Abstract
Anaplastic carcinoma of the thyroid gland (ATC) is one of the most aggressive cancers in humans. With insufficient treatment, the disease most often leads to death in suffocation. From 2002, our treatment strategy has been hyperfractionated accelerated radiotherapy (HART) with high doses (64 Gy) to the neck, followed by surgery 4–8 weeks later if feasible, with the aim to gain control in the neck. After a pathology review, 51 patients were diagnosed with ATC in the period 2002–2014 in the south-east of Norway. Thirty-one received HART, and we present a study of these patients, with death in suffocation as the primary endpoint and survival as the second. No patients treated with HART died in suffocation. Six had a tracheostomy during their course of disease, of whom four were dependent on a tracheal cannula when they died. The best median survival, 19 months, was obtained in the 13 patients where both radiotherapy and surgery were possible as primary treatments. Only surgery came out as a prognostic factor for survival in multivariate analysis. Patients surviving more than 2 years were characterised by having surgery with R0 resection and no or small residual foci of ATC in the specimens. Stage 4C patients survived 3 months only.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Anaplastic carcinoma of the thyroid gland (ATC) accounts for less than 5% of thyroid cancers, and is highly aggressive with a median time to death reported to be 3.9 months overall, and 10.5 months with multimodal treatment, no improvement being made over the last 20 years [1].
With insufficient treatment to the neck, patients eventually die in suffocation. Efficient treatment for metastatic disease is still lacking.
The rationale for hyperfractionation in ATC is the high regenerative capability of the tumour clonogens during treatment. In addition, the normal tissue will to a higher extent be spared with lower fraction doses [2]. Simpson [3] reported in 1980 for the first time results using hyperfractionation in ATC, and later Tennvall [4] treated 55 ATC patients with hyperfractionated and hyperfractionated accelerated radiotherapy (HART) with weekly doxorubicin preoperatively in three different protocols, showing a better outcome and control in the neck for HART as a preoperative treatment.
Changing from previous practice [5], we adopted HART followed by surgery if feasible as our institutional strategy in 2002, the main goal being to avoid death in suffocation. The current study is retrospective and encompasses 31 patients receiving HART with or without surgery. The primary endpoint was death in suffocation, the secondary survival.
Patients and methods
Patients
Patients with ATC from the south-east region of Norway (2.95 million inhabitants) are regularly referred to Oslo University Hospital (OUH). A multidisciplinary team (MDT) for thyroid nodules and cancer was established in 2002, and from the same time we adopted preoperative HART followed by surgery 4–8 weeks later if feasible, as our treatment strategy for ATC.
We reviewed all patients treated at OUH through the years 2002–2014. The study was approved by the Regional Ethical Committee and the hospital’s local authorities, and data stored accordingly. The patients were identified from the hospital’s pathology and radiotherapy registers. Clinical data were retrieved from the patients’ medical records, and letters were sent to local hospitals and general practitioners to collect follow-up data until the time of death. WHO performance status (WHO ps) was not followed, and health-related quality of life questionnaires (HRQLQ) were not used.
Patients who had ATC on review by two senior thyroid cancer pathologists were eligible. Data from patients undergoing HART with or without surgery were studied in detail.
The diagnostic work-up consisted of clinical and ultrasound examination of the neck with fine needle aspiration and/or core needle biopsy (CNB) from the thyroid gland, CT scans of the neck, chest and abdomen, and for some a PET-CT scan. The tumours were staged according to the UICC TNM classification version 7. All patients were discussed in the MDT before decision on therapy, and the MDT would also decide on surgery 4–8 weeks post-radiotherapy. Referral of a patient with, or with suspected ATC, was considered an emergency, and radiotherapy was to start within a week following diagnosis.
Surgical patients were followed up at OUH. Follow-ups of non-surgical HART patients were performed at OUH or the local ENT or oncological unit. The absence of suffocation was defined as no stridor at the time of death. Cutoff was set to 31st December 2015 for living patients.
Histopathology
The diagnosis was established in accordance with the WHO classification of 2004 [6]. Histology and cytology reports, as described by the Norwegian National guidelines for diagnosis and management of thyroid diseases, were used for reporting results from examinations of surgical specimens [7].
Radiotherapy
HART was administered to a total dose of 64 Gy in 4 weeks, fraction dose 1.6 Gy twice daily at least 6 h apart, Monday till Friday, together with weekly 20 mg doxorubicin [4]. Patients diagnosed with ATC on surgical specimen only were given postoperative HART.
Pre- and postoperative radiotherapy was defined as being part of planned primary treatment. Since local tumour control in the neck was our main concern, the presence of stage IV disease did not necessarily preclude high-dose radiotherapy, but depended on the patient’s general condition.
A plastic mesh was individually fit for fixation. Gross tumour volume (GTV) was drawn according to the findings in the CT and PET-CT scans. Clinical target volume (CTV) and internal target volume (ITV) were considered identical, and drawn with a 5 mm margin to GTV. Elective ITV (e-ITV) consisted of lymph node levels II–VI, and treated to 48 Gy. A cumulative dose to the spinal cord equivalent to 50 Gy in 2 Gy’s fractions and corrected for total time and fractionation was accepted. Modifications of volumes or total dose were made with very large tumour sizes. 3D dose planning was done using multiple fields.
Surgery
Surgery was performed either as thyroidectomy in one or two stages, or as an extended procedure with, e.g., sternum split, thoracoscopy or tracheal resection reconstructed with myoperiostal flap. Surgical resection was classified as either R0 resection (complete resection of the tumour with no microscopic residual disease), R1 resection (residual microscopic disease), and R2 resection (residual macroscopic disease).
Statistics
Descriptive statistics were provided as frequencies, proportions and medians with ranges. Survival was calculated by the Kaplan–Meier method. Cox regression models were performed to identify factors associated with survival. Parameters significant at a level of 0.2 in univariate analyses were included in the multivariate analysis, in addition to age. The presumptions of proportional hazards were adequately met. Results are presented as hazard ratios (HR) with 95% confidence intervals (CI). SPSS version 21 [SPSS IBM, Chicago, USA] was used for all data handling and analyses.
Results
Results are presented in Tables 1, 2 and 3. Fifty-one patients diagnosed between 2002 and 2014 had confirmed ATC on histopathology review. Thirty-one of these (61%) were treated with HART, and comprise the study. Thirteen underwent surgical treatment as part of primary treatment, and one had surgery on a recurrence after 93 months.
Fine-needle aspiration cytology (FNAC) was performed in 28 cases revealing malignant cells consistent with ATC in 14 cases, malignant cells/carcinoma not otherwise specified (NOS) in nine cases, PTC in three cases, follicular neoplasia in one case and poorly differentiated thyroid carcinoma in one case. Twenty-three of the 31 HART patients were additionally diagnosed as ATC by core needle biopsy (CNB) and one case as thyroid carcinoma NOS on CNB.
All surgery was done with curative intent. Six cases were diagnosed as ATC by examination of the surgical specimen only, and had postoperative radiotherapy. All except one patient (48 Gy) were planned for 64 Gy. If less than half dose was administered, the patient was allocated to the no HART group (n = 2, total doses 16 and 19.2 Gy).
Five patients did not receive concurrent doxorubicin because of heart disease, general condition or high age.
In spite of a grave disease, most patients had a good performance status (WHO ps 0: n = 21, WHO ps 1: n = 8) at diagnosis. WHO ps was not followed after treatment.
Early adverse events after radiotherapy were seen in all patients and were treated accordingly in an inpatient unit. All patients were in need of a nasogastric feeding tube. Swallow difficulties that resulted in PEG occurred in two patients and left carotid artery stenosis > 95% after 7 years occurred in one patient, which led to his death.
Six patients had postoperative complications: four in the preoperative group, and two in the postoperative group. Two patients had a postoperative bleeding, of which one was lethal. Five patients had a postoperative infection, of whom three had postoperative fistulae. One patient had postoperative bilateral recurrent laryngeal nerve paralysis, one had a chylous leak.
By the end of the study, 27 of 31 patients had died, none of them in suffocation. Six patients had had a tracheostomy during their course of disease, two of them from the time of diagnosis, and four were dependent on a tracheal cannula until they died.
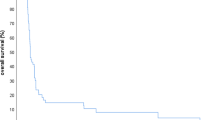
Survival is presented in Fig. 1. Median survival for the 31 patients was 9 (2–106) months. The best median survival, 19 months, was obtained in the 13 patients where both radiotherapy and surgery were possible as primary treatments. HART patients with distant metastases at diagnosis (stage 4C, n = 9) survived 3 (2–15) months only.
Six patients, of whom five had surgery, survived more than two years (30–106 months). They were characterized by having R0 resections, one with total regression of ATC and two with only small foci remaining. Three had preoperative radiotherapy, two postoperative and one no surgery as part of primary treatment.
Univariate analyses showed a 23% increase in the risk of death by each unit (cm) increase in tumour diameter (HR 1.23, 95% CI 1.01–1.48), and patient not receiving surgery had a fivefold risk of dying compared to patients who received surgery (HR 4.95, 95% CI 2.01–12.2). In multivariate analysis, Table 4, only surgery remained as a statistically significant prognostic factor for survival (HR 4.78, 95% CI 1.92–11.9).
Discussion
We studied patient and tumour characteristics, treatment and course of disease in 31 ATC patients referred to OUH and receiving high dose HART between 2002 and 2014. Our treatment strategy proved successful in the respect that no patient died in suffocation, and the survival compared well with the literature.
According to some studies, ATC can arise as an anaplastic transformation, or dedifferentiation of pre-existing well-differentiated thyroid carcinoma (WDTC) [8]. However, an indication of development of tumour cells de novo, was found in one study revealing that only 13.5% in a group of 126 cases of ATC contained foci of WDTC [9]. A limited number of immunohistochemical stains are usually sufficient to make a diagnosis of ATC when the clinical features and morphology in HE-stained slides are consistent with ATC. In the present study, ATC was correctly diagnosed on FNAC in 14 out of 28 cases (50%), while 23 out of 24 cases were correctly diagnosed on CNB. According to our experience, CNB provide useful information compared to FNAC, including the possibility to evaluate growth pattern and necrosis. Furthermore, immunohistochemistry on CNB is favourable for the purpose of excluding other malignancies. The fact that ATC was found unexpectedly at surgery in six patients illustrates the importance of thorough and correct preoperative diagnosis.
In the literature, local control is reported as response rates or local control over time. We chose another endpoint which is of clinical importance and mirrors the rate of local control: death in suffocation.
Giving more than one radiotherapy fraction a day is demanding on a hospital’s resources. It is, therefore, not surprising that the literature on hyperfractionated RT and HART is limited. When comparing radiotherapy regimens, Wang [10] found improved median survival among patients who received hyperfractionation compared to conventional fractionation (13.6 vs 10.3 months).
Tennvall et al. [4] have shown the benefit of preoperative radiotherapy 48 Gy administered in twice daily fractions of 1.6 Gy in ATC with a local control rate of 100%. Later Segerhammar et al. [11] presented a 13-year patient series of ATC in one institution, administering the same preoperative regimen to 34 of 59 diagnosed patients. The surgical procedures varied. Median survival or follow-up for all 59 patients was 3.3 months, and for patients who underwent surgery after radiotherapy 5.5 months. No patients who died from distant metastases had any sign of local relapse in the thyroid bed, and of the 59 patients only 5 died from local tumour growth. The authors conclude that progress in achieving local control has been made in recent decades following the development of multimodal treatment, although survival rates remain low.
Glaser et al. [12] published a register study of 3552 patients with ATC using the US National Cancer Database. In a multivariate analysis, total thyroidectomy and high-dose radiotherapy (59.4 Gy or more) came out as predictive for survival. Different from our study apart from the large number of patients and variables was that local control was not addressed nor was radiotherapy fractionation, and pathology review was not performed.
Swaak-Kragten et al. [13] reported CR of 50% in the neck after HART, and a significantly better locoregional control in patients who had undergone R0/R1 resection or chemoradioherapy, and a significantly better survival when total doses exceeded 40 Gy.
In one study by de Crevoisier et al. [14], 30 patients were treated with chemotherapy and HART, and 24 underwent thyroid surgery. Twenty of the surgical patients received postoperative CRT. This study showed a median survival of 10 months, and an overall survival rate at 3 years of 27%. Two-thirds of the patients died from their ATC. They conclude that high long-term survival was obtained when CRT was given after complete surgery. Death was mainly caused by distant metastases.
Dandekar et al. [15] published the results of HART up to 60 Gy in twice daily fractions, fraction dose 1.8 or 2 Gy in 31 ATC patients, a protocol initiated to improve survival and limit toxicities. They reported a median survival of 70 days and a high degree of toxicity. The protocol was abandoned due to the poor results.
Sherman et al. [16] reported on the Memorial Sloan-Kettering Cancer Center results of 37 ATC patients with locoregional disease and a pathology review, treated to a median dose of 57.6 Gy hyperfractionated or in once daily fractions. One year outcomes were 45% locoregional progression-free survival, and 28% overall survival. The results are inferior to prior results from the same institution, and they assume that this may at least in part be due to more accurate histology in their present study.
Nachalon et al. [17] reported on 26 patients undergoing chemotherapy and radiotherapy according to stage, and surgery if operable, local disease. Radiotherapy dose for patients who did not undergo surgery was 70 Gy, and was associated with a significantly longer survival than palliative radiotherapy. The radiotherapy was administered as a 4-week hyperfractionated regimen. They conclude that although having a grave prognosis, ATC patients may have a survival benefit when given high doses of radiotherapy. One outcome measure was quality of life (QLQ); this was presented as frequencies of percutaneous gastrostomies and tracheal cannulas.
Foote et al. reported on 25 ATC patients of which 10 with locoregional disease were presented in detail [18]. Eight of these had a pathological review. After individualized surgery radiotherapy to total doses of 57.6–70 Gy was applied using IMRT technique and HART or conventional fractionation together with chemotherapy (doxorubicin or taxanes), in some patients followed by chemoradiotherapy. Median follow-up was 36 months (range 4–89). Long-term locoregional control was achieved in 7 patients, and median Kaplan–Meier survival was 60 months in this selected series. There were no treatment-related deaths. They conclude that this aggressive approach with multimodal therapy demonstrates encouraging long-term survival with acceptable, though significant toxicity.
Apart from two [4, 14], these studies are all retrospective in design. Most studies look at survival, which is still grim, but some also look on locoregional control in one way or another. Patient-reported outcomes (PRO) have not been used in any of the studies we have seen.
A Confounding factors in comparing survival between studies are is the lack of randomised trials, the selection of fit patients to optimal treatment, and that all studies except two [4, 14] are retrospective.
In our series, the 13 surgical patients were selected on the basis of having no distant metastases, and being found operable before and/or after HART. They tended to have smaller tumours and better performance status at diagnosis. Whether pre- or postoperative radiotherapy should be preferred cannot be answered from our data, but R0 resections being associated with a better survival can be an argument for preoperative radiotherapy. The literature, however, is not conclusive on this matter. American Thyroid Association (ATA) recommends in its guidelines for ATC postoperative radiotherapy after R0 or R1 resections [19]. There seems to be an agreement that doses higher than 40 Gy lead to better local control [12, 13].
As expected in accelerated radiotherapy, the acute toxicity was severe, and needed to be handled in an inpatient unit. That most, and most severe, postoperative complications occurred in patients who received preoperative radiotherapy was not an unexpected finding. Radiotherapy makes tissue prone to poorer healing, and even more so when the time interval to surgery exceeds 6 weeks. However, the interval between radiotherapy and surgery was similar to between our preoperative and postoperative groups. Few other studies present surgical complications in irradiated ATC patients.
Since many patients are inoperable at the time of diagnosis, and even after radiotherapy, we chose to give a maximum tolerated radiotherapy doses to all. ATC occurs more often in patients of advanced age, which also limits the therapeutic choices [1, 10].
The best survival seen in patients selected to both HART and surgery is in accordance with the literature [1]. Patients surviving 2 years or more were characterized by having surgery with R0 resection and no or small ATC foci in the surgical specimens. The short survival of the stage 4C patients calls for a different therapeutic approach.
The WHO ps is the doctor’s opinion of the patient’s performance status, and does not take into account his or her own opinion of their quality of life (QLQ). We did not follow WHO performance status of our patients, and only WHO ps at start of therapy was found in the medical records.
In light of the patients’ short life time and the heavy burden of symptoms from disease and treatment, QLQ ought to be a focus of interest. Searching the literature, we have not found any papers which address QLQ in ATC. We have not performed any investigations using HRQLQ ourselves. There are now tools available in many languages for such studies in thyroid cancer [20].
The strengths of this study are that we have seen all ATC patients in one geographical region, and a thorough pathology review. The limitations is are the retrospective nature of a study on a rare disease, and that patients were selected to treatment, which makes comparisons between groups treated in different ways biased.
Conclusion
No patients died in suffocation when treated with HART to 64 Gy with or without surgery. Four patients were dependant on a tracheal cannula when they died.
Survival compares well with literature reports. Patients selected to surgery survived longer than patients not having surgery (median 19 versus 9 months), and only surgery remained as a prognostic factor for survival in a multivariate analysis.
Patients surviving 2 years or more were characterized by having R0 resections and only small foci of ATC in the surgical specimens. Stage 4C patients survived 3 months only.
If HART can be tolerated and R0 resection can be achieved, the prognosis may not be as grim as expected, but efficient treatment for metastatic disease is needed.
References
Bisof V, Rakusic Z, Despot M (2015) Treatment of patients with anaplastic thyroid cancer during the last 20 years: whether any progress has been made? Eur Arch Otorhinolaryngol 272:1553–1567
Withers HR (1985) Biologic basis for altered fractionation schemes. Cancer 55:2086–2095
Simpson WJ (1980) Anaplastic thyroid carcinoma: a new approach. Can J Surg 23:25–27
Tennvall J, Lundell G, Wahlberg P, Bergenfelz A, Grimelius L, Akerman M et al (2002) Anaplastic thyroid carcinoma: three protocols combining doxorubicin, hyperfractionated radiotherapy and surgery. Br J Cancer 86:1848–1853
Hoie J, Brennhovd IO, Host H, Stenwig AE (1986) Anaplastic thyroid carcinomas. Tidsskr Nor Laegeforen 106:2133–2136
DeLellis RA, Lloyd RV, Heitz PU et al (eds) (2004) World health Organization classification of tumours. Pathology and genetics of tumours of endocrine organs. IARC Press, Lyon
The Norwegian Pathology Association (2007) Nasjonale retningslinjer for diagnostikk og behandling av differensiert cancer thyreoidea. http://www.google.no/url?sa=t&rct=j&q=&esrc=s&source=web&cd=1&cad=rja&uact=8&ved=0ahUKEwjU8v681qjRAhUCDywKHasoC24QFggaMAA&url=http%3A%2F%2Flegeforeningen.no%2FFagmed%2FNorsk-endokrinologisk-forening%2FVeiledere-%2FNasjonale-retningslinjer-for-diagnostikk-behandling-og-oppfolging-av-differensiert-cancer-thyreoidea%2F&usg=AFQjCNHXj-ds5nbTD7W127mmYVaEEUYS2g
Wiseman SM, Loree TR, Rigual NR et al (2003) Anaplastic transformation of thyroid cancer: review of clinical, pathologic, and molecular evidence provides new insights into disease biology and future therapy. Head Neck 25:662–670
Wallin G, Bäckdahl M, Tallroth-Ekman E, Lundell G, Auer G, Löwhagen T (1989) Co-existent anaplastic and well differentiated thyroid carcinomas: a nuclear DNA study. Eur J Surg Oncol 15:43–48
Wang Y, Tsang R, Asa S, Dickson B, Arenovich T, Brierley J (2006) Clinical outcome of anaplastic thyroid carcinoma treated with radiotherapy of once- and twice-daily fractionation regimens. Cancer 107:1786–1792
Segerhammar I, Larsson C, Nilsson IL, Backdahl M, Hoog A, Wallin G et al (2012) Anaplastic carcinoma of the thyroid gland: treatment and outcome over 13 years at one institution. J Surg Oncol 106:981–986
Glaser SM, Mandish SF, Gill BS, Balasubramani GK, Clump DA, Beriwal S (2016) Anaplastic thyroid cancer: prognostic factors, patterns of care, and overall survival. Head Neck 38:2083–2090
Swaak-Kragten AT, de Wilt JH, Schmitz PI, Bontenbal M, Levendag PC (2009) Multimodality treatment for anaplastic thyroid carcinoma-treatment outcome in 75 patients. Radiother Oncol 92:100–104
De Crevoisier R, Baudin E, Bachelot A, Leboulleux S, Travagli JP, Caillou B et al (2004) Combined treatment of anaplastic thyroid carcinoma with surgery, chemotherapy, and hyperfractionated accelerated external radiotherapy. Int J Radiat Oncol Biol Phys 60:1137–1143
Dandekar P, Harmer C, Barbachano Y, Rhys-Evans P, Harrington K, Nutting C et al (2009) Hyperfractionated accelerated radiotherapy (HART) for anaplastic thyroid carcinoma: toxicity and survival analysis. Int J Radiat Oncol Biol Phys 74:518–521
Sherman EJ, Lim SH, Ho AL, Ghossein RA, Fury MG, Shaha AR et al (2011) Concurrent doxorubicin and radiotherapy for anaplastic thyroid cancer: a critical re-evaluation including uniform pathologic review. Radiother Oncol 101:425–430
Nachalon Y, Stern-Shavit S, Bachar G et al (2015) Aggressive palliation and survival in anaplastic thyroid carcinoma. JAMA Otolaryngol Head Neck Surg 141:1128–1132
Foote RL, Molina JR, Kasperbauer JL, Lloyd RV, McIver B, Morris JC et al (2011) Enhanced survival in locoregionally confined anaplastic thyroid carcinoma: a single-institution experience using aggressive multimodal therapy. Thyroid 21:25–30
Smallridge RC, Ain KB, Asa SL, Bible KC, Brierley JD, Burman KD et al (2012) American thyroid association guidelines for management of patients with anaplastic thyroid cancer. Thyroid 22:1104–1139
Susanne Singer Susan, Jordan Laura D, Locati et al (2017) The EORTC module for quality of life in patients with thyroid cancer: phase III. Endocr Relat Cancer 24:197–207
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
The study was approved by the Regional Ethical Committee and the hospital’s local authorities, and the patients are fully anonymized in the paper. Permission was given from the Regional Ethical Committee to omit informed consent from the patients, since most of them were dead at the time of data collection.
Conflict of interest
The authors report no conflicts of interest.
Funding
The research was done within the frames of Oslo University Hospital with no external grants.
Rights and permissions
About this article
Cite this article
Jacobsen, AB., Grøholt, K.K., Lorntzsen, B. et al. Anaplastic thyroid cancer and hyperfractionated accelerated radiotherapy (HART) with and without surgery. Eur Arch Otorhinolaryngol 274, 4203–4209 (2017). https://doi.org/10.1007/s00405-017-4764-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00405-017-4764-8