Abstract
Background
Many Chinese patients who experience chronic rhinosinusitis with nasal polyps (CRSwNP) have been shown to exhibit specifically enhanced TH1/TH17 responses and excessive neutrophil accumulation without demonstrating significant eosinophilia. These patients may be subject to different pathologies and therapies compared to Western patients. YKL40 can be produced by neutrophil and is associated with many inflammatory diseases, while its role in the pathogenesis of chronic rhinosinusitis (CRS) has yet to be determined.
Objective
The aim of this study was to investigate the relationship between the expression level and biologic role of YKL40 in CRS.
Methods
YKL40 expression was examined via quantitative reverse transcriptase polymerase chain reaction (qRT-PCR), immunohistochemistry, and Western blot. Human nasal epithelia cells (HNECs) were isolated to detect YKL40 expression in response to specific inflammatory stimulation.
Results
YKL40 expression levels were significantly higher in NP patients compared to the turbinates of CRSsNP/CRSwNP and the control group and can be strongly activated by stimulation with IL-4 in vitro and suppressed by the other pro-inflammatory cytokines; lipopolysaccharide (LPS) and dexamethasone also caused significant decreases in YKL40 expression in HNECs.
Conclusions
YKL40 may play a significant role in Chinese patients with CRSwNP. The molecular mechanisms identified here may aid in the design of new therapeutic strategies for improving the clinical outcomes of Chinese patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic rhinosinusitis (CRS) is broadly divided into CRS with nasal polyps (CRSwNP) and CRS without nasal polyps (CRSsNP). CRSwNP can be further classified into eosinophilic and non-eosinophilic subsets in White individuals [1, 2]. However, published data indicate that many Chinese patients with CRSwNP do not demonstrate significant eosinophilia [3]. Moreover, Chinese patients with CRSwNP have been shown to exhibit specifically enhanced TH1/TH17 responses and excessive neutrophil accumulation [4]. These findings indicate different pathologies between Chinese patients with CRSwNP and their White counterparts.
YKL40, also called Chitinase-3-Like-1 (CHI3L1), is a secreted mammalian glycoprotein that is approximately 40 kDa in size and has a chitin-binding ability but lacks the enzymatic activity to catalyze chitin [5]. YKL40 can be produced by neutrophil and is associated with many inflammatory diseases [6], such as inflammatory bowel disease [7], osteomyelitis, and lung diseases; for example, chronic obstructive pulmonary disease (COPD) [8], lung fibrosis [9], and asthma [10]. It also has been proved to be up-regulated in mild and moderate/severe persistent allergic rhinitis, and its expression can be regulated differentially by different cytokines, possibly contributing to the remodeling of nasal mucosa in allergic rhinitis [10]. While YKL40 has been found to be involved in the pathogenesis of allergic rhinitis, a few reports have been published on the role of YLK40 in CRS patients. As it may be a more suitable marker for Chinese patients, our group investigated YKL40 expression in different subsets of CRS patients and primary modulation under allergy-like conditions in vitro.
Subjects and patients
All the study participants (n = 79) were recruited from the Department of Ophthalmology, Eye and ENT Hospital of Fudan University, Shanghai, China. The CRS patients who had not taken oral and/or topical corticosteroids or any other sinonasal medications for at least 1 month prior to the study were diagnosed based on previously published criteria [11]. Nasal polyps (NPs) were obtained from CRSwNP patients (n = 31), and nasal mucosal tissues were obtained from the middle turbinates of CRSsNP patients and from the inferior turbinates of control patients (n = 14) who had no clinical or radiographic evidences of CRS and had undergone septoturbinoplasty. Allergies were diagnosed or documented based on positive skin-prick test results. The subjects’ demographic data are summarized in Table 1. Exclusion criteria for the study group included the following: age < 18 or > 80 years, patients with a diagnosis of cystic fibrosis, Churg–Strauss syndrome, immunodeficiency, or autoimmune diseases.
This study obtained permission from the local ethical committee of the Otolaryngology-Head and Neck Surgery, Eye, Ear, Nose, and Throat Hospital, Fudan University, and every participant provided an informed consent.
Materials and methods
RNA extraction and real-time polymerase chain reaction
Tissue specimens obtained during surgery were immediately cut into several portions and homogenized in liquid nitrogen. Total RNA was isolated using TRIzol Reagent (invitrogen) according to the manufacturer’s instructions. The quality and concentration of RNA was measured using an A260/A280 ratio. We then used PrimeScript RT Master Mix (Takara) to synthesize complementary DNA (cDNA) following the protocol. The qRT-PCR was performed via the use of SYBR Green chemistry in the ABI 7900 Sequence Detection (ABI Applied Biosystems). Primers were designed and synthesized (Sangon Biotech, Table 2). Each sample was analyzed in triplicate. Gene expression was calculated using the comparative threshold cycle (2−ΔΔCT) method.
Immuohistochemistry (IHC) staining
Fresh tissues were fixed in 4% paraformaldehyde, embedded in paraffin, and sectioned (4 µm). An immunohistochemistry analysis was performed using a streptavidin biotin complex (SABC) kit (Weiao Biological Technology). After incubation with a primary antibody (polyclonal rabbit anti-human YKL40; Abcam; 1:200 dilution) at 4° overnight, the sections were incubated with an anti-rabbit secondary antibody for 30 min, and 3′3-diaminobenzidine (DAB) was applied for visualization. The analysis was performed by two pathologists in a double-blind analysis. The results of the staining were scored according to the following immunostaining intensities: 0 = absent, 1 = mild, 2 = moderate, and 3 = marked. The average numbers of immunopositive cells within the samples were determined in at least five areas at 400× magnification. The percentage of immunopositive cells was scored on a scale of 0 (0–9%), 1 (10–25%), 2 (26–50%), 3 (51–75%), and 4 (> 76%). For each sample, the scores from the two scoring systems were multiplied to get a final point score. The maximum combined score was 12, and the minimum combined score was 0. The immunohistochemical expression of YKL40 was therefore defined according to the following scores: negative, combined score of 0; low expression, combined score of 1–8; high expression, combined score of 9–12.
Western blots
Tissue specimens obtained during surgery were immediately frozen in liquid nitrogen. The protein concentration was measured using a bicinchoninic acid assay (BCA assay) kit (Thermo). Protein samples (30 μg) were separated by electrophoresis using 10% sodium dodecyl sulfate polyacrylamide gels and then transferred to polyvinylidene difluoride (PVDF) membranes. After incubation with an anti-YKL40 (1:1000, Abcam) antibody, the membranes were incubated with a horseradish peroxidase-conjugated goat anti-rabbit secondary antibody (Santa Cruz). The bands were subjected using an ECL (enhanced chemiluminescence) kit (Millipore). Data were quantified via densitometry using ImageJ analysis and processing software, and expressed as densitometry units (DUs). The protein expression for each sample was normalized against the expression of β-actin as a loading control.
Cell culture and stimulation
HNECs isolated from nasal polyps of CRSwNP patients were cultured as described previously [12]. Following the washing of nasal specimens with PBS, they were briefly immersed in DMEM/F12 (HyClone) containing 1.4 mg/mL protease K and 0.1 mg/mL Dnase for a 1.5 h-incubation at 37 °C, and protease activity was blocked by 10% FBS (Gbico). All cells were then collected and immersed in DMEM/F12 (HyClone) containing 1% ITS for 2 h at 37 °C and cultured in BEBM medium (Lonza).
When an 80–90% confluence was reached, the epithelial cells were washed with PBS (37 °C, pH 7.4), and fresh media without hydrocortisone were added in the presence of the following stimulators or control PBS for 24 h: the recombinant cytokines human IFN-γ (100 ng/mL), IL-4 (100 ng/mL), IL-13 (100 ng/mL), IL-17A (100 ng/mL), TGF-β (10 ng/mL), and IL-1α (100 ng/mL), all purchased from Peprotech; the TLR agonist LPS (500 ng/mL, Sigma), and the glucocorticoids dexamethasone (10 μg/mL, Sigma). After stimulation, cell pellets were collected for qRT-PCR and Western blot analysis.
Statistical analysis
Statistical analysis was performed with SPSS v22.0 software (IBM Corporation). The data were expressed as a mean ± standard deviation. Differences between groups were determined by one-way analysis of variance, and the two independent samples via a t test or the Mann–Whitney U test. Pearson correlations were used to determine variable relationships. If not normally distributed, the Spearman correlation coefficient was selected. p < 0.05 was defined as statistically significant.
Results
Expression levels of YKL40 in nasal tissues of patients with different CRS types
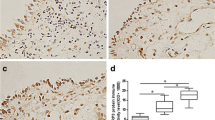
As shown in Fig. 1a, a significant upregulation of YKL40 mRNA levels was found in the NP (p < 0.01) and CRSsNP (p < 0.05) groups compared with the control group. However, there were no significant differences between the turbinates of the CRSwNP group and the control group. To verify the results at the protein level, Western blots were performed. Strong bands of YKL40 were observed in the NP and CRSsNP groups, whereas weaker bands were found in the turbinates of the CRSwNP and control groups (Fig. 1c). The DU analysis (Fig. 1b) showed that the protein expression level of YKL40 was significantly higher in the NP (p < 0.0001) and CRSsNP (p < 0.01) groups compared to that of the control group. Moreover, there was also a significant difference between the NP and CRSsNP groups (p < 0.001). In a comparison of the turbinates of the CRSwNP and control groups, the YKL40 expression showed no difference.
These findings indicate that YKL40 mRNA and protein expression differ in different types of CRS patients.
Expression position of YKL40 in the nasal mucosa of patients with different CRS types
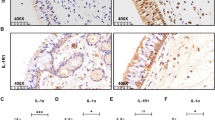
Immunohistochemistry was performed to further confirm the results pertaining to the protein levels of YKL40 in the various samples. As indicated by immunohistochemistry staining (Fig. 2a), YKL40 was significantly overexpressed in the NP group compared with the other three groups. The cytoplasmic or nuclear staining of YKL40 was mainly located at the nasal epithelium cells and submucosal inflammatory cells and glands. A quantitative analysis of YKL40 revealed an obvious elevation in the immuno-labeling of YKL40 in the NP group compared with the control group (p < 0.01), the turbinates of the CRSsNP group (p < 0.05), and the CRSwNP group (p < 0.05) (Fig. 2b).
Expression position and producing cells of YKL40 in nasal tissues. a YKL40 protein expression and distribution in nasal tissue detected by immunohistochemistry staining; b YKL40 was significantly overexpressed in the NP group compared with the other three groups; c–g correlation of YKL40 with cell-specific markers in nasal mucosa of CRSsNP patients; the expression of YKL40 and cell-specific markers was analyzed by real-time PCR. The Pearson correlation test was used, and R values indicate Pearson correlation coefficients; *p < 0.05, **p < 0.01
Detection of YKL40-producing cells in nasal mucosa
The immunohistochemistry staining revealed that YKL40+ cells were highly accumulated in the submucosal region of NP tissue (Fig. 2a). After the immunohistochemistry staining, the relationship between YKL40 expression and the markers of inflammatory cells in the NP group was assessed by qRT-PCR. The inflammatory cell markers include CD3 (T cells), tryptase (mast cells), Charot–Leyden crystal (eosinophils), CXCR1 (neutrophils), and CD68 (macrophages). The results demonstrated that the mRNA expression levels of YKL40 in the NP tissues were significantly and positively related to the expression of CD3 (r = 0.3703; p < 0.05), CXCR1 (r = 0.3581; p < 0.05), and CD68 (r = 0.3737; p < 0.05) but not to tryptase and Charot–Leyden crystal (CLC), which indicated that the YKL40 expression may derive from T cells, neutrophils, and macrophages (Fig. 2c–g).
Pro-inflammatory cytokines, LPS, and dexamethasone differentially regulate YKL40 expression in HNECs
To further determine the role and mechanism of the YKL40 signaling pathway in the nasal mucosa of NP patients, the effects of pro-inflammatory cytokine stimulation were examined in cultured human nasal epithelial cells (HNECs). As presented in Fig. 3a, mRNA expression levels of YKL40 were significantly increased after stimulating the HNECs with IL-4 (p < 0.001) and decreased after IFN-γ stimulation (p < 0.05). The deregulated protein levels of YKL40 detected via Western blot are shown in Fig. 3c. The DU analysis (Fig. 3b) revealed data that represented the means ± SEM from three independent experiments. YKL40 protein expression levels in HNECs were significantly higher after stimulation with IL-4 (p < 0.0001), while they were significantly lower after stimulation with other pro-inflammatory cytokines, including IL-17 (p < 0.0001), IFN-γ (p < 0.0001), TGF-β (p < 0.0001), and IL-1α (p < 0.001), compared to the control group. The TLR agonist LPS was also used to stimulate the HNECs and caused a significant decrease in YKL40 expression (p < 0.0001).
YKL40 mRNA and protein expression in cultured HNECs in response to pro-inflammatory cytokines, LPS, and dexamethasone. a YKL40 mRNA expression after a 24 h stimulation; b densitometric analysis; c different expression of YKL40 after pro-inflammatory cytokines, LPS and dexamethasone stimulation. *p < 0.05, ***p < 0.001, ****p < 0.0001
Because glucocorticoid treatment is recommended as one of the primary treatments of choice for patients with NPs, the effects of glucocorticoid on YKL40 expression in HNECs were investigated. After incubating cells with dexamethasone, YKL40 mRNA and protein expression was downregulated compared to that of the control group (p < 0.5).
Discussion
The results of the present study indicated that YKL40 expression levels were significantly higher in the NP patients compared to the turbinates of participants in the CRSsNP, CRSwNP, and control groups. Moreover, in the submucosa of the NP tissues, YKL40+ cells were identified to be T cells, neutrophils, and macrophages, a finding that is consistent with a previous study [6]. In addition, YKL40 expression can be strongly activated by stimulation with IL-4 in vitro and suppressed by other pro-inflammatory cytokines, such as IL-17, IFN-γ, TGF-β, and IL-1α. LPS and dexamethasone also caused a significant decrease in YKL40 expression in HNECs.
While CRSsNP is characterized by Th1-biased inflammation and an elevated expression of transforming growth factor (TGF-β1), CRSwNP in White individuals is characterized by a Th2 dominance [13]. Significant numbers of eosinophils accumulate in the mucosa of Western patients’ NP tissue, while the NPs of Chinese patients are characterized by a Th1/Th17 dominance. These differences are reflected in a distinguished distinct eosinophil granulocyte and neutrophil activation bias in the polyps of Western and Chinese patients, respectively [14].
The NPs of White patients represent an often severe and difficult-to-treat eosinophilic airway inflammation, which is frequently linked to comorbid asthma [15]. The typical inflammation shares many similar features with asthma, such as increased numbers of mucosal eosinophils and activated T cells that produce a TH2-biased cytokine profile, including IL-5 and IgE formation [16]. A few patients with severe asthma are largely unresponsive to treatment with systemic corticosteroids because of neutrophil recruitment [17]. This phenomenon also exists in NP patients. Wen et al. [4] compared the polyp size score, nasal resistance, nasal congestion score, and TNSS (total nasal system score) between a neutrophil-negative NP group and a neutrophil-positive NP group after 1 week of treatment with oral prednisone. The results of the study showed that the neutrophil-positive NP group was less sensitive to prednisone therapy compared to the neutrophil-negative NP group.
Because neutrophils and Th17 cells [18] are YKL40-producing cells and these two types of cells largely infiltrate the mucosa of NP tissues in Chinese patients, YKL40 expression levels should be higher in China’s NP patients, a speculation identified in our data.
Moreover, YKL40 has already been identified as a biomarker of asthma and correlates to asthma phenotypes [19]. The circulating level of YKL40 is associated with the severity of asthma [20] and can participate in airway remodeling in both asthma and allergic rhinitis [10]. Lai et al. found that patients with elevated levels of YKL40 had significantly greater corticosteroid use than patients with lower levels [20]. The authors’ results indicated that a high YKL40 expression level was associated with a resistance to present asthma treatment. Therefore, YKL40 levels could also be a credible marker in evaluating the therapeutic effects of NPs.
It has been reported that YKL40 secretion is stimulated by the pro-inflammatory cytokines IL-6, IL-17, and IL-18 released from neutrophils, vascular smooth muscle, macrophages, chondrocytes, and cancer cells [21]. In the present study, considerable data showed that only the Th2 cytokine IL-4 could significantly increase the YKL40 expression level in HNECs in vitro. IL-5 and IL-13 could also activate YKL40 expression, but a comparison with the control group demonstrated no statistical significance. However, YKL40 expression was suppressed by stimulation with IL-17 and IFN-γ, a finding that is in contrast with a previous study [10]. We think that this disparity may be caused by the source of HNECs. We used NPs to isolate epithelium cells, while Park et al. [10] used allergic nasal mucosa. Further studies are needed to confirm the results of stimulation with pro-inflammatory cytokines in different sources of HNECs.
Notably, we found that LPS also caused a significant decrease in YKL40 expression in HNECs. This result is consistent with the low YKL40 expression in CRSsNP. We also observed that dexamethasone could decrease YKL40 expression in NPs.
In summary, the present study revealed that YKL40 is highly expressed in Chinese patients with NPs and that IL-4 may play a significant role in the YKL40 signaling pathway in NP patients. Further investigation will concentrate on new therapy for high YKL40 expression and neutrophil infiltration NPs types. The therapy will be different from the one performed on eosinophil infiltration NPs types. The molecular mechanisms identified here will help to clarify the pathogenic processes involved in these CRS subsets as well as aid in the design of novel therapeutic strategies to improve clinical outcomes.
References
Van Zele T, Claeys S, Gevaert P, Van Maele G, Holtappels G, Van Cauwenberge P, Bachert C (2006) Differentiation of chronic sinus diseases by measurement of inflammatory mediators. Allergy 61(11):1280–1289. https://doi.org/10.1111/j.1398-9995.2006.01225.x
Tomassen P, Van Zele T, Zhang N, Perez-Novo C, Van Bruaene N, Gevaert P, Bachert C (2011) Pathophysiology of chronic rhinosinusitis. Proc Am Thorac Soc 8(1):115–120. https://doi.org/10.1513/pats.201005-036RN
Cao PP, Li HB, Wang BF, Wang SB, You XJ, Cui YH, Wang DY, Desrosiers M, Liu Z (2009) Distinct immunopathologic characteristics of various types of chronic rhinosinusitis in adult Chinese. J Allergy Clin Immunol 124(3):478–484, 484.e471–472. https://doi.org/10.1016/j.jaci.2009.05.017
Wen W, Liu W, Zhang L, Bai J, Fan Y, Xia W, Luo Q, Zheng J, Wang H, Li Z, Xia J, Jiang H, Liu Z, Shi J, Li H, Xu G (2012) Increased neutrophilia in nasal polyps reduces the response to oral corticosteroid therapy. J Allergy Clin Immunol 129(6):1522–1528.e1525. https://doi.org/10.1016/j.jaci.2012.01.079
Meng G, Zhao Y, Bai X, Liu Y, Green TJ, Luo M, Zheng X (2010) Structure of human stabilin-1 interacting chitinase-like protein (SI-CLP) reveals a saccharide-binding cleft with lower sugar-binding selectivity. J Biol Chem 285(51):39898–39904. https://doi.org/10.1074/jbc.M110.130781
Kawada M, Hachiya Y, Arihiro A, Mizoguchi E (2007) Role of mammalian chitinases in inflammatory conditions. Keio J Med 56(1):21–27
Mizoguchi E (2006) Chitinase 3-like-1 exacerbates intestinal inflammation by enhancing bacterial adhesion and invasion in colonic epithelial cells. Gastroenterology 130(2):398–411. https://doi.org/10.1053/j.gastro.2005.12.007
Lai T, Wu D, Chen M, Cao C, Jing Z, Huang L, Lv Y, Zhao X, Lv Q, Wang Y, Li D, Wu B, Shen H (2016) YKL-40 expression in chronic obstructive pulmonary disease: relation to acute exacerbations and airway remodeling. Respir Res 17:31. https://doi.org/10.1186/s12931-016-0338-3
Zhou Y, Peng H, Sun H, Peng X, Tang C, Gan Y, Chen X, Mathur A, Hu B, Slade MD, Montgomery RR, Shaw AC, Homer RJ, White ES, Lee CM, Moore MW, Gulati M, Lee CG, Elias JA, Herzog EL (2014) Chitinase 3-like 1 suppresses injury and promotes fibroproliferative responses in Mammalian lung fibrosis. Sci Transl Med 6(240):240ra276. https://doi.org/10.1126/scitranslmed.3007096
Park SJ, Jun YJ, Kim TH, Jung JY, Hwang GH, Jung KJ, Lee SH, Lee HM, Lee SH (2013) Increased expression of YKL-40 in mild and moderate/severe persistent allergic rhinitis and its possible contribution to remodeling of nasal mucosa. Am J Rhinol Allergy 27(5):372–380. https://doi.org/10.2500/ajra.2013.27.3941
Rosenfeld RM, Piccirillo JF, Chandrasekhar SS, Brook I, Kumar KA, Kramper M, Orlandi RR, Palmer JN, Patel ZM, Peters A, Walsh SA, Corrigan MD (2015) Clinical practice guideline (update): adult sinusitis executive summary. Otolaryngol Head Neck Surg Off J Am Acad Otolaryngol Head Neck Surg 152(4):598–609. https://doi.org/10.1177/0194599815574247
Wang H, Bai J, Ding M, Liu W, Xu R, Zhang J, Shi J, Li H (2013) Interleukin-17A contributes to the expression of serum amyloid A in chronic rhinosinusitis with nasal polyps. Eur Arch Otorhinolaryngol Off J Eur Fed Otorhinolaryngol Soc (EUFOS) Affil Ger Soc Otorhinolaryngol Head Neck Surg 270(6):1867–1872. https://doi.org/10.1007/s00405-012-2295-x
Van Bruaene N, Derycke L, Perez-Novo CA, Gevaert P, Holtappels G, De Ruyck N, Cuvelier C, Van Cauwenberge P, Bachert C (2009) TGF-beta signaling and collagen deposition in chronic rhinosinusitis. J Allergy Clin Immunol 124(2):253–259, 259.e251–252. https://doi.org/10.1016/j.jaci.2009.04.013
Zhang N, Van Zele T, Perez-Novo C, Van Bruaene N, Holtappels G, DeRuyck N, Van Cauwenberge P, Bachert C (2008) Different types of T-effector cells orchestrate mucosal inflammation in chronic sinus disease. J Allergy Clin Immunol 122(5):961–968. https://doi.org/10.1016/j.jaci.2008.07.008
Meltzer EO, Hamilos DL, Hadley JA, Lanza DC, Marple BF, Nicklas RA, Bachert C, Baraniuk J, Baroody FM, Benninger MS, Brook I, Chowdhury BA, Druce HM, Durham S, Ferguson B, Gwaltney JM, Kaliner M, Kennedy DW, Lund V, Naclerio R, Pawankar R, Piccirillo JF, Rohane P, Simon R, Slavin RG, Togias A, Wald ER, Zinreich SJ (2004) Rhinosinusitis: establishing definitions for clinical research and patient care. J Allergy Clin Immunol 114(6 Suppl):155–212. https://doi.org/10.1016/j.jaci.2004.09.029
Lui JK, Lutchen KR (2017) The role of heterogeneity in asthma: a structure-to-function perspective. Clin Transl Med 6(1):29. https://doi.org/10.1186/s40169-017-0159-0
Hew M, Bhavsar P, Torrego A, Meah S, Khorasani N, Barnes PJ, Adcock I, Chung KF (2006) Relative corticosteroid insensitivity of peripheral blood mononuclear cells in severe asthma. Am J Respir Crit Care Med 174(2):134–141. https://doi.org/10.1164/rccm.200512-1930OC
Capone M, Maggi L, Santarlasci V, Rossi MC, Mazzoni A, Montaini G, Cimaz R, Ramazzotti M, Piccinni MP, Barra G, De Palma R, Liotta F, Maggi E, Romagnani S, Annunziato F, Cosmi L (2016) Chitinase 3-like-1 is produced by human Th17 cells and correlates with the level of inflammation in juvenile idiopathic arthritis patients. Clin Mol Allergy CMA 14:16. https://doi.org/10.1186/s12948-016-0053-0
Specjalski K, Chelminska M, Jassem E (2015) YKL-40 protein correlates with the phenotype of asthma. Lung 193(2):189–194. https://doi.org/10.1007/s00408-015-9693-y
Lai T, Chen M, Deng Z, L Y, Wu D, Li D, Wu B (2015) YKL-40 is correlated with FEV1 and the asthma control test (ACT) in asthmatic patients: influence of treatment. BMC Pulm Med 15:1. https://doi.org/10.1186/1471-2466-15-1
Zhu Z, Zheng T, Homer RJ, Kim YK, Chen NY, Cohn L, Hamid Q, Elias JA (2004) Acidic mammalian chitinase in asthmatic Th2 inflammation and IL-13 pathway activation. Science 304(5677):1678–1682. https://doi.org/10.1126/science.1095336
Acknowledgements
We appreciate all the authors who have made efforts in the whole program, and also thank all the researchers of the primary studies.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Yue Ma declares that she has no conflict of interest. Chunquan Zheng declares that he has no conflict of interest. Le Shi declares that she has no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Ma, Y., Zheng, C. & Shi, L. The role of YKL40 in the pathogenesis of CRS with nasal polyps. Eur Arch Otorhinolaryngol 275, 431–438 (2018). https://doi.org/10.1007/s00405-017-4859-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00405-017-4859-2