Abstract
Introduction
The primary aim of the current study was to examine the usefulness of our proposed olfactory scoring system in chronic rhinosinusitis (CRS) patients with olfactory disorders (n = 213) receiving endoscopic sinus surgery (ESS).
Materials and methods
Analyzed patients were divided into two groups: an eosinophilic CRS (ECRS) group (n = 153); and a non-ECRS group (n = 60). The T&T recognition threshold test was used to evaluate olfaction at baseline and at 3 and 12 months after ESS. Patients with mean recognition threshold < 2.0 at 3 or 12 months or with a decrease of ≥ 1.0 as compared with baseline were defined as showing clinical improvement. We scored mucosal conditions as normal (0 points), edema (1 point), and polyp (2 points) at the canopy of olfactory cleft (OC), middle and superior turbinates, superior nasal meatus, and sphenoethmoidal recess during ESS. The total score of OCs (SOCs) was calculated (range 0–20 points). We compared SOCs between ECRS and non-ECRS groups. Factors related to olfactory improvement were also investigated using uni- and multivariate analyses.
Results
SOCs in the ECRS and non-ECRS groups showed significant correlations with mean recognition thresholds at baseline and at 3 and 12 months. In the multivariate analysis for predicting improvement of mean recognition threshold, lower SOCs were significantly associated with olfactory improvement factors at 3 and 12 months postoperatively in the ECRS group.
Conclusion
SOCs appears promising for estimating olfactory prognosis after ESS in CRS patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Olfactory impairment is a common complaint and is recognized in 60–80% of patients with chronic rhinosinusitis (CRS) [1,2,3]. Olfactory dysfunction is likely due to a combination of mechanical obstruction from edematous mucosa or polyposis (preventing smell molecules from reaching the olfactory nerve) and injury to the olfactory neuroepithelium caused by chronic inflammation, which can result in sensorineural disorders and inhibition of neo-genesis of the olfactory nerve over prolonged periods [1, 2, 4]. Clinically, focusing on olfactory dysfunction is important for the treatment of CRS. In particular, olfactory function at the region where the olfactory nerve is mainly distributed should be evaluated.
The treatment method for patients with CRS includes initial medical management prior to the consideration of surgery [5,6,7]. In Japan, the number of cases of eosinophilic CRS (ECRS) has been increasing [6]. ECRS patients suffer from olfactory impairment in the early phase. Medical management includes macrolide antibiotics and corticosteroid therapy [5,6,7]. Among CRS patients refractory to medical management, endoscopic sinus surgery (ESS) can achieve olfactory improvement [8,9,10,11]. However, it is not possible to achieve olfactory improvement some cases receiving ESS. Age, disease duration, presence of asthma, presence of polyp at the olfactory cleft (OC), ethmoid sinus lesions and higher levels of non-specific immunoglobulin (Ig)E have been reported as predictors linked to outcomes for olfactory function [3, 12,13,14,15].
Olfactory epithelium in humans is mainly located in the OC, and is widely distributed around the superior turbinate, middle turbinate, and nasal septum [16,17,18,19]. In Japan, the average areas of OC in adult individuals have reported as: 3.20 cm2 (right side) and 2.84 cm2 (left side) laterally, and 1.10 cm2 (right side) and 1.15 cm2 (left side) medially, respectively [20,21,22]. Thus, to evaluate olfactory function, precise assessment according to sites in the OC will be required [23]. Attempts to quantify the severity of inflammatory lesions in CRS patients and to evaluate the relationship with olfactory impairment have revolved around computed tomography (CT) staging. However, the most commonly used CT scoring system focuses on the paranasal sinuses alone and does not assess disease severity at the OC [24,25,26].
Since 1996, we have routinely assessed olfactory function in CRS patients receiving ESS using an olfactory scoring system we developed focusing on macroscopic findings at the OC during surgery. The primary aim of the current study was to examine the utility of our proposed scoring system for olfactory function in CRS patients receiving ESS.
Patients and methods
Patients
Between June 2008 and September 2016, a total of 990 CRS patients received ESS in our department. Of these, 213 patients with preoperative mean recognition threshold > 2.2 as assessed by T&T olfactometer (Takasago Industry, Tokyo, Japan) were analyzed (mean ± standard deviation (SD) age = 53.4 ± 14.2 years; 132 males, 81 females). Categorization of CRS into subgroups may harbor essential implications for the treatment and expected long-term clinical outcomes [27]. Thus, based on the diagnostic criteria from the Japanese Epidemiological Survey of Refractory Eosinophilic Chronic Rhinosinusitis Study [28], analyzed subjects were divided into two groups: an ECRS group (n = 163; mean ± SD age = 53.4 ± 16.4 years; 91 males, 62 females), and a non-ECRS group (n = 50, mean ± SD age = 55.3 ± 13.1 years; 41 males, 19 females).
The ethics committee meeting in our institution approved all study protocols (approval number, 1512) and this study strictly followed all regulations of the Declaration of Helsinki.
Our proposed scoring system for olfactory function and study endpoints
In our department, we have routinely focused on five relevant olfactory nerve distribution areas at OCs for patients receiving ESS: (1) canopy of the OC; (2) middle turbinate; (3) superior turbinate; (4) superior meatus; and (5) sphenoethmoidal recess. We scored each area by following macroscopic mucosal findings: normal, 0 points; edema, 1 point; and polyp, 2 points. The sum of points in the five areas on both sides (score of OCs, SOCs) was calculated, ranging from 0 to 20 points (Fig. 1). SOCs in this study were determined through discussion with three experienced, expert rhinologists during ESS.
We retrospectively examined the relationship between SOCs and olfactory disorder. We also compared baseline characteristics (laboratory data, SOCs, olfactory tests, respiratory function and presence of underlying diseases such as asthma, etc.). Furthermore, variables related to the improvement of mean recognition thresholds after ESS were investigated using uni- and multivariate analyses.
Olfactory tests
Olfactory tests were performed using the T&T olfactometer and intravenous olfaction test, both of which are covered by health insurance and are commonly used for olfactory examination in Japan.
The T&T olfactory test consists of five odorants: (A) b-phenyl ethyl alcohol, which smells like a rose; (B) methyl cyclopentenolone, which smells like burning; (C) iso-valeric acid, which smells like sweat; (D) g-undecalactone, which smells like fruit; and (E) skatole, which smells like garbage. Recognition thresholds were determined for each odorant. The mean value for these five recognition thresholds was defined as olfactometry function [29].
Postoperative olfactory function was evaluated at 3 and 12 months using the T&T olfactometer. Postoperative olfactory changes were determined by subtraction (∆T&T = preoperative mean recognition threshold—postoperative mean recognition threshold) as reported in a previous study [3]. Patients were defined as two groups: “improvement group”, when postoperative mean recognition threshold was ≤ 2.0, and/or when ∆T&T was ≥ 1.0; and “unchanged group”, when the finding was other than those described above.
The intravenous olfactory test has also seen wide use for assessing olfactory function [30]. The intravenous olfactory test was performed using prosultiamine, providing a garlic or onion smell (alinamin; Takeda Pharmaceutical Company, Osaka, Japan). A dose of 10 mg (2 ml) of alinamin was injected into an antecubital vein at a constant rate over 20 s. Patients who did and did not recognize the alinamin odor were categorized to the response and non-response groups.
Respiratory function test
Patients with respiratory disorder were defined as those with following conditions as assessed by spirometry: (1) percentage predicted vital capacity < 80%; and/or (2) percentage predicted forced expiratory volume in 1.0 s < 70%.
Statistical analysis
Categorical parameters were compared using Fisher’s exact test. Continuous parameters were compared by Welch’s t test, the Mann–Whitney U test or Spearman’s rank correlation coefficient rs, as applicable. For predicting treatment outcomes (i.e., improvement or unchanged), candidate variables were selected from univariate analysis; parameters showing values p < 0.10 were entered into multivariate logistic regression analysis. The following parameters potentially related to outcomes from ESS in mean recognition thresholds were examined in univariate analyses: age, sex, preoperative mean recognition threshold, intravenous olfactory test, presence of asthma, respiratory dysfunction, blood eosinophil count (%), total IgE level, presence of perennial or seasonal allergic rhinitis, presence of mucosal lesions at ethmoid sinus or sphenoethmoidal sinus, and SOCs. Clinical data are shown as mean (± SD) unless otherwise mentioned. Statistical significance was set at p < 0.05. All statistical analyses were performed using StatFlex® version 6 (Atec, Osaka, Japan).
Results
Data from ECRS and non-ECRS groups
In baseline characteristics, in terms of age and sex, no significant difference was found between the ECRS (n = 153) and non-ECRS groups (n = 60). Mean SOCs and recognition thresholds in the two groups were 12.97 ± 5.36 and 5.16 ± 1.05, respectively, in the ECRS group, and 6.57 ± 6.12 and 4.30 ± 1.43, respectively, in the non-ECRS group. Significant differences between groups were seen for both SOCs and mean recognition thresholds (p < 0.001 each). According to analysis of each assessment site in SOCs, the superior meatus showed the highest score in both groups (Fig. 2). In all assessment sites, SOCs was significantly higher in the ECRS group than in the non-ECRS group. SOCs correlated significantly with preoperative mean recognition thresholds in both ECRS (r s = 0.515, p < 0.001) and non-ECRS groups (r s = 0.398, p < 0.001) (Fig. 3). Similarly, as for the relationship between SOCs and postoperative mean recognition thresholds, SOCs correlated significantly with ECRS at 3 months (r s = 0.347, p < 0.001), ECRS at 12 months (r s = 0.342, p = 0.002), non-ECRS at 3 months (r s = 0.408, p = 0.007) and non-ECRS at 12 months (r s = 0.617, p = 0.001) (Fig. 4). We also examined the relationship between preoperative mean recognition thresholds and SOCs according to assessment site (Table 1). In the ECRS group, significant correlations were found for the sphenoethmoidal recess (r s = 0.262, p = 0.016) and OC canopy (r s = 0.418, p = 0.001), while in the non-ECRS group, significant correlations were found for the superior turbinate (r s = 0.440, p = 0.007), superior meatus (r s = 0.511, p = 0.001) and OC canopy (r s = 0.554, p = 0.001).
SOCs in five assessment sites in the ERCS and non-ECRS groups. At all assessment points, SOCs was significantly higher in the ECRS group than in the non-ECRS group. Numbers above each bar graph indicate SOCs and those below each bar graph indicate percentage. Asterisks indicate significant differences in each site between ECRS and non-ECRS (p < 0.05)
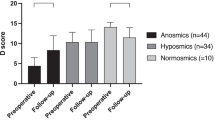
Comparison of SOCs between improvement and unchanged groups
SOCs were analyzed in relation to postoperative olfactory changes. In comparing SOCs between the improvement and unchanged groups, significantly higher scores were observed in the unchanged group in the ECRS at 3 and 12 months and in the non-ECRS at 12 months (Fig. 5). According to analysis of SOCs at each assessment site, sphenoethmoidal recess and OC canopy in ECRS at 3 months were significantly higher in the unchanged group, and those in the middle turbinate, sphenoethmoidal recess and OC canopy at ECRS 12 months were significantly higher in the unchanged group (Supplementary Fig. 1), while those in the superior meatus in the non-ERCS group at 3 months and those in the superior meatus and OC canopy in the non-ERCS at 12 months were significantly higher in the unchanged group (Supplementary Figs. 1, 2).
Uni- and multivariate analyses
Results for univariate analyses in terms of treatment outcomes (improvement or unchanged) are shown in Table 2. Variables showing values of p < 0.10 in univariate analyses were entered into logistic regression analyses. In ECRS at 3 months, presence of respiratory dysfunction (odds ratio (OR) 3.084, p = 0.025) and SOCs (OR 1.094, p = 0.029), and in ECRS at 12 months, mean recognition threshold (OR 2.266, p = 0.006) and SOCs (OR 1.134, p = 0.017) were identified as significant predictors (Table 3). On the other hand, in the non-ECRS group, no significant variables were found although near-significance of SOCs was observed in non-ECRS at 3 months (p = 0.058) (Table 3).
Discussion
The current study primarily sought to examine the usefulness of our scoring system (SOCs) in CRS patients undergoing ESS, focusing on macroscopic findings at the OC during surgery. In our results, SOCs correlated with both pre- and postoperative mean recognition thresholds, and in multivariate analyses, SOCs was significant in the ECRS group. These results denoted that our proposed olfactory scoring system may be helpful for predicting treatment outcomes in CRS patients undergoing ESS.
The SOCs focused on the mucosal condition of olfactory neuroepithelium area that consisted of the nasal septum up to the canopy, middle turbinate, superior turbinate, superior nasal meatus, and sphenoethmoidal recess [16,17,18,19]. These relevant sites at the OC require intensive operation in CRS patients with olfactory disorder. The SOCs has three grading scales (0, 1, and 2 points), allowing unification with previous reports about endoscopic scores [31,32,33]. Furthermore, the significant correlation of SOCs with both pre- and postoperative severity of olfactory disorder can provide useful information for the management in CRS patients undergoing ESS. We therefore believe that the SOCs offers a valid and useful scoring system.
One of the major findings in our study was that in the examination of SOCs according to assessment sites, results differed between the ECRS and non-ECRS groups. In other words, higher SOCs in the ECRS group were prominent at all assessment points, indicating differences in pathophysiology between the two groups. Previous cross-sectional studies have demonstrated that mucosal eosinophilia infiltration correlated significantly with worse disease severity in CRS patients and that eosinophilic cationic protein and eosinophilic-derived neurotoxin can directly affect the neurological function [34,35,36]. Such findings may be associated with our current results.
In our results, in the ECRS group, SOCs of the sphenoethmoidal recess and OC canopy correlated significantly with baseline mean recognition thresholds and significantly higher SOCs of sphenoethmoidal recess and OC canopy were found at 3 and 12 months after ESS in the unchanged group. Presence of nasal polyps located vertically from the OC canopy to the sphenoethmoidal recess may account for these results. Ventilatory disturbance to the olfactory mucosa caused by nasal polyps and eosinophilic infiltration related to direct olfactory mucosal injury can lead to olfactory impairment [37].
In surgical treatment for OC lesions, complete eradication of these inflamed mucosal lesions is an important treatment strategy [11]. However, the presence of olfactory neuroepithelium can make this surgical procedure difficult. From the perspective of maintaining olfactory function, preservation of olfactory mucosa may be desirable [11]. Recently, the usefulness of an absorbable gelatin dressing impregnated with triamcinolone within the OC on polypoid rhinosinusitis smell disorders in patients with CRS undergoing ESS has been reported [38]. This technique has also been used in our department.
Significantly higher SOCs of the middle turbinate in ECRS were also found at 12 months after ESS in the unchanged group. A recent CT analysis of the OC in CRS patients demonstrated that the percent opacification as determined by two- and three-dimensional, computerized volumetric analysis of the anterior plane displayed the strongest correlations with objective olfaction [23]. These reports may be linked to our current results.
In the non-ECRS group, SOCs of the superior turbinate and superior meatus (located horizontally in the olfactory nerve distribution area) correlated significantly with baseline mean recognition thresholds and significantly higher SOCs for the superior meatus was found at 3 and 12 months after ESS in the unchanged group. The near-significance of posterior ethmoid sinus lesions and sphenoidal sinus lesions in univariate analyses may account for our results at 12 months. In several non-ECRS patients, due to olfactory nerve injury caused by inflammatory infiltration in the paranasal sinus such as posterior ethmoid sinus and the related olfactory impairment, olfactory function may not improve even after ESS.
We acknowledge several limitations to this study. First, this was a single-center, retrospective study. Second, in both ECRS and non-ECRS groups, missing data after ESS may have potentially led to bias. Third, the current study was based on CRS patients from a certain ethnic background, and additional investigations on different ethnic populations are required to further verify the usefulness of SOCs. However, our results indicated that SOCs correlated with olfactory function pre- and post-ESS and were significant in the ECRS group in multivariate analysis.
In conclusion, clinicians need to be aware of the importance of macroscopic findings at OC in ESS from the viewpoint of patient olfactory prognosis. Our proposed olfactory scoring system during ESS appears promising for estimating olfactory prognosis after ESS for patients with CRS.
Abbreviations
- CRS:
-
Chronic rhinosinusitis
- ESS:
-
Endoscopic sinus surgery
- CT:
-
Computed tomography
- SD:
-
Standard deviation
- ECRS:
-
Eosinophilic chronic rhinosinusitis
- OC:
-
Olfactory cleft
- SOC:
-
Score of olfactory clefts
- OR:
-
Odds ratio
References
Kohli P, Naik AN, Harruff EE, Nguyen SA, Schlosser RJ, Soler ZM (2017) The prevalence of olfactory dysfunction in chronic rhinosinusitis. Laryngoscope 127(2):309–320
Mattos JL, Schlosser RJ, Storck KA, Soler ZM (2017) Understanding the relationship between olfactory-specific quality of life, objective olfactory loss, and patient factors in chronic rhinosinusitis. Int Forum Allergy Rhinol 7(7):734–740
Oka H, Tsuzuki K, Takebayashi H, Kojima Y, Daimon T, Sakagami M (2013) Olfactory changes after endoscopic sinus surgery in patients with chronic rhinosinusitis. Auris Nasus Larynx 40(5):452–457
Kern RC (2000) Chronic sinusitis and anosmia: pathologic changes in the olfactory mucosa. Laryngoscope 110(7):1071–1077
Shimizu T, Suzaki H (2016) Past, present and future of macrolide therapy for chronic rhinosinusitis in Japan. Auris Nasus Larynx 43(2):131–136
Ishitoya J, Sakuma Y, Tsukuda M (2010) Eosinophilic chronic rhinosinusitis in Japan. Allergol Int 59(3):239–245
Bachert C, Pawankar R, Zhang L, Bunnag C, Fokkens WJ, Hamilos DL, Jirapongsananuruk O, Kern R, Meltzer EO, Mullol J, Naclerio R, Pilan R, Rhee CS, Suzaki H, Voegels R, Blaiss M (2014) ICON: chronic rhinosinusitis. World Allergy Organ J 7(1):25
Kohli P, Naik AN, Farhood Z, Ong AA, Nguyen SA, Soler ZM, Schlosser RJ (2016) Olfactory outcomes after endoscopic sinus surgery for chronic rhinosinusitis: a meta-analysis. Otolaryngol Head Neck Surg 155(6):936–948
Rudmik L (2017) Economics of chronic rhinosinusitis. Curr Allergy Asthma Rep 17(4):20
Hull BP, Chandra RK (2017) Refractory chronic rhinosinusitis with nasal polyposis. Otolaryngol Clin N Am 50(1):61–81
Eloy JA, Marchiano E, Vázquez A (2017) Extended Endoscopic and Open Sinus Surgery for Refractory Chronic Rhinosinusitis. Otolaryngol Clin N Am 50(1):165–182
Banglawala SM, Oyer SL, Lohia S, Psaltis AJ, Soler ZM, Schlosser RJ (2014) Olfactory outcomes in chronic rhinosinusitis with nasal polyposis after medical treatments: a systematic review and meta-analysis. Int Forum Allergy Rhinol 4(12):986–994
Ichinose M, Sugiura H, Nagase H, Yamaguchi M, Inoue H, Sagara H, Tamaoki J, Tohda Y, Munakata M, Yamauchi K, Ohta K; Japanese Society of Allergology (2017) Allergol Int 66(2):163–189
Hong CJ, Tsang AC, Quinn JG, Bonaparte JP, Stevens A, Kilty SJ (2015) Anti-IgE monoclonal antibody therapy for the treatment of chronic rhinosinusitis: a systematic review. Syst Rev 4:166
Grgić MV, Ćupić H, Kalogjera L, Baudoin T (2015) Surgical treatment for nasal polyposis: predictors of outcome. Eur Arch Otorhinolaryngol 272(12):3735–3743
Morrison EE, Costanzo RM (1990) Morphology of the human olfactory epithelium. J Comp Neurol 297:1–13
Paik SI, Lehman MN, Seiden AM, Duncan HJ, Smith DV (1992) Human olfactory biopsy. The influence of age and receptor distribution. Arch Otolaryngol Head Neck Surg 118:731–738
Leopold DA, Hummel T, Schwob JE, Hong SC, Knecht M, Kobal G (2000) Anterior distribution of human olfactory epithelium. Laryngoscope 110:417–421
Feron F, Perry C, McGrath JJ, Mackay-Sim A (1998) New techniques for biopsy and culture of human olfactory epithelial neurons. Arch Otolaryngol Head Neck Surg 124:861–866
Mori E, Matsuwaki Y, Mitsuyama C, Okushi T, Nakajima T, Moriyama H (2013) Risk factors for olfactory dysfunction in chronic rhinosinusitis. Auris Nasus Larynx 40(5):465–469
Masaki M, Tanaka Y (1998) Nasal polyps in the olfactory cleft. Laryngoscope 108(8 Pt 1):1243–1246
Sakuma Y, Ishitoya J, Komatsu M, Shiono O, Hirama M, Yamashita Y, Kaneko T, Morita S, Tsukuda M (2011) New clinical diagnostic criteria for eosinophilic chronic rhinosinusitis. Auris Nasus Larynx 38(5):583–588
Kohli P, Schlosser RJ, Storck K, Soler ZM (2016) Olfactory cleft computed tomography analysis and olfaction in chronic rhinosinusitis. Am J Rhinol Allergy 30(6):402–406
Chang H, Lee HJ, Mo JH, Lee CH, Kim JW (2009) Clinical implication of the olfactory cleft in patients with chronic rhinosinusitis and olfactory loss. Arch Otolaryngol Head Neck Surg 135:988–992
Pallanch JF, Yu L, Delone D, Robb R, Holmes DR 3rd, Camp J, Edwards P, McCollough CH, Ponikau J, Dearking AC, Lane J, Primak A, Shinkle A, Hagan J, Frigas E, Ocel JJ, Tombers N, Siwani R, Orme NM, Reed KB, Jerath N, Dhillon R, Kita H (2013) Three-dimensional volumetric computed tomographic scoring as an objective outcome measure for chronic rhinosinusitis: Clinical correlations and comparison to Lund-Mackay scoring. Int Forum Allergy Rhinol 3:963–972
Soler ZM, Pallanch JF, Sansoni ER, Jones CS, Lawrence LA, Schlosser RJ, Mace JC, Smith TL (2015) Volumetric computed tomography analysis of the olfactory cleft in patients with chronic rhinosinusitis. Int Forum Allergy Rhinol 5:846–854
Smith TL, Mendolia-Loffredo S, Loehrl TA, Sparapani R, Laud PW, Nattinger AB (2005) Predictive factors and outcomes in endoscopic sinus surgery for chronic rhinosinusitis. Laryngoscope 115(11):2199–2205
Tokunaga T, Sakashita M, Haruna T, Asaka D, Takeno S, Ikeda H, Nakayama T, Seki N, Ito S, Murata J, Sakuma Y, Yoshida N, Terada T, Morikura I, Sakaida H, Kondo K, Teraguchi K, Okano M, Otori N, Yoshikawa M, Hirakawa K, Haruna S, Himi T, Ikeda K, Ishitoya J, Iino Y, Kawata R, Kawauchi H, Kobayashi M, Yamasoba T, Miwa T, Urashima M, Tamari M, Noguchi E, Ninomiya T, Imoto Y, Morikawa T, Tomita K, Takabayashi T, Fujieda S (2015) Novel scoring system and algorithm for classifying chronic rhinosinusitis: the JESREC Study. Allergy 70:995–1003
Shiga H, Taki J, Okuda K, Watanabe N, Tonami H, Nakagawa H, Kinuya S, Miwa T (2017) Prognostic value of olfactory nerve damage measured with thallium-based olfactory imaging in patients with idiopathic olfactory dysfunction. Sci Rep 7(1):3581
Horikiri K, Kikuta S, Kanaya K, Shimizu Y, Nishijima H, Yamasoba T, Kondo K (2017) Intravenous olfactory test latency correlates with improvement in post-infectious olfactory dysfunction. Acta Otolaryngol 137(10):1083–1089
Lund VJ, Kennedy DW (1995) Quantification for staging sinusitis. The staging and therapy group. Ann Otol Rhinol Laryngol Suppl 167:17–21
Wright ED, Agrawal S (2007) Impact of perioperative systemic steroids on surgical outcomes in patients with chronic rhinosinusitis with polyposis: evaluation with the novel perioperative sinus endoscopy (POSE) scoring system. Laryngoscope 117:1–28
Tsuzuki K, Hinohira Y, Takebayashi H, Kojima Y, Yukitatsu Y, Daimon T, Sakagami M (2014) Novel endoscopic scoring system after sinus surgery. Auris Nasus Larynx 41:450–454
Soler ZM, Sauer DA, Mace J, Smith TL (2009) Relationship between clinical measures and histopathologic findings in chronic rhinosinusitis. Otolaryngol Head Neck Surg 141:454–461
Durack DT, Ackerman SJ, Loegering DA, Gleich GJ (1981) Purification of human eosinophil-derived neurotoxin. Proc Natl Acad Sci USA 78:5165–5169
Fredens K, Dahl R, Venge P (1982) The Gordon phenomenon induced by the eosinophil cationic protein and eosinophil protein X. J Allergy Clin Immunol 70:361–366
Cho SW, Kim DW, Kim JW, Lee CH, Rhee CS (2017) Classification of chronic rhinosinusitis according to a nasal polyp and tissue eosinophilia: limitation of current classification system for Asian population. Asia Pac Allergy 7(3):121–130
Bardaranfar MH, Ranjbar Z, Dadgarnia MH, Atighechi S, Mirvakili A, Behniafard N, Sadeghi M, Abbaslu F, Baradaranfar A (2014) The effect of an absorbable gelatin dressing impregnated with triamcinolone within the olfactory cleft on polypoid rhinosinusitis smell disorders. Am J Rhinol allergy 28:172–175
Acknowledgements
We gratefully acknowledge the help of our technical assistants, Ms. Yumi Kida and Mrs. Midori Tanide.
Author information
Authors and Affiliations
Contributions
Conception and design of the study: KT, KO, YM, and MS; acquisition of data: KO, KT, KH, HT, HO, YK, and YY; analysis and interpretation of data: KO, KT, KH, MS, and HN; drafting of the article: KO, KT, and HN.
Corresponding author
Ethics declarations
Funding
This work was partly supported by Grants-in-Aid for Scientific Research (Japan Society for the Promotion of Science, KAKENHI: numbers JP25462671 and JP16K11220) from the Japan Society for the Promotion of Science, and the Practical Research Project for Rare/Intractable Diseases from the Japan Agency for Medical Research and Development (AMED).
Conflict of interest
All authors declare that they have no conflicts of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The ethics committee meeting in our institution approved all study protocols (approval number 1512). Written informed consent from the participants was waived due to the retrospective nature of this study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
405_2017_4831_MOESM1_ESM.pptx
Supplementary figure 1 SOCs in the improvement and unchanged groups at five assessment sites in the ECRS group at 3 months (A) and 12 months (B). Supplementary figure 2 SOCs in the improvement and unchanged groups at five assessment sites in the non-ECRS group at 3 months (A) and 12 months (B). (PPTX 14676 KB)
Rights and permissions
About this article
Cite this article
Okazaki, K., Tsuzuki, K., Hashimoto, K. et al. Usefulness of our proposed olfactory scoring system during endoscopic sinus surgery in patients with chronic rhinosinusitis. Eur Arch Otorhinolaryngol 275, 415–423 (2018). https://doi.org/10.1007/s00405-017-4831-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00405-017-4831-1