. Ann Nucl Med 22:177–184. https://doi.org/10.1007/s12149-007-0097-9
Norling R, Buron BMD, Therkildsen MH et al (2014) Staging of cervical lymph nodes in oral squamous cell carcinoma: adding ultrasound in clinically lymph node negative patients may improve diagnostic work-up. PLoS One 9:1–6. https://doi.org/10.1371/journal.pone.0090360
Imani Moghaddam M, Davachi B, Mostaan LV et al (2011) Evaluation of the sonographic features of metastatic cervical lymph nodes in patients with head and neck malignancy. J Craniofac Surg 22:2179–2184. https://doi.org/10.1097/SCS.0b013e3182324166
Nutting CM, Morden JP, Harrington KJ et al (2011) Parotid-sparing intensity modulated versus conventional radiotherapy in head and neck cancer (PARSPORT): a phase 3 multicentre randomised controlled trial. Lancet Oncol 12:127–136. https://doi.org/10.1016/S1470-2045(10)70290-4
Jellema AP, Slotman BJ, Doornaert P et al (2007) Unilateral versus bilateral irradiation in squamous cell head and neck cancer in relation to patient-rated xerostomia and sticky saliva. Radiother Oncol 85:83–89. https://doi.org/10.1016/j.radonc.2007.03.002
Al-Mamgani A, Van Rooij P, Tans L et al (2013) A prospective evaluation of patient-reported quality-of-life after (chemo)radiation for oropharyngeal cancer: which patients are at risk of significant quality-of-life deterioration? Radiother Oncol 106:359–363. https://doi.org/10.1016/j.radonc.2012.12.014
Liu C, Dutu G, Peters LJ et al (2014) Tonsillar cancer: the Peter MacCallum experience with unilateral and bilateral irradiation. Head Neck 36:317–322. https://doi.org/10.1002/hed.23297
Jensen K, Overgaard M, Grau C (2007) Morbidity after ipsilateral radiotherapy for oropharyngeal cancer. Radiother Oncol 85:90–97. https://doi.org/10.1016/j.radonc.2007.06.005
Al-Mamgani A, van Werkhoven E, Navran A et al (2017) Contralateral regional recurrence after elective unilateral neck irradiation in oropharyngeal carcinoma: a literature-based critical review. Cancer Treat Rev 59:102–108. https://doi.org/10.1016/j.ctrv.2017.07.004
Rackley TP, Namelo WC, Palaniappan N et al (2017) Unilateral radiotherapy for surgically resected lateralized squamous cell carcinoma of the tonsil. Head Neck 39:17–23. https://doi.org/10.1002/hed.24541
Schilling C, Stoeckli SJ, Haerle SK et al (2015) Sentinel European Node Trial (SENT): 3-year results of sentinel node biopsy in oral cancer. Eur J Cancer 51:2777–2784. https://doi.org/10.1016/j.ejca.2015.08.023
Höft S, Maune S, Muhle C et al (2004) Sentinel lymph-node biopsy in head and neck cancer. Br J Cancer 91:124–128. https://doi.org/10.1038/sj.bjc.6601877
Foote RL, Schild SE, Thompson WM et al (1994) Tonsil cancer. Patterns of failure after surgery alone and surgery combined with postoperative radiation therapy. Cancer 73:2638–2647. https://doi.org/10.1002/1097-0142(19940515)73:10%3C2638::AID-CNCR2820731028%3E3.0.CO;2-H
Buckley JG, MacLennan K (2000) Cervical node metastases in laryngeal and hypopharyngeal cancer: a prospective analysis of prevalence and distribution. Head Neck 22:380–385
Böttcher A, Olze H, Thieme N et al (2017) A novel classification scheme for advanced laryngeal cancer midline involvement: implications for the contralateral neck. J Cancer Res Clin Oncol 143:1605–1612. https://doi.org/10.1007/s00432-017-2419-1
Al-Mamgani A, Verheij M, van den Brekel MWM (2017) Elective unilateral nodal irradiation in head and neck squamous cell carcinoma: a paradigm shift. Eur J Cancer 82:1–5. https://doi.org/10.1016/j.ejca.2017.05.035
Alkureishi LWT, Ross GL, Shoaib T et al (2010) Sentinel node biopsy in head and neck squamous cell cancer: 5-year follow-up of a European multicenter trial. Ann Surg Oncol 17:2459–2464. https://doi.org/10.1245/s10434-010-1111-3
Haerle SK, Hany TF, Strobel K et al (2009) Is there an additional value of SPECT/CT over planar lymphoscintigraphy for sentinel node mapping in oral/oropharyngeal squamous cell carcinoma? Ann Surg Oncol 16:3118–3124. https://doi.org/10.1245/s10434-009-0632-0
Tomifuji M, Shiotani A, Fujii H et al (2008) Sentinel node concept in clinically n0 laryngeal and hypopharyngeal cancer. Ann Surg Oncol 15:2568–2575. https://doi.org/10.1245/s10434-008-0008-x
Colnot DR, Nieuwenhuis EJ, van den Brekel MW et al (2001) Head and neck squamous cell carcinoma: US-guided fine-needle aspiration of sentinel lymph nodes for improved staging-initial experience. Radiology 218:289–293. https://doi.org/10.1148/radiology.218.1.r01dc01289
Haerle SK, Stoeckli SJ (2011) SPECT/CT for lymphatic mapping of sentinel nodes in early squamous cell carcinoma of the oral cavity and oropharynx. Int J Mol Imaging 2011:1–6. https://doi.org/10.1155/2011/106068
Agrawal A, Civantos FJ, Brumund KT et al (2015) [99mTc]tilmanocept accurately detects sentinel lymph nodes and predicts node pathology status in patients with oral squamous cell carcinoma of the head and neck: results of a phase III multi-institutional trial. Ann Surg Oncol 22:3708–3715. https://doi.org/10.1245/s10434-015-4382-x
American Joint Committee on Cancer (2010) AJCC cancer staging manual, 7th edn. Springer, New York
Werner JA, Dünne AA, Ramaswamy A et al (2004) The sentinel node concept in head and neck cancer: solution for the controversies in the NO neck? Head Neck 26:603–611. https://doi.org/10.1002/hed.20062
Flach GB, Bloemena E, Klop WMC et al (2014) Sentinel lymph node biopsy in clinically N0 T1–T2 staged oral cancer: the Dutch multicenter trial. Oral Oncol 50:1020–1024. https://doi.org/10.1016/j.oraloncology.2014.07.020
Stoeckli SJ (2007) Sentinel node biopsy for oral and oropharyngeal squamous cell carcinoma of the head and neck. Laryngoscope 117:1539–1551. https://doi.org/10.1097/MLG.0b013e318093ee67
Bilde A, Von Buchwald C, Mortensen J et al (2006) The role of SPECT-CT in the lymphoscintigraphic identification of sentinel nodes in patients with oral cancer. Acta Otolaryngol 126:1096–1103. https://doi.org/10.1080/00016480600794453
Haddad RI, Shin DM (2008) Recent advances in head and neck cancer. N Engl J Med 359:1143–1154. https://doi.org/10.1056/NEJMra0707975
Lawson G, Matar N, Nollevaux MC et al (2010) Reliability of sentinel node technique in the treatment of N0 supraglottic laryngeal cancer. Laryngoscope 120:2213–2217. https://doi.org/10.1002/lary.21131
Lim YC, Lee SY, Lim J-Y et al (2005) Management of contralateral N0 neck in tonsillar squamous cell carcinoma. Laryngoscope 115:1672–1675. https://doi.org/10.1097/01.mlg.0000184791.68804.0b
Olzowy B, Tsalemchuk Y, Schotten K-J et al (2011) Frequency of bilateral cervical metastases in oropharyngeal squamous cell carcinoma: a retrospective analysis of 352 cases after bilateral neck dissection. Head Neck 33:239–243. https://doi.org/10.1002/hed.21436
Weiss MH, Harrison LB, Isaacs RS (1994) Use of decision analysis in planning a management strategy for the stage N0 neck. Arch Otolaryngol Head Neck Surg 120:699–702
Okura M, Aikawa T, Sawai NY et al (2009) Decision analysis and treatment threshold in a management for the N0 neck of the oral cavity carcinoma. Oral Oncol 45:908–911. https://doi.org/10.1016/j.oraloncology.2009.03.013
O’Sullivan B, Warde P, Grice B et al (2001) The benefits and pitfalls of ipsilateral radiotherapy in carcinoma of the tonsillar region. Int J Radiat Oncol Biol Phys 51:332–343
Vermeeren L, Klop WMC, Van Den Brekel MWM et al (2009) Sentinel node detection in head and neck malignancies: innovations in radioguided surgery. J Oncol. https://doi.org/10.1155/2009/681746
den Toom IJ, van Schie A, van Weert S et al (2017) The added value of SPECT-CT for the identification of sentinel lymph nodes in early stage oral cancer. Eur J Nucl Med Mol Imaging 44:998–1004. https://doi.org/10.1007/s00259-017-3613-8
Funding
This research was partially funded by a Top Consortium Knowledge and Innovation of the sector Life Sciences and Health (LSH-TKI Foundation) public–private partnership grant (LSHM15036) in collaboration with Elekta (SE).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Rights and permissions
About this article
Cite this article
de Veij Mestdagh, P.D., Jonker, M.C.J., Vogel, W.V. et al. SPECT/CT-guided lymph drainage mapping for the planning of unilateral elective nodal irradiation in head and neck squamous cell carcinoma. Eur Arch Otorhinolaryngol 275, 2135–2144 (2018). https://doi.org/10.1007/s00405-018-5050-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00405-018-5050-0